Abstract
The possibility that exposures to environmental agents are associated with reproductive disorders in human populations has generated much public interest recently. Phthalate esters are used most commonly as plasticizers in the food and construction industry, and di-(2-ethylhexyl) phthalate (DEHP) is the most abundant phthalate in the environment. Daily human exposure to DEHP in the U.S. is significant, and occupational and clinical exposures from DEHP-plasticized medical devices, e.g., blood bags, hemodialysis tubing, and nasogastric feeding tubes, increase body burden levels. We investigated the effects of chronic exposures to low environmentally relevant DEHP levels on testicular function. Our data show that prolonged exposures to this agent induced high levels of the gonadotropin luteinizing hormone and increased the serum concentrations of sex hormones [testosterone and 17β-estradiol (E2)] by >50%. Increased proliferative activity in Leydig cells was evidenced by enhanced expression of cell cycle proteins, as determined by RT-PCR. The numbers of Leydig cells in the testis of DEHP-treated rats were 40–60% higher than in control rats, indicating induction of Leydig cell hyperplasia. DEHP-induced elevations in serum testosterone and E2 levels suggest the possibility of multiple crosstalks between androgen, estrogen, and steroid hormone receptors, whereas the presence of estrogen receptors in nonreproductive tissues, e.g., cardiovascular system and bones, implies that the increases in serum E2 levels have implications beyond reproduction, including systemic physiology. Analysis of the effects of phthalate exposures on gonadotropin and steroid hormone levels should form part of overall risk assessment in human populations.
Reports of a higher incidence of urogenital anomalies of the newborn, such as cryptorchidism, hypospadias, and reproductive abnormalities in wild life exposed to high levels of chemicals in the environment, have generated public concern that these agents may impair human reproductive health (1, 2). Phthalates are used as plasticizers in certain infant toys and consumer products (e.g., containers for soaps, shampoos, and perfumes) and medical devices such as tubings and catheters. The U.S. Department of Health and Human Services in 1985 (3) estimated the total daily human consumption of di-(2-ethylhexyl) phthalate (DEHP) from all sources of exposure at 5.8 mg in the U.S. In a report just published by the Center for Disease Control and Prevention, the urinary levels of mono-(ethylhexyl) phthalate (MEHP) (micrograms per liter), which is the chief metabolite of DEHP, ranged from 3.26 to 4.15 in males and 2.93 to 3.51 in females; these levels are thought to represent only one-tenth of the ingested DEHP dose within the previous 24 h (4). In a recent review of laboratory studies, the U.S. National Toxicology Program's Center for the Evaluation of Risks to Human Reproduction Expert Panel concluded that DEHP has the potential to produce adverse reproductive effects in humans (5). Indeed, several proposed mechanisms by which this agent affects testicular function in rats and mice are relevant to humans, e.g., depletion of testicular iron and zinc, alteration of antioxidant status, and inhibition of phospholipase A2 (6, 7).
Previous studies of phthalates were generally conducted with high doses and using short exposure periods. However, acute exposure paradigms do not approximate real-life situations when human populations are subjected to prolonged low-level exposures. Moreover, chronic exposures (a duration of 4 wk or longer in the present study) provide a better model to study the effects of endocrine disruptors on the hypothalamo–pituitary–testicular (HPT) axis, which regulates reproductive function. Gonadal steroids, acting at the pituitary level to regulate gonadotropin secretion and on the hypothalamus to affect gonadotropin-releasing hormone (GnRH) pulses, modulate the HPT axis. Hypothalamic control of gonadotropin secretion is exerted via the GnRH receptor in gonadotropes (8). Leydig cells are the only binding sites for luteinizing hormone (LH) in the testis, and LH is the primary tropic hormone that stimulates Leydig cell function in the postnatal testis (9). We previously observed simultaneous elevations in serum LH and testosterone (T) levels and increased Leydig cell steroidogenesis in rats that were treated with 10 and 100 mg/kg per day DEHP for 28 days (10). The higher-than-normal LH levels seen after DEHP treatment, occurring in the presence of elevated serum T concentrations, implies that regulatory pathways in the HPT axis were disrupted.
High-serum LH levels stimulate androgen biosynthesis in Leydig cells in the short term but may cause premature deterioration of steroidogenic capacity if prolonged and/or induce Leydig cell proliferation (11). Therefore, the present study was designed to (i) determine the effect of DEHP-induced LH overstimulation on Leydig cell steroidogenesis and (ii) investigate whether LH overstimulation affects Leydig cell numbers. We show herein that prolonged DEHP exposures impair Leydig cell steroidogenesis and induce Leydig cell hyperplasia. DEHP-induced LH overstimulation, in concert with Leydig cell hyperplasia, caused chronic elevations in serum T levels. Other studies have demonstrated that males with persistently high LH and T levels have increased risk of precocious puberty and testicular tumors (12, 13).
