Abstract
Researchers working in the burgeoning field of adult stem cell biology seek to understand the signals that regulate the behavior and function of stem cells during normal homeostasis and disease states. The understanding of adult stem cells has broad reaching implications for the future of regenerative medicine1. For example, better knowledge about adult stem cell biology can facilitate the design of therapeutic strategies in which organs are triggered to heal themselves or even the creation of methods for growing organs in vitro that can be transplanted into humans1. The zebrafish has become a powerful animal model for the study of vertebrate cell biology2. There has been extensive documentation and analysis of embryonic development in the zebrafish3. Only recently have scientists sought to document adult anatomy and surgical dissection techniques4, as there has been a progressive movement within the zebrafish community to broaden the applications of this research organism to adult studies. For example, there are expanding interests in using zebrafish to investigate the biology of adult stem cell populations and make sophisticated adult models of diseases such as cancer5. Historically, isolation of the zebrafish adult kidney has been instrumental for studying hematopoiesis, as the kidney is the anatomical location of blood cell production in fish6,7. The kidney is composed of nephron functional units found in arborized arrangements, surrounded by hematopoietic tissue that is dispersed throughout the intervening spaces. The hematopoietic component consists of hematopoietic stem cells (HSCs) and their progeny that inhabit the kidney until they terminally differentiate8. In addition, it is now appreciated that a group of renal stem/progenitor cells (RPCs) also inhabit the zebrafish kidney organ and enable both kidney regeneration and growth, as observed in other fish species9-11. In light of this new discovery, the zebrafish kidney is one organ that houses the location of two exciting opportunities for adult stem cell biology studies. It is clear that many outstanding questions could be well served with this experimental system. To encourage expansion of this field, it is beneficial to document detailed methods of visualizing and then isolating the adult zebrafish kidney organ. This protocol details our procedure for dissection of the adult kidney from both unfixed and fixed animals. Dissection of the kidney organ can be used to isolate and characterize hematopoietic and renal stem cells and their offspring using established techniques such as histology, fluorescence activated cell sorting (FACS)11,12, expression profiling13,14, and transplantation11,15. We hope that dissemination of this protocol will provide researchers with the knowledge to implement broader use of zebrafish studies that ultimately can be translated for human application.
Keywords: Developmental Biology, Issue 54, kidney, blood, zebrafish, regeneration, adult stem cell, dissection
Protocol
1. Dissection of the adult zebrafish kidney from an unfixed animal sample
Select an adult zebrafish between 3-6 months in age for dissection.
Euthanize the fish by placing it into a dish with 0.2% Tricaine pH 7.0 for approximately 4-5 minutes, watching carefully to see that the gills stop moving and the heart stops beating.
Using a spoon, lift the fish from the Tricaine bath in a small amount of solution, decant the solution and gently place the animal on a paper towel.
Use a sharp pair of dissection scissors to remove the head of the animal, making a cut just behind the gill operculum (Figure 1, path A). Discard the head in a biohazard waste container.
Use the dissection scissors to open the body of the animal, by making a long ventral incision, starting from the head and terminating at the tail at the base of the caudal fin (Figure 1, path B).
Remove the internal organs of the animal using a pair of dissection tweezers/forceps, and place them in biohazard waste. Be careful not to brush the dorsal body wall, as this is the location of the kidney (Figure 2). If you have selected a female fish, you will need to scoop developing eggs out of the body cavity, which can be most easily done using an angled pair of forceps or tweezers.
Use dissection needles to pin open the body walls to a dissection tray, and angle the pins so that the kidney can be visualized. The kidney will possess a characteristic shape (head, trunk/saddle, tail) and color, being interspersed with black hued pigmentation (Figure 2). You may also see the aorta filled with peripheral blood, which runs down the midline of the organ structure.
Use fine forceps to detach the kidney from the dorsal body wall, using one pair of forceps to lift the kidney as you separate the organ from the connective tissues that underlie it.
Gently place the kidney into the desired vehicle for further live cell studies, such as a microcentrifuge tube containing 1x PBS for FACS preparation.
2. Preparation and dissection of the adult zebrafish kidney from a fixed animal sample
Prepare a solution of 4% paraformaldehyde/1X PBST. Boil the solution to dissolve the PFA into solution, then cool the mixture to 4°C and add 0.1% dimethylsulfoxide. We have had the most success with tissue preservation from using freshly made PFA solutions as opposed to frozen aliquots. Make enough solution to submerge the adult zebrafish during dissection.
Fill a dissection tray with enough fixation solution to submerge the adult zebrafish during the dissection. We perform the subsequent steps with this tray positioned under the microscope, which can be placed in a chemical hood to minimize exposure to fixation odors.
