Abstract
The ability to control/regulate emotions is an important coping mechanism in the face of emotionally stressful situations. Although significant progress has been made in understanding conscious/deliberate emotion regulation (ER), less is known about non-conscious/automatic ER and the associated neural correlates. This is in part due to the problems inherent in the unitary concepts of automatic and conscious processing1. Here, we present a protocol that allows investigation of the neural correlates of both deliberate and automatic ER using functional magnetic resonance imaging (fMRI). This protocol allows new avenues of inquiry into various aspects of ER. For instance, the experimental design allows manipulation of the goal to regulate emotion (conscious vs. non-conscious), as well as the intensity of the emotional challenge (high vs. low). Moreover, it allows investigation of both immediate (emotion perception) and long-term effects (emotional memory) of ER strategies on emotion processing. Therefore, this protocol may contribute to better understanding of the neural mechanisms of emotion regulation in healthy behaviour, and to gaining insight into possible causes of deficits in depression and anxiety disorders in which emotion dysregulation is often among the core debilitating features.
Protocol
1. Task Design, Stimuli, and Experimental Protocol
The overarching protocol involves assessments of emotional ratings of pictures (immediate impact), and of the memory for these pictures (long-term impact), as a result of inducing the goal to regulate emotional responses. The goal to regulate is induced explicitly or implicitly, and the immediate and long-term impact is assessed relative to ratings of and memory for pictures presented during baseline blocks/runs that precede the ER manipulations (pre-ER induction baseline); see Figure 1 for a diagram of the overall experimental design. Below we provide details regarding all these aspects.
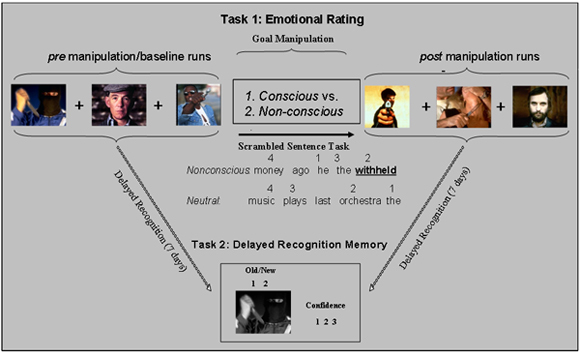 Figure 1. Diagram of the protocol. The ER goal is induced in each participant both consciously/explicitly and non-consciously/implicitly, but the order of induction is different and counterbalanced across participants - i.e., those assigned to explicit ER in the first part complete the implicit manipulation in the second part, and vice versa; each manipulation is preceded by its own baseline runs. See below for detailed descriptions of the Rating and Memory tasks.
Figure 1. Diagram of the protocol. The ER goal is induced in each participant both consciously/explicitly and non-consciously/implicitly, but the order of induction is different and counterbalanced across participants - i.e., those assigned to explicit ER in the first part complete the implicit manipulation in the second part, and vice versa; each manipulation is preceded by its own baseline runs. See below for detailed descriptions of the Rating and Memory tasks.
Emotion Regulation Manipulation
Explicit ER manipulation. In the explicit ER condition, the emotion rating task is preceded by instructions to suppress emotional experience and expression of emotional responses. Previous evidence suggests that explicit ER strategies can reliably influence emotional responding2.
Implicit ER manipulation. For this, an adaptation of the Scrambled Sentence Task (SST)3 is used to non-consciously induce the goal to regulate emotions. In this task, participants have to construct 20 four-word grammatically correct sentences from five-word jumbles that have embedded words that convey the idea of emotion control (e.g., "restrains", "stable", "covered"), and thus prime the participants to suppress their emotional response4. The emotion control words were taken from previous studies4, and selected by asking undergraduate students to "list the 20 words that come to your mind when you think of the concept 'emotion control'."
Of note, to maintain the same structure of the task in both ER conditions, a SST is also performed in the explicit ER condition, but in this case participants are presented with 20 sentences containing only neutral terms regarding the goal to control emotions.
Assessments of the Immediate Impact of ER manipulation: The Emotional Rating Task
Selection of Stimuli. Stimuli consist of emotional and neutral pictures selected from the International Affective Picture System (IAPS)5, based on their normative scores for emotional valence and arousal. Careful attention should be paid to balancing the emotional content of picture across all study runs, and hence avoid confounding effects due to possible differences in these basic properties.