Materials and Methods
General. All animal procedures were performed in accordance with the policies of The Rockefeller University's Animal Care and Use Committee. In all experiments, each treatment group consisted of at least 10 rats. Dosages (0, 10, or 100 mg/kg per day DEHP) were selected based on our previously determined lowest-observed-effect level in pubertal rats (10). In the present study, Long-Evans rats were gavaged with DEHP or the corn oil vehicle daily, from postnatal day (PND) 21, i.e., at weaning, to day 48, 90, or 120. Exposures to 0, 10, or 100 mg/kg per day DEHP caused no overt toxicity, as determined by body (555 ± 24, 548 ± 14, and 571 ± 24 g) and paired testes weights (3.9 ± 0.08, 3.7 ± 0.14, and 3.8 ± 0.05 g) measured at the end of the longest exposure period, i.e., PND 21–120. All data were analyzed by one-way ANOVA with multiple comparisons performed by the Duncan multiple range tests to identify differences between groups. Data are presented as mean ± SEM. Differences were considered significant at P ≤ 0.05.
Effect of Chronic DEHP Exposures on Androgen Biosynthesis. To determine the effect of chronic DEHP exposures on Leydig cell T production, male prepubertal Long-Evans rats were assigned to one of three groups and gavaged with 0, 10, or 100 mg/kg per day DEHP from PND 21–90 or 120 (Experiment I). This 70- or 100-day exposure period is 2.5- to 3.5-fold longer than that used in our initial study (28 days) (10). At the end of treatment, serum hormone levels (LH and T) were measured by RIA. Measurement of T production by Leydig cells was done ex vivo.
Evaluation of Proliferative Activity. Because increased LH levels promote Leydig cell hyperplasia, we explored the possibility of increased proliferative capacity in Leydig cells by using three criteria: (i) expression of cell division cycle markers, (ii) tritiated thymidine incorporation, and (iii) changes in cell number. In a second set of experiments (Experiment II), rats were assigned to one of three groups and gavaged with 0, 10, or 100 mg/kg DEHP from PND 21–90 or 120. Using RT-PCR and primer sets based on their published sequences, we measured steady-state mRNA levels for a number of cell cycle regulators, namely proliferating cell nuclear antigen (PCNA), cyclins D3 and G1, and the tumor suppressor protein p53, at the end of DEHP treatment. PCNA initiates DNA replication and is a marker of decreased doubling time, whereas cyclin D3 mediates the G1 to S transition phase during the cell cycle (14, 15). Induction of the p53 tumor suppressor protein is the cellular response to several potentially damaging extracellular stimuli (16), and cyclin G1 is a down-stream mediator of p53 (17). Purified Leydig cells were labeled with [3H]thymidine during a 3-h incubation period to determine the rate of thymidine incorporation into DNA at mitosis. Finally, the number of Leydig cells in the testis of DEHP-treated rats vs. controls was enumerated by stereology.
Evaluation of Estrogen Biosynthesis. To test the hypothesis that DEHP-induced LH overstimulation induced estrogen biosynthesis, rats were assigned to one of three groups and gavaged with 0, 10, or 100 mg/kg per day DEHP from 21 to 90 days of age (Experiment III). We then measured serum 17β-estradiol (E2) levels, Leydig cell E2 production, and aromatase gene (Cyp 19) expression in Leydig cells at two time points: days 48 and 90. Conversion of androgen to estrogen in all tissues is catalyzed by the aromatase enzyme (18).
Hormone Assays. Serum LH concentrations were measured by using 125I rat LH (Covance Laboratories, Vienna, VA) and materials obtained from the National Hormone and Pituitary Program, namely, rat antibody NIDDK-anti-rLH-S11 (NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases), and LH reference standards (NIDDK-rLF-RP-3). The secondary IgG antiserum was supplied by ICN. The lower limit of detection for this assay is 0.12 ng/ml, and LH values were expressed in relation to the standards. The intra- and interassay coefficients of variation were 5% and 10%, respectively. Steroid hormone (T and E2) concentrations were measured by a previously described tritium-based RIA validated for use with rat antiserum (10).
Analysis of Serum MEHP and DEHP Levels by HPLC. Ingestion of large doses of DEHP potentially overwhelms the hydrolytic ability of the gut to convert DEHP into MEHP, and both can be measured in the blood. Therefore, we measured the serum levels of both compounds by HPLC, as described (19). Di-n-heptyl phthalate (DNHP, 1 μg) was included in each serum sample as the internal standard. Elution was performed by using a mobile phase consisting of acetonitrile-aqueous buffer (0.08% triethylamine adjusted to pH 2.8 with 1 M phosphoric acid) at a flow rate of 1.0 ml/min. MEHP, DNHP, and DEHP were eluted at 5.1, 15.1, and 19.9 min, respectively (Shimadzu). Serum levels were calculated by using the peak ratio (MEHP or DEHP/DNHP peak area) on the calibration curves obtained during the validation of methods. The limit of detection for MEHP and DEHP was 100 ng/ml.
Purification of Leydig Cells. Leydig cells were isolated from the testes by a previously published procedure (10, 20). Incubations were conducted at a temperature of 34°C for 3 h without (basal) and with a maximally stimulating dose of ovine LH (100 ng/ml). Ovine LH was provided by the National Hormone and Pituitary Program (NIDDK, Rockville, MD). T and E2 production values were normalized to ng/106 cells.