Select an adult zebrafish between 3-6 months in age for dissection.
Euthanize the fish by placing it into a dish with 0.2% Tricaine pH 7.0 for approximately 5 minutes, watching carefully to see that the gills and heart stop beating.
Using a spoon, lift the fish from the Tricaine bath in a small amount of solution, decant the solution and gently place the animal on a paper towel.
Use a sharp pair of dissection scissors to remove the head of the animal, making a cut just behind the gill operculum (Figure 1, path A). Discard the head in a biohazard waste container, and immediately place the animal into the PFA-containing dissection tray.
Use the dissection scissors to open the body of the animal, by making a long ventral incision, starting from the head and terminating at the tail at the base of the caudal fin (Figure 1, path B), and keep the body partially submerged during the procedure.
Remove the internal organs of the animal using a pair of dissection tweezers/forceps, and place them in biohazard waste. Be careful not to brush the dorsal body wall, as this is the location of the kidney (Figure 2).
Use dissection needles to pin open the body walls, and angle the pins so that the kidney can be visualized. The kidney will possess a characteristic shape (head, saddle, tail) and color, being interspersed with black hued pigmentation (Figure 2). You may also see the aorta filled with peripheral blood, which runs down the midline of the organ structure.
Fix the sample overnight, placing the tray at 4°C when you are done with arranging the desired samples.
The next day, remove the PFA solution from the tray and replace with 1x PBST.
Use fine forceps to carefully detach the kidney from the dorsal body wall, using one pair of forceps to lift the kidney as you separate the organ from the connective tissues that underlie it. The kidney tissue will be soft and pliable from the DMSO in the fixative solution, making it possible to dissect the entire kidney organ in one piece.
Use a wide-bore transfer pipet to place the kidney into a microcentrifuge tube.
The kidney can now be processed further to conduct histological or whole mount in situ gene expression studies based on the desired study.
3. Representative results:
The steps that are diagrammed in Figure 1 indicate the dissection procedure. The kidney can be identified based on its characteristic shape and coloration, and anatomical location on the dorsal wall of the animal’s body cavity, as shown in Figure 2. After dissection of a fixed kidney sample, whole mount in situ hybridization can be used to localize the expression of a gene of interest, as shown in Figure 3.
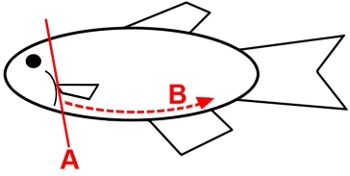 Figure 1: Procedure that enables direct access for dissecting the zebrafish adult kidney. (A) Euthanized zebrafish is dissected in a stepwise fashion (indicated by numbers and arrows) to give the researcher the easiest access to the complete kidney organ. Images of the abdominal cavity before (B) and after (C) removal of the abdominal organs.
Figure 1: Procedure that enables direct access for dissecting the zebrafish adult kidney. (A) Euthanized zebrafish is dissected in a stepwise fashion (indicated by numbers and arrows) to give the researcher the easiest access to the complete kidney organ. Images of the abdominal cavity before (B) and after (C) removal of the abdominal organs.
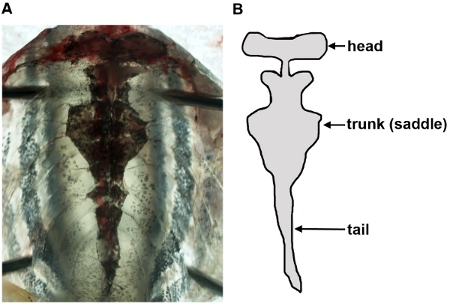 Figure 2: Visualization of the zebrafish kidney in an unfixed sample. Following removal of organs in the body cavity, the kidney appears as a single, flattened organ that is adherent to the dorsal body wall via connective tissues (A), and has been schematized (B) to show its anatomical shape.
Figure 2: Visualization of the zebrafish kidney in an unfixed sample. Following removal of organs in the body cavity, the kidney appears as a single, flattened organ that is adherent to the dorsal body wall via connective tissues (A), and has been schematized (B) to show its anatomical shape.