Timing of the task. Each picture is presented on the screen for 4 seconds, and then is removed to minimize confounding effects of eye movements associated with prolonged scanning of images6; a fixation cross follows each picture for 12 seconds. Participants' task is to watch the picture and rate their subjective emotional experience triggered by the pictures on an 8-point scale (1 = neutral, 8 = extremely negative). To avoid mood induction, pictures are pseudo-randomized so that no more than three pictures of the same valence are consecutively presented.
Assessment of the Long-Term Impact of ER manipulation: The Memory Retrieval Task
One week later, participants view an equal number of Old (seen in the scanner) and New pictures (never seen before), which are presented in black and white to prevent ceiling performance. Following an Old/New decision based on whether they remember seeing the pictures while in the scanner or not, participants also rate the confidence of their responses on a 3-point scale (1 = low; 2 = medium; 3 = high confidence).
2. Preparing the Subject for the Scan
All participants provide written informed consent prior to running the experimental protocol, which is approved by an Ethics Board. They are also warned that many of the pictures depict distressing events, such as acts of violence or trauma, and are provided with printed examples of representative pictures.
Before Scanning
On the day of scanning, participants' current state of mind is assessed7, to control for the effect of mood on the emotional experience and ability to control emotions. In conjunction with post-scanning assessments, these initial evaluations can be also used to screen for changes in mood as a result of study participation. Assessments of personality traits, such those indexing habitual engagement of emotion regulation strategies8 can also be made to investigate their possible influences on the success of the ER manipulation and the associated neural correlates.
Prior to the scan, the participants are informed in detail of the scan procedures, and are given specific instructions for the behavioural task. To avoid discomfort and to create increased familiarity with the task, participants are also given abbreviated practice runs for both the emotion rating task and the SST.
In the Scanning Room
Participants are instructed to lie supine on the scanning bed, with additional cushioning for the head to ensure comfort during the scan and minimize movement. The non-adhesive side of a length of tape may be wrapped lightly around the participants' forehead to further minimize head movement. Participants are given ear protection as well as isolation headphones to communicate with the experimenter during the MRI scan.
Subjects' right hand is positioned comfortably on the response box, thus allowing the left hand to be used for support or for other measurements (e.g., skin conductance responses). An emergency stop button is placed nearby so that the subject may indicate any urgent need to stop the scanner. Before starting data collection, it is critical to make sure that the subject can see the screen projection clearly for stimulus presentation and that the response buttons work properly.
The study is divided into runs/blocks of trials, to allow participants time to rest, and to avoid massive data loss in case of equipment malfunction. Run order is counterbalanced between participants, and each run begins with six seconds of fixation, to allow for MR signal stabilisation.
3. Data Recording and Processing
Scanning Parameters
We collected MRI data from 24 young healthy participants, using a 1.5 Tesla Siemens Sonata scanner for MRI recordings. Our anatomical images are 3D MPRAGE anatomical series (repetition time (TR) = 1600 ms, echo time (TE) = 3.82 ms; number of slices = 112; voxel size = 1x1x1mm). The functional images consist of series of 28 functional slices (voxel size = 4x4x4 mm), acquired axially using an echoplanar sequence (TR = 2000 ms; TE = 40 ms; field of view FOV = 256x256mm), thus allowing for full-brain coverage.
Data Analysis
We use Statistical Parametric Mapping (SPM: http://www.fil.ion.ucl.ac.uk/spm/) in combination with in-house MATLAB-based tools. Pre-processing involves typical steps: quality assurance, TR alignment, motion correction, co-registration, normalization, and smoothing (8 mm3 kernel)9. Individual and group-level statistical analyses may involve comparisons of brain activity according to ER manipulation (ER vs. baseline runs; see Figure 2), emotional valence (negative vs. neutral), arousal (low vs. high), and memory performance (remembered vs. forgotten). Correlations of brain imaging data with behavioural data (e.g., picture ratings) and/or scores indexing personality measures (e.g., personality traits indexing habitual engagement of ER strategies) can also be performed, to investigate how brain activity co-varies with individual differences in behaviour and personality.