Determination of Leydig Cell Number. Testes were obtained after whole-body perfusion of control and DEHP-treated rats with Bouin's fixative and stored until embedded in paraffin. Testicular tissue was processed for histological evaluation by light microscopy. To enumerate Leydig cell numbers, sampling of testicular tissue was done according to the fractionator technique (21). Identification of Leydig cells was facilitated by processing tissue for immunocytochemistry and staining with a polyclonal antibody specific for the steroidogenic enzyme 3β-HSD (generously donated by Van Luu-The, University of Laval, Quebec, PQ, Canada). Approximately 10 sections were sampled from each testis. The total number of Leydig cells was calculated by multiplying the number of Leydig cells counted in a known fraction of the testis by the inverse of the sampling probability.
RT-PCR Analysis. Four hundred nanograms of total RNA was reverse transcribed with avian myeloblastosis virus reverse transcriptase, random primers, and deoxy-NTPs at 37°C for 75 min, and heating at 95°C for 5 min terminated the reaction. Target cDNA amplification was performed by PCR and using published sequences (14–18, 22) conducted four to five times for each gene of interest. Densitometric signals from individual bands were divided by the respective density for rat ribosomal protein S16 to correct for differences in gel loading (Kodak).
Results
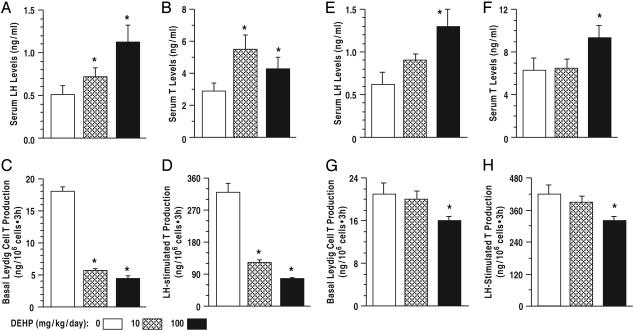
DEHP Exposure Induces High-Serum LH and T Levels, but T Biosynthesis Was Decreased. After DEHP treatment from 21 to 90 days of age, serum LH and T levels were higher in DEHP-treated rats compared to control (Fig. 1 A and B; P < 0.01), signifying increased LH stimulation of Leydig cells in vivo. In contrast, basal and LH-stimulated T production per Leydig cell, measured ex vivo, was reduced to <50% of control (Fig. 1 C and D; P < 0.01). Similarly, serum LH levels were elevated after DEHP treatment from days 21 to 120 (Fig. 1E), whereas serum T was elevated only in rats exposed to 100 mg/kg DEHP (Fig. 1F). Basal and LH-stimulated T production by Leydig cells from control and rats treated with 10 mg/kg DEHP was comparable but was decreased after exposure to 100 mg/kg DEHP (Fig. 1 G and H). We hypothesize that elevated serum T levels were due to higher numbers of Leydig cells in DEHP-treated rats (Table 1). However, there is a possibility that increased steroidogenesis in extragonadal tissues, e.g., the adrenals, contributes to the rise in serum androgen levels.
Fig. 1.
Effect of DEHP treatment (A–D, PND 21–90; E–H, PND 21–120) on serum LH and T levels and Leydig cell T production. Serum LH (A and E)andT(B and F) levels were higher in DEHP-treated rats than control, but basal and LH-stimulated T production per Leydig cell was decreased (C, D, and G, H). Thus, elevated serum T levels in the presence of reduced steroidogenic capacity is presumably due to Leydig cell hyperplasia. Leydig cell T production, normalized to nanograms per 106 cells, was measured by RIA in aliquots of the spent media after incubation of Leydig cells for 3 h. n = 10; *, P < 0.01 compared to control. Data are presented as mean ± SEM.
Table 1. Patterns of change in serum hormone levels and Leydig cell steroidogenesis during DEHP treatment.
| Treatment period
|
|||
|---|---|---|---|
| Parameter | PND 21-48* | PND 21-90 | PND 21-120 |
| Serum hormones | |||
| LH | ↑ | ↑ | ↑ |
| T | ↑ | ↑ | ↑ |
| E2 | ↑ | — | ND |
| Leydig cell steroidogenesis | |||
| T | ↑ | ↓ | ↓ |
| E2 | ↑ | ↓ | ND |
| Number of Leydig cells in the testis | ND | ↑ | ↑ |
↑, increase; ↓, decrease; —, unchanged; ND, not determined.
Observations from ref. 10, except E2 measurements (present study).