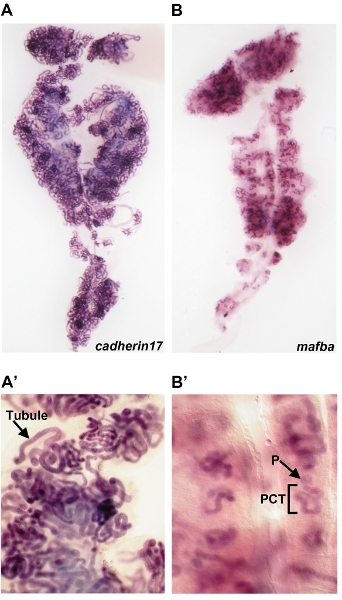 Figure 3: Whole mount in situ hybridizations performed on the whole adult kidney organ. (A)cadherin17 transcripts are detected in the tubule of adult kidney nephrons, enlarged in (A’). (B)
mafba transcripts are localized proximal nephron cell types in the adult kidney (compare to entire tubule in panel A). (B’) An enlarged view of mafba expression reveals that transcripts are specifically localized to the podocyte (P) cells of the blood filter (glomerulus) and proximal convoluted tubule (PCT).
Figure 3: Whole mount in situ hybridizations performed on the whole adult kidney organ. (A)cadherin17 transcripts are detected in the tubule of adult kidney nephrons, enlarged in (A’). (B)
mafba transcripts are localized proximal nephron cell types in the adult kidney (compare to entire tubule in panel A). (B’) An enlarged view of mafba expression reveals that transcripts are specifically localized to the podocyte (P) cells of the blood filter (glomerulus) and proximal convoluted tubule (PCT).
Discussion
Adult stem cells are dynamic and essential components that maintain the adult body form1. Adult stem cells can also serve to counteract damage that the body incurs during disease states, and the dysfunction or loss of adult stem cells can lead to the decline of organ function and has been implicated to drive cancer malignancies and contribute to aging. Adult stem cells exhibit cellular properties that distinguish them from differentiated cell types. Upon cell division, adult stem cells are capable of self-renewal and the production of progenitor cells that in turn can give rise to distinct differentiated offspring. There is great interest in understanding the pathways that regulate the cell fate decisions and potency of adult stem cells because of their important roles in tissue homeostasis1. For example, many scientists currently seek to identify the signals that maintain various adult stem cell populations in their niche, or specialized microenvironment, and how this niche is impacted by humoral factors.
Adult hematopoietic stem cells (HSCs) are pluripotent cells that fuel the continual rejuvenation of blood cells in animals8. Hematopoiesis is a vibrant process that is constantly ongoing during the life of a vertebrate organism because the mature differentiated blood cell types are short-lived and hence must be replenished regularly. As they divide, HSCs supply this crucial demand by self-renewing and producing a series of progenitors that give rise to the erythroid, megakaryocyte, lymphoid and myeloid lineages. Each of these blood cell types performs unique functions in the circulation, including providing gas exchange to tissue, a way for sealing injuries to blood vessels by means of clot formation, or defense mechanisms against invading pathogens. While HSCs have been recognized for many years, a plethora of fascinating questions remain unanswered about these amazing cells. Recent studies have identified subclasses of HSCs with different long-term self-renewal properties, and the elucidation of niche components and signals that modulate HSC behavior8. The zebrafish is now among the cornerstones of hematopoiesis research paradigms, based on the genetic and molecular attributes of this animal model8. Research utilizing the zebrafish has led to novel insights about HSC biology that are conserved with higher vertebrates like mammals, emphasizing the biomedical relevance of fish blood investigations8.
Historically, knowledge about HSCs has paved the way for the expeditions seeking to discover if other body organs are maintained by adult stem cells. Numerous adult stem cell populations are now appreciated to exist, ranging across diverse structures from the brain to the intestine, skin, and skeletal muscle. Continued efforts in the stem cell field seek to identify how adult stem cells and even other differentiated cell types can display enhanced potency in various settings.
With respect to the kidney, there has been great debate among nephrologists as to whether renal stem cells exist16. Independent findings have documented populations of kidney cells in mammals that possess varying degrees of regenerative potential, but a cohesive understanding of these reports has yet to be achieved. Interestingly, there is strong evidence to support the notion that many fish species possess cells that can robustly fuel regeneration of damaged nephrons, as well as grow entirely new nephrons during adulthood9. Recent work has identified the molecular hallmarks of adult kidney stem cells in the zebrafish10,11, and demonstrated the self-renewal potency of these so-called renal stem/progenitor cells (RPCs)11. Future studies are needed to ascertain whether analogous kidney cells are present in mammals. Nevertheless, continued investigations about the cellular and molecular mechanisms that regulate fish RPCs may provide insight into how regenerative feats may be stimulated in the mammalian kidney. It is evident that much remains to be understood about RPCs, and that the many tools now available to the zebrafish researcher can be implemented to tackle these intriguing questions.