4. Representative Results:
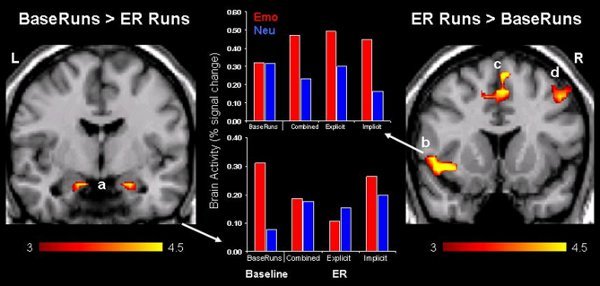 Figure 2. Decreased amygdala activity and increased prefrontal cortex activity following ER manipulation. Inducement of the ER goal was associated with reduced activity in brain areas associated with emotion processing, including the amygdala (a), and increased activity in brain regions associated with cognitive control and emotion regulation10, including ventrolateral prefrontal cortex (PFC) (b), dorsolateral PFC (d), and medial PFC (c)11,12. As shown in the bar graph illustrating the vlPFC activity, explicit and implicit ER were associated with similar patterns of response, suggesting that these changes reflect joint contribution of explicit and implicit ER. The "activation maps" are superimposed on high resolution brain images displayed in coronal views; the color bars indicate the gradient of t values of the activation maps, based on group statistics (N = 24), reflecting brain activity in voxels that show decreased (left panel) or increased (right panel) activity as a result of ER manipulation. It should be noted that activation blobs in the PFC may cover more areas, as follows: vlPFC/Insula (b) and dorsal Anterior Cingulate Cortex/Premotor Cortex (c). The bar graphs illustrate percent signal changes in response to emotional (Emo) and neutral (Neu) pictures before (i.e., BaseRuns) and after the induction of the ER goals (i.e., Combined ER = Explicit and Implicit ER averaged together, Explicit ER, and Implicit ER), as extracted from voxels showing pre- to post-ER differences. L = Left; R = Right.
Figure 2. Decreased amygdala activity and increased prefrontal cortex activity following ER manipulation. Inducement of the ER goal was associated with reduced activity in brain areas associated with emotion processing, including the amygdala (a), and increased activity in brain regions associated with cognitive control and emotion regulation10, including ventrolateral prefrontal cortex (PFC) (b), dorsolateral PFC (d), and medial PFC (c)11,12. As shown in the bar graph illustrating the vlPFC activity, explicit and implicit ER were associated with similar patterns of response, suggesting that these changes reflect joint contribution of explicit and implicit ER. The "activation maps" are superimposed on high resolution brain images displayed in coronal views; the color bars indicate the gradient of t values of the activation maps, based on group statistics (N = 24), reflecting brain activity in voxels that show decreased (left panel) or increased (right panel) activity as a result of ER manipulation. It should be noted that activation blobs in the PFC may cover more areas, as follows: vlPFC/Insula (b) and dorsal Anterior Cingulate Cortex/Premotor Cortex (c). The bar graphs illustrate percent signal changes in response to emotional (Emo) and neutral (Neu) pictures before (i.e., BaseRuns) and after the induction of the ER goals (i.e., Combined ER = Explicit and Implicit ER averaged together, Explicit ER, and Implicit ER), as extracted from voxels showing pre- to post-ER differences. L = Left; R = Right.
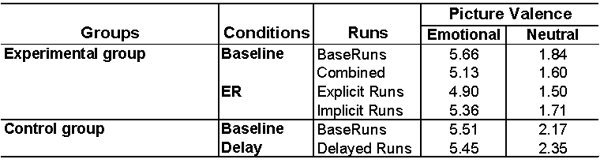 Table 1. Decreased emotional ratings following ER manipulation. Similar to the fMRI results, there was a reduction of emotional ratings following ER induction, and this effect resulted from decreases following both explicit and implicit ER. Importantly, emotional ratings did not change significantly in a control group that performed the same task following similar delays but in the absence of ER goal induction. Supporting these ideas, a 2 (Manipulation: Baseline vs. ER Induction) x 2 (Valence: Emotional vs. Neutral) repeated-measures ANOVA on data from the experimental group* yielded a significant main effect of Manipulation (F[1, 19] = 11.21, p < 0.002) and a significant Manipulation x Valence Interaction (F[1, 19] = 3.02, p < 0.05), and a similar 2-way ANOVA on data from the control group (N = 9) did not yield a significant main effect of Delay (Baseline vs. Delayed Runs) or a significant Delay x Valence interaction. These findings suggest that the decrease in ratings in the experimental group was due to the induction of the goal to control emotions rather than due to habituation following repeated exposure to emotional stimulation. *because of data attrition due to common causes (e.g., technical failure), behavioral analyses in the experimental group were based on data from 20 participants.