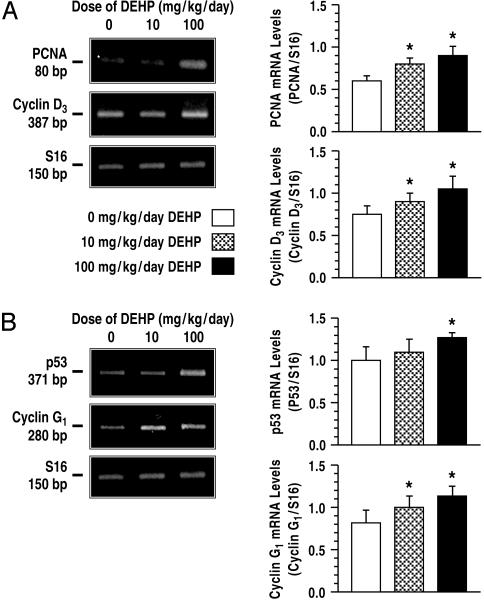
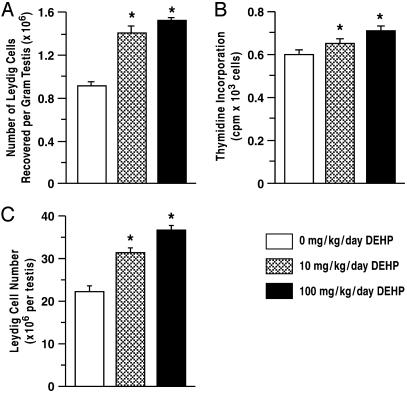
DEHP Exposure Increased Proliferative Activity in Leydig Cells. Compared to control, steady-state mRNA levels for PCNA and cyclin D3 in Leydig cells were increased after DEHP treatment from days 21 to 90 (Fig. 2A; P < 0.05). These findings support the hypothesis that PCNA and cyclin D3 are expressed at higher levels in proliferating than in nondividing Leydig cells (23). Because cyclin G1 is transcriptionally activated by p53 and acts as a downstream mediator, it is not surprising that cyclin G1 is expressed at higher levels in DEHP-treated Leydig cells, compared to control, in parallel with p53 (Fig. 2B; P < 0.05). Consistent with a higher number of Leydig cells in the testis of DEHP-treated rats, cell yields from the isolation procedure after treatment with 10 or 100 mg/kg per day DEHP were >50% greater than control (Fig. 3A; P < 0.01). Although mature rat Leydig cells typically do not undergo mitosis beyond 60 days of age (24), a modest but significant increase in DNA incorporation of thymidine by DEHP-treated Leydig cells was observed after treatment from days 21 to 120 (Fig. 3B; P < 0.01). Moreover, a 40–60% increase in the numbers of Leydig cells was observed in the testes of DEHP-treated rats compared to controls (Fig. 3C; P < 0.01), indicating that chronic exposures to DEHP induce Leydig cell hyperplasia.
Fig. 2.
Effect of DEHP treatment on expression of cell cycle proteins. DEHP treatment from 21 to 90 days of age increased expression of several cell cycle proteins [PCNA and cyclins D3 (A) and the tumor suppressor protein p53 and cyclin G1 (B)]. Increased proliferative activity in Leydig cells is therefore due to induction of cell cycle proteins by DEHP treatment. Total RNA was obtained from two separate experiments, and rat ribosomal protein S16 (S16) was used as internal control for PCR amplification. p53, tumor suppressor protein; *, P < 0.05 compared to control. Data are presented as mean ± SEM.
Fig. 3.
Effect of DEHP treatment on Leydig cell numbers. After DEHP treatment of rats from 21 to 90 days of age (n = 10), the number of Leydig cells recovered from the testis was increased (A). DEHP treatment from PND 21 to 120 increased thymidine incorporation by Leydig cells (B), and the numbers of Leydig cells, counted in both testes from four animals in each group, were increased by as much as 40–60% in DEHP-treated rats compared to control (C). These observations confirm that chronic DEHP exposures induced Leydig cell hyperplasia. *, P < 0.01 compared to control. Data are presented as mean ± SEM.
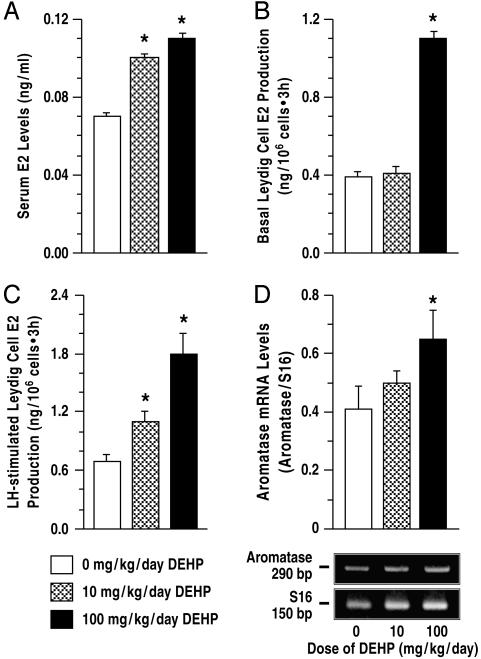
DEHP Exposure Increased Estradiol Biosynthesis in Leydig Cells. Serum E2 levels (ng/ml) were elevated by as much as 50% above control levels after DEHP treatment from days 21 to 48 (Fig. 4A). Increased serum E2 levels are the result of enhanced E2 biosynthesis, because basal and LH-stimulated E2 production by Leydig cells after exposure to 10 and 100 mg/kg per day DEHP was ≈50% and 150% greater than control (Figs. 4 B and C; P < 0.01). Aromatase gene expression was also expectedly higher in Leydig cells (Fig. 4D; P < 0.05). By day 90, however, serum E2 levels were equivalent in control (0.19 ± 0.01 ng/ml) and DEHP-treated rats (0.18 ± 0.01 and 0.21 ± 0.01; P > 0.05), although LH-stimulated Leydig cell E2 production was decreased (1.74 ± 0.14, 1.02 ± 0.06, and 0.86 ± 0.08 (ng/106 cells·3 h; P < 0.01). Thus, DEHP-treated Leydig cells produced more E2 than control, i.e., on day 48, implying that testicular tissue, including Leydig cells, was exposed to unusually high estrogen levels in the course of DEHP treatment. The presence of similar serum E2 concentrations in all groups of rats after E2 production per Leydig cell was decreased, i.e., on day 90, is indicative of increased numbers of Leydig cells in DEHP-treated rats.