In this protocol, we describe our method for the anatomical identification and dissection of the adult zebrafish kidney. Alternative steps can be used to dissect the kidney, such as opening the abdominal cavity with a ventral incision using dissection scissors; however, we have encountered the most success in accessing the entire kidney when the abdominal wall is pinned open and the head is removed. Blood and kidney researchers alike who wish to isolate and work with these cell types can use either dissection method. It should be noted that the method we describe for isolating and working with the zebrafish adult kidney is certainly not limited to scientists who wish to pursue questions of adult stem cell biology, as the dissection of this organ can facilitate studies for those researchers pursuing investigations on topics ranging from physiology to aging. Further, this method can be coupled with other technologies such as transgenics, so as to molecularly label and then purify and study discrete subpopulations of blood or kidney cells from wildtype or genetically mutant zebrafish11. Looking forward, such detailed purification procedures of stem and progenitor cell types is vital to gaining knowledge about how their behavior is regulated, as operational tests such as transplantation or progenitor culturing constitute the basis by which the potency and identity of these cells is defined. Recent advances in hematopoietic progenitor culture methods open many new avenues for study, and may be adapted to culturing of renal populations17. Zebrafish chemical genetics have been successful in the identification of pathways that modulate HSCs and renal progenitors in various contexts18-20, and will continue to be a useful avenue to test in combination with the above cell biology techniques. Taken together, there have been a number of groundbreaking contributions to the fields of hematology and nephrology using the zebrafish model, and continued research in these arenas promises to wield further transformative insights in the coming years.
Disclosures
No conflicts of interest declared.
Acknowledgments
R.A.W. wishes to thank members of the Zon and Davidson research laboratories for their innumerable contributions to helping to develop strategies that have progressively shaped this dissection technique in adult zebrafish over the past decade. In addition, we wish to express our gratitude to the staff members of the Notre Dame Center for Zebrafish Research for providing excellent ongoing husbandry care for our zebrafish colony. R.A.W. garnered research funding from the NIH-NIDDK grant award DK083512, and generous laboratory start-up funding from the University of Notre Dame.
References
- Stocum DL, Zupanc GK. Stretching the limits: stem cells in regeneration science. Dev. Dyn. 2008;237:3648–3671. doi: 10.1002/dvdy.21774. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullman B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Gupta T, Mullins MC. Dissection of organs from the adult zebrafish. J Vis Exp. 2010. [DOI] [PMC free article] [PubMed]
- Mione MC, Trede NS. The zebrafish as a model for cancer. Dis. Model Mech. 2010;3:517–523. doi: 10.1242/dmm.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon LI. Developmental biology of hematopoiesis. Blood. 1995;86:2876–2891. [PubMed] [Google Scholar]
- Davidson AJ, Zon LI. The 'definitive' (and 'primitive') guide to zebrafish hematopoiesis. Oncogene. 2004;23:7233–7246. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimschuessel R. A fish model of renal regeneration and development. ILAR J. 2001;42:285–291. doi: 10.1093/ilar.42.4.285. [DOI] [PubMed] [Google Scholar]
- Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am. J. Physiol. Renal Physiol. 2010;299:F1040–F1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CQ, Ma D, Holm T, Naylor R, Arora N, Wingert R, Bollig F, Djordjevic G, Lichman B, Zhu H. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- Weber GJ, Choe SE, Dooley KA, Paffett-Lugassy NN, Zhou Y, Zon LI. Mutant-specific gene programs in the zebrafish. Blood. 2005;106:521–530. doi: 10.1182/blood-2004-11-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Jorniak T, Lin S. Identification of novel vascular endothelial-specific genes by the microarray analysis of the zebrafish cloche mutants. Blood. 2005;106:534–541. doi: 10.1182/blood-2004-12-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MH, Bertram JF. Is there such a thing as a renal stem cell. J. Am. Soc. Nephrol. 2009;20:2112–2117. doi: 10.1681/ASN.2009010066. [DOI] [PubMed] [Google Scholar]
- Stachura DL, Reyes JR, Bartunek P, Paw BH, Zon LI, Traver D. Zebrafish kidney stromal cell lines support multilineage hematopoiesis. Blood. 2009;114:279–289. doi: 10.1182/blood-2009-02-203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, Davidson AJ. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3:1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groh ED, Swanhart LM, Cosentino CC, Jackson RL, Dai W, Kitchens CA, Day BW, Smithgall TE, Hukriede NA. Inhibition of histone deacetylase expands the renal progenitor cell population. J. Am. Soc. Nephrol. 2010;21:794–802. doi: 10.1681/ASN.2009080851. [DOI] [PMC free article] [PubMed] [Google Scholar]