Table 1. Decreased emotional ratings following ER manipulation. Similar to the fMRI results, there was a reduction of emotional ratings following ER induction, and this effect resulted from decreases following both explicit and implicit ER. Importantly, emotional ratings did not change significantly in a control group that performed the same task following similar delays but in the absence of ER goal induction. Supporting these ideas, a 2 (Manipulation: Baseline vs. ER Induction) x 2 (Valence: Emotional vs. Neutral) repeated-measures ANOVA on data from the experimental group* yielded a significant main effect of Manipulation (F[1, 19] = 11.21, p < 0.002) and a significant Manipulation x Valence Interaction (F[1, 19] = 3.02, p < 0.05), and a similar 2-way ANOVA on data from the control group (N = 9) did not yield a significant main effect of Delay (Baseline vs. Delayed Runs) or a significant Delay x Valence interaction. These findings suggest that the decrease in ratings in the experimental group was due to the induction of the goal to control emotions rather than due to habituation following repeated exposure to emotional stimulation. *because of data attrition due to common causes (e.g., technical failure), behavioral analyses in the experimental group were based on data from 20 participants.
Taken together, these behavioral and brain imaging findings validate the present experimental design, which can be used to compare explicit and implicit inductions of the goal to regulate emotions. It should be noted that the present report emphasizes aspects that result from the joint contribution of the Explicit and Implicit ER manipulations, but this does not exclude the possibility that their effects may be dissociable at behavioral and/or brain level.
Discussion
We described a protocol that involves explicit and implicit manipulation of the goal to regulate emotion, and allows investigation of the associated neural correlates. This design has the potential to advance our knowledge of how the brain regulates emotions, being well suited for comparisons of explicit ER to implicit ER, which is unintentional and can happen without participant's insight and awareness13,14. Hence, the protocol can be particularly useful for investigations of habitual opposite affective biases, such as those observed in healthy aging (positivity bias15) and depression (negativity bias16), which are associated with enhanced automatic engagement of ER mechanisms (older adults17,18) and impaired ability to regulate emotions (depressed patients19,20).
Disclosures
No conflicts of interest declared.
Acknowledgments
This research was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression and a CPRF Award from the Canadian Psychiatric Research Foundation (to FD). The authors wish to thank Trisha Chakrabarty and Peter Seres for assistance with fMRI data collection and Kristina Suen for assistance with data analysis.
References
- Bargh JA, Williams LE. In: Handbook of emotion regulation. Gross JJ, editor. New York, NY: The Guilford Press; 2007. pp. 429–445. [Google Scholar]
- McRae K. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srull TK, Wyer RS. The role of category accessibility in the interpretation of information about persons: Some determinants and implications. Journal of Personality and Social Psychology. 1979;37:1660–1672. [Google Scholar]
- Mauss IB, Cook CL, Gross JJ. Automatic emotion regulation during anger provocation. Journal of Experimental Social Psychology. 2007;43 [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL. Gaze fixations predict brain activation during the voluntary regulation of picture induced negative affect. NeuroImage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS Scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Elsevier; 2006. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Using neuroscience to broaden emotion regulation: Theoretical and methodological considerations. Social and Personality Compass. 2009;3:475–493. doi: 10.1111/j.1751-9004.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Currents Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole SL, Rothermund K. I Feel Better but I Don't Know Why": The Psychology of Implicit Emotion Regulation. Cognition and Emotion. 2011;25:389–399. doi: 10.1080/02699931.2010.550505. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: A dual-process framework. Cognition and Emotion. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders IV. Washinton, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Jacques P, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: a network analysis of fMRI data. Neurobiol Aging. 2010;31:315–327. doi: 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol Sci. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: Changes with behavioral cognitive therapy and predictors of treatment response. Journal of Psychiatric Research. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddington KM. Neural correlates of idiographic goal priming in depression: goal-specific dysfunctions in the orbitofrontal cortex. Soc Cogn Affect Neurosci. 2009;4:238–246. doi: 10.1093/scan/nsp016. [DOI] [PMC free article] [PubMed] [Google Scholar]