Fig. 4.
Effect of DEHP treatment on estrogen biosynthesis. Serum E2 levels (A) were higher due to increased Leydig cell E2 production (B and C) and increased aromatase gene expression (D) after DEHP treatment of rats from 21 to 48 days of age (n = 10). E2 production was normalized to nanograms per 106 cells and measured by RIA in aliquots of the spent media after incubation of Leydig cells for 3 h. S16, rat ribosomal protein S16; *, P < 0.01 compared to control. Data are presented as mean ± SEM.
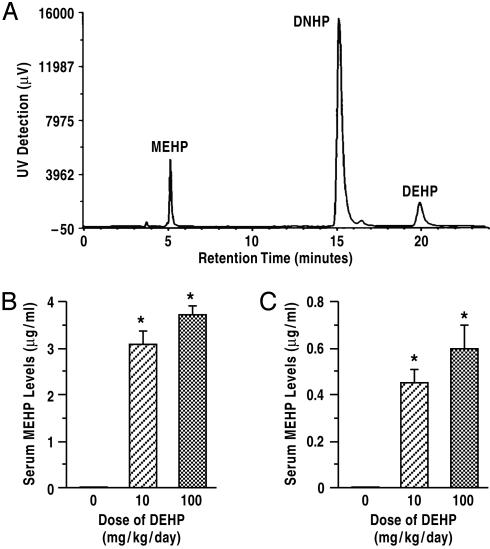
DEHP Effects on Leydig Cell Function Are Due to MEHP Action. We measured serum MEHP and DEHP levels at two time points, i.e., after 28- or 70-day DEHP treatment periods, corresponding to DEHP exposures from PND 21–48 or 90. Only MEHP was detectable in the blood at both time points, and higher levels were measured on PND 48 (Fig. 5B) than on day 90 (Fig. 5C). These measurements imply that DEHP effects were due mostly to the action of MEHP and its metabolites in Leydig cells.
Fig. 5.
Serum MEHP levels in DEHP-treated rats (n = 10). HPLC chromatogram of MEHP, DNHP, and DEHP is shown in A, indicating retention times of 5.1, 15.1, and 19.9 min, respectively. Serum MEHP levels measured on PND 48 after a 28-day DEHP treatment period (B) were much higher than was measured on PND 90 after a 70-day treatment period (C). DNHP was used as internal standard. *, P < 0.01 compared to control. Data are presented as mean ± SEM.
Discussion
The present study demonstrates that DEHP induces an increase in the population of Leydig cells associated with chronically elevated serum LH and T levels (Table 1). The decrease in T biosynthesis together with increased E2 production is apparently due to LH induction of aromatase activity in Leydig cells. Disturbances in testicular steroidogenesis and Leydig cell hyperplasia have also been reported in transgenic mice overexpressing human chorionic gonadotropin, an analogue of LH (25, 26). Transgenic female mice with chronically elevated LH levels are characterized by precocious puberty and elevated serum estrogen levels with granulosa cell tumors (27), and high-serum E2 levels are known to induce cell proliferation in estrogen-dependent tissues (28). Similarly, males with persistently high LH and T levels are known to develop precocious puberty (13). The present observations thus provide a potential mechanism for an environmentally induced precocious puberty in males analogous to the premature mammary gland development in young girls associated with high-serum phthalate levels (29).
We propose that increased Leydig cell E2 production (Fig. 4), acting in an autocrine fashion and in concert with LH over-stimulation (Figs. 1 and 6), stimulated activity of cyclin proteins to achieve increased Leydig cell numbers (Fig. 3). The number of cells in a tissue is determined by the rate of mitosis as well as programmed cell death (apoptosis) (30). Other studies have suggested that Leydig cell hyperplasia is associated with disturbances in paracrine relationships between Sertoli and Leydig cells due to germ cell loss and testicular atrophy (31). In this regard, the dosages used in the present study caused no histological changes in the testis, as determined by light microscopy (data not shown). However, exposure of rats to di(n-butyl)phthalate, a compound closely related to DEHP, increased expression of two antiapoptotic genes in the testis: T-repressed prostate message-2 and bcl-2 (14). Therefore, testicular damage and/or inhibition of the apoptotic pathway may contribute to phthalate induction of Leydig cell hyperplasia.
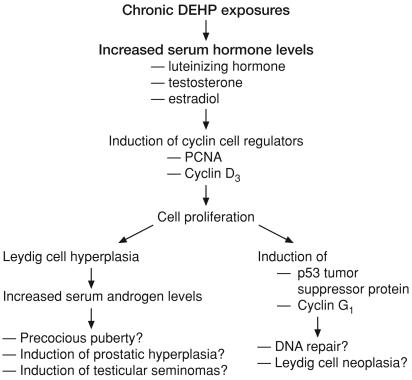
Fig. 6.
A schema summarizing DEHP effects on Leydig cell function. Chronic DEHP exposures increased the serum levels of the gonadotropin LH and the sex steroids T and estradiol. These effects presumably induce cell cycle proteins and cause Leydig cell proliferation and hyperplasia. Increased serum androgen levels in DEHP-treated rats may result in precocious puberty and induce hyperplasia of the prostatic epithelium as well as promote development of testicular seminomas. Furthermore, induction of the tumor suppressor protein p53 is suggestive of DNA repair activity and represents a potential for neoplasia.
Although Leydig cell adenomas represent the most common tumors of the gonadal stroma in humans (32), it is not known whether phthalates have a role in their etiology, given divergent phthalate metabolic pathways in primates and rodents (33). DEHP is metabolized by intestinal lipases to MEHP, which is glucuronized and rapidly excreted from the body with little or no tissue accumulation (34). Primates, including humans, are known to have higher capacities for glucuronidation than rodents (35). It has also been suggested that the pharmacokinetics of phthalate esters protects humans from toxic effects seen in rodents, e.g., the pathway mediated by peroxisome proliferator-activated receptor-α, but such protective effects, if any, may not apply to testicular toxicity (36). Nevertheless, it remains to be determined whether the effects of chronic DEHP exposures are reversed or mitigated when exposure is terminated or reduced. Measurement of smaller amounts of MEHP at 90 days, compared to 48 days (Fig. 5), suggests that lesser amounts of this agent get into systemic circulation with advancing age; this probably explains the greater decrease in Leydig cell androgen biosynthesis seen on day 90 (Fig. 1 C and D) compared to day 120 (Fig. 1 G and H). Prenatal DEHP exposures decreased serum LH and T levels at 21 and 35 days, but these effects were absent at 90 days (10). Together, these observations indicate reversibility of some DEHP effects after a period and suggest that the pubertal period, compared to adulthood, represents a period of greater vulnerability to DEHP effects. However, DEHP effects that occur in the pubertal period may impact reproductive tract development and function at a later time. Moreover, the rat Leydig cell has fewer LH receptors, ≈10% of the human (37), implying that enhanced serum LH concentrations could stimulate Leydig cells to a greater extent in humans than in the rat. The increase in p53 levels in DEHP-treated Leydig cells suggests increased DNA repair activity and constitutes a potential for induction of neoplasia (38).
Acceptable human exposures to specific chemicals are calculated by reducing the no-observed-adverse-effect levels (NOAEL) from animal experiments by uncertainty factors to account for susceptible populations and interspecies differences (39). The NOAEL for DEHP in pubertal rats was previously determined to be 1 mg/kg per day (10). Extrapolating from our data, acceptable human daily intake would be 10 μg/kg per day, which is far lower than the levels of human daily exposures estimated at 82.9 μg/kg for a 70-kg man (5.8 mg/day) (3). Our data indicate that the rat gut is able to hydrolyze DEHP into MEHP efficiently, as suggested (40). On the other hand, the serum levels of DEHP measured in thelarche patients (187–2,098 μg/liter) were much higher than MEHP levels (6.3–38) (29). These observations imply that DEHP is less actively hydrolyzed and more easily absorbed from the gut, at least in young individuals, and DEHP-related effects in humans may result not from a downstream metabolite such as is the case in rats but rather from direct DEHP action (41). Altogether, the presence of measurable amounts of DEHP in human tissues given the homology between organ systems affected by DEHP in animal models and human systems indicates a potential for adverse effects on sexual development, particularly in children exposed to DEHP.
In conclusion, the present study highlights two issues that are relevant to the action of endocrine disruptors in reproductive tissues. First, the effects of acute exposures to DEHP may differ significantly from those associated with chronic exposures. For example, elevations in serum sex steroid levels were not observed until after 4 wk of DEHP exposure in the present study. Because exposures in vivo may affect multiple sites within the hypothalamo–pituitary–testicular axis, chronic exposures can provide a model in which latent effects that arise, e.g., in the pituitary, in response to events in other organs, e.g., in the testis, become apparent at a later time. Therefore, effects associated with chronic exposures of laboratory species to chemical agents, rather than acute exposures, are probably more related to the pattern of human exposures. Second, our observations support the hypothesis that several chemicals have both estrogenic and antiandrogenic activity (42). Although DEHP and other phthalates do not bind the androgen receptor but suppress androgen-stimulated sexual differentiation, they are considered prototype antiandrogens (43). Herein, we show that DEHP is indirectly estrogenic because it increased serum E2 levels, presumably due to LH induction of aromatase activity in Leydig cells. Simultaneous elevations in serum T and E2 levels suggest the possibility of multiple crosstalks between androgen, estrogen, and steroid hormone receptors, indicating that the mechanisms of chemical-induced effects may be more complex than previously thought. Moreover, estrogen receptors are present in other tissues, e.g., cardiovascular system and bones; therefore, DEHP-induced increases in serum E2 levels have implications beyond reproduction, including systemic physiology (44). In summary, analysis of the effects of phthalate exposures on gonadotropin and steroid hormone levels should form part of overall risk assessment in human populations.
Acknowledgments
We are grateful to Dr. Guimin Wang and Michael Holmes for technical help. Many thanks also to Evan Read for help with manuscript preparation. This work was supported in part by National Institute of Environmental Health Sciences Grant ES 10233.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DEHP, di-(2-ethylhexyl) phthalate; MEHP, mono-(ethylhexyl) phthalate; LH, luteinizing hormone; T, testosterone; E2, 17β-estradiol; PCNA, proliferating cell nuclear antigen; DNHP, di-n-heptyl phthalate; PND, postnatal day.
References
- 1.Sharpe, R. M. (2001) Toxicol. Lett. 120, 221–232. [DOI] [PubMed] [Google Scholar]
- 2.Akingbemi, B. T. & Hardy, M. P. (2001) Ann. Med. 33, 391–403. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services (1985) Fourth Annual Report on Carcinogenesis (U.S. Dept. of Health and Human Services, Washington, DC), National Toxicology Program Publication No. 85-002.
- 4.U.S. Centers for Disease Control and Prevention (2003) Human Exposures to Environmental Chemicals (U.S. Centers for Disease Control and Prevention, Atlanta).
- 5.National Toxicology Program Center for the Evaluation of Risks to Human Reproduction (2000) NTP-CERHR Expert Panel Report NTP-CERHR-DEHP-00 (National Toxicology Program Center for the Evaluation of Risks to Human Reproduction, Washington, DC). [DOI] [PubMed]
- 6.Labow, R. S., Meek, E., Adams, G. A. & Rock, G. (1988) Environ. Health Perspect. 78, 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters, J. M., Taubeneck, M. W., Keen, C. L. & Gonzalez, F. J. (1997) Teratology 56, 311–316. [DOI] [PubMed] [Google Scholar]
- 8.Shupnik, M. A. (1996) Biol. Reprod. 54, 279–286. [DOI] [PubMed] [Google Scholar]
- 9.Ewing, L. L., Wing, T. Y., Cochran, R. C., Kromann, N. & Zirkin, B. R. (1983) Endocrinology 112, 1763–1769. [DOI] [PubMed] [Google Scholar]
- 10.Akingbemi, B. T., Youker, R. T., Sottas, C. M., Ge, R., Katz, E., Klinefelter, G. R., Zirkin, B. R. & Hardy M. P. (2001) Biol. Reprod. 65, 1252–1259. [DOI] [PubMed] [Google Scholar]
- 11.Neumann, F. (1991) Mutat. Res. 248, 341–356. [DOI] [PubMed] [Google Scholar]
- 12.Laue, L., Chan, W. Y., Hsueh, A. J., Kudo, M., Hsu, S. Y., Wu, S. M., Blomberg, L. & Cutler, G. B., Jr. (1995) Proc. Natl. Acad. Sci. USA 92, 1906–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin, M. M., Wu, S. M., Martin, A. L., Rennert, O. M. & Chan, W. Y. (1998) Eur. J. Endocrinol. 139, 101–106. [DOI] [PubMed] [Google Scholar]
- 14.Shultz, V. D., Phillips, S., Sar, M., Foster, P. M. & Gaido, K. W. (2001) Toxicol. Sci. 64, 233–242. [DOI] [PubMed] [Google Scholar]
- 15.Qian, X., Kulig, E., Jin, L. & Lloyd, R. V. (1998) Endocrinology 139, 2058–2067. [DOI] [PubMed] [Google Scholar]
- 16.Zakut-Houri, R., Oren, M., Bienz, B., Lavie, V., Hazum, S. & Givol, D. (1983) Nature 306, 594–597. [DOI] [PubMed] [Google Scholar]
- 17.Tamura, K., Kanaoka, Y., Jinno, S., Nagata, A., Ogiso, Y., Shimizu, K., Hayakawa, T., Nojima, H. & Okayama, H. (1993) Oncogene 8, 2113–2118. [PubMed] [Google Scholar]
- 18.Genissel, C. & Carreau, S. (2001) Mol. Cell Endocrinol. 178, 141–146. [DOI] [PubMed] [Google Scholar]
- 19.Kambia, K., Dine, T., Gressier, B., Germe, A. F., Luyckx, M., Brunet, C., Michaud, L. & Gottrand, F. (2001) J. Chromatogr. 755, 297–303. [DOI] [PubMed] [Google Scholar]
- 20.Payne, A. H., Downing, J. R. & Wong, K. L. (1980) Endocrinology 106, 1424–1429. [DOI] [PubMed] [Google Scholar]
- 21.Petersen, P. M. & Pakkenberg, B. (2000) Image Anal. Stereol. 19, 215–218. [Google Scholar]
- 22.Chan, Y. L., Paz, V., Olvera, J. & Wool, I. G. (1990) FEBS Lett. 263, 85–88. [DOI] [PubMed] [Google Scholar]
- 23.Sriraman, V., Rao, V. S., Sairam, M. R. & Rao, A. J. (2000) Mol. Cell Endocrinol. 162, 113–120. [DOI] [PubMed] [Google Scholar]
- 24.Hardy, M. P., Gelber, S. J., Zhou, Z. F., Penning, T. M., Ricigliano, J. W., Ganjam, V. K., Nonneman, D. & Ewing, L. L. (1991) Ann. NY Acad. Sci. 637, 152–163. [DOI] [PubMed] [Google Scholar]
- 25.Matzuk, M. M., DeMayo, F. J., Hadsell, L. A. & Kumar, T. R. (2003) Biol. Reprod. 69, 338–346. [DOI] [PubMed] [Google Scholar]
- 26.Rulli, S. B., Kuorelahti, A., Karaer, O., Pelliniemi, L. J., Poutanen, M. & Huhtaniemi, I. (2002) Endocrinology 143, 4084–4095. [DOI] [PubMed] [Google Scholar]
- 27.Risma, K. A., Clay, C. M., Nett, T. M., Wagner, T., Yun, J. & Nilson, J. H. (1995) Proc. Natl. Acad. Sci. USA 92, 1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosland, M. C. (1996) Prog. Clin. Biol. Res. 394, 309–352. [PubMed] [Google Scholar]
- 29.Colon, I., Caro, D., Bourdony, C. J. & Rosario, O. (2000) Environ. Health Perspect. 108, 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunez, G., Benedict, M. A., Hu, Y. & Inohara, N. (1998) Oncogene 17, 3237–3245. [DOI] [PubMed] [Google Scholar]
- 31.Foster, P. M., Mylchreest, E., Gaido, K. W. & Sar, M. (2001) Hum. Reprod. Update 7, 231–235. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins, C. & Miaskowski, C. (1996) Oncol. Nurs. Forum 23, 1203–1211. [PubMed] [Google Scholar]
- 33.Rhodes, C., Orton, T. C., Pratt, I. S., Batten, P. L., Bratt, H., Jackson, S. J. & Elcombe, C. R. (1986) Environ. Health Perspect. 65, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albro, P. W., Jordan, S. T., Schroeder, J. L. & Corbett, J. T. (1982) J. Chromatogr. 244, 65–79. [DOI] [PubMed] [Google Scholar]
- 35.Huber, W. W., Grasl-Kraupp, B. & Schulte-Hermann, R. (1996) Crit. Rev. Toxicol. 26, 365–481. [DOI] [PubMed] [Google Scholar]
- 36.Ward, J. M., Peters, J. M., Perella, C. M. & Gonzalez, F. J. (1998) Toxicol. Pathol. 26, 240–246. [DOI] [PubMed] [Google Scholar]
- 37.Min, L. & Ascoli, M. (2000) Mol. Endocrinol. 14, 1797–1810. [DOI] [PubMed] [Google Scholar]
- 38.Vaslet, C. A., Messier, N. J. & Kane, A. B. (2002) Toxicol. Sci. 68, 331–338. [DOI] [PubMed] [Google Scholar]
- 39.Moore, R. W., Rudy, T. A., Lin, T. M., Ko, K. & Peterson, R. E. (2001) Environ. Health Perspect. 109, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albro, P. W., Chapin, R. E., Corbett, J. T., Schroeder, J. & Phelps, J. L. (1989) Toxicol. Appl. Pharmacol. 100, 193–200. [DOI] [PubMed] [Google Scholar]
- 41.Barr, D. B., Silva, M. J., Kato, K., Reidy, J. A., Malek, N. A., Hurtz, D., Sadowski, M., Needham, L. L. & Calafat, A. M. (2003) Environ. Health Perspect. 111, 1148–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maness, S. C., McDonnell, D. P. & Gaido, K. W. (1998) Toxicol. Appl. Pharmacol. 151, 135–142. [DOI] [PubMed] [Google Scholar]
- 43.Gray, L. E. Jr., Wolf, C., Lambright, C., Mann, P., Price, M., Cooper, R. L. & Ostby, J. (1999) Toxicol. Ind. Health 15, 94–118. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson, S. & Gustafsson, J. A. (2002) Crit. Rev. Biochem. Mol. Biol. 37, 1–28. [DOI] [PubMed] [Google Scholar]