Abstract
Peripheral neuropathic pain is a severe chronic pain condition which may result from trauma to sensory nerves in the peripheral nervous system. The spared nerve injury (SNI) model induces symptoms of neuropathic pain such as mechanical allodynia i.e. pain due to tactile stimuli that do not normally provoke a painful response [1].
The SNI mouse model involves ligation of two of the three branches of the sciatic nerve (the tibial nerve and the common peroneal nerve), while the sural nerve is left intact [2]. The lesion results in marked hypersensitivity in the lateral area of the paw, which is innervated by the spared sural nerve. The non-operated side of the mouse can be used as a control. The advantages of the SNI model are the robustness of the response and that it doesn’t require expert microsurgical skills.
The threshold for mechanical pain response is determined by testing with von Frey filaments of increasing bending force, which are repetitively pressed against the lateral area of the paw [3], [4]. A positive pain reaction is defined as sudden paw withdrawal, flinching and/or paw licking induced by the filament. A positive response in three out of five repetitive stimuli is defined as the pain threshold.
As demonstrated in the video protocol, C57BL/6 mice experience profound allodynia as early as the day following surgery and maintain this for several weeks.
Keywords: Neuroscience, Issue 54, Sciatic, Injury, PNS, Mechanical allodynia, Neuropathic pain, von Frey
Protocol
1. von Frey baseline measurements prior to surgery
For von Frey testing procedure, please refer to section 5.
2. Anesthesia/preparation
Anesthetize the animals (intraperitoneal injection of a mixture of 100mg/kg ketamine and 15 mg/kg Xylazine).
Place the animal in a quiet place until fully anesthetized (e.g. covered with a paper towel).
Check reflexes by pinching the tip of the tail and the paws with a pair of tweezers. Be sure that the animals are unresponsive before proceeding.
Subcutaneously inject (back shoulder area) 0.5ml isotonic saline with antibiotics, e.g. ampicillin (to avoid dehydration and prevent infection, although this is a minor surgical procedure).
Using an electrical shaver, shave the operative field from slightly below the knee area to the hip area (for right-handed persons the left hind limb is recommended).
Apply ophthalmic ointment to the eyes with a cotton-wool bud.
Place the animal on its right side and place the left hindlimb on a small platform to keep it elevated. Secure the leg with adhesive tape.
Disinfect the operative field with alternating scrubs of ethanol and betadine from surgical site out.
3. Surgery
Locate the knee with the thumb of your left hand and use a scalpel to make an app. 1 cm incision in the longitudinal direction proximal to the knee.
Open the skin by blunt dissection using the tip of a pair of sterile scissors.
Separate the muscle layer by blunt dissection right next to the clearly visible blood vessel, close to the thigh bone (femur). If done correctly, the muscle layers will easily separate without any bleeding, revealing the sciatic nerve right below the muscles. If bleeding occurs e.g. by damage to a blood vessel close to the knee, use sterile cotton-wool buds or pieces of gaze to absorb the blood. Press until the bleeding stops.
Place the mouse under a stereo microscope and carefully separate the muscles with a pair of sterile tweezers (nr. 2) to visualize the sciatic nerve. Retractors may also be applied.
Identify the area where the sural nerve branches from the sciatic nerve. The sural nerve is the smallest of the three branches, branching to the right in the left leg.
Apply suture (6-0 suture) around the other two branches which are still running in parallel (the tibial and common peroneal nerves), being very careful not to touch the sural branch. This is a critical step as the sural nerve has to be left completely intact.
Make a tight surgical knot. If the first knot is tight, contractions of the limb will be observed.
Grab the nerves to be cut below the suture with a pair of tweezers (nr. 5) and cut the nerves above and below tweezers with a small pair of scissors. By first cutting above the pair of tweezers, pulling of the nerves is avoided.
Cut off suture ends with a pair of micro scissors and gently close the muscle layer. Add a drop of lidocaine to the wound and suture with surgical knots.
4. After surgery
Check if eye ointment is still sufficient.
Place the mouse in a clean cage under a paper towel in a comfortable posture. If the room is cold, place a heat pad under a part of the cage (only under part of the cage as the animal should be able to escape to a colder area if preferred).
Ensure easily accessible water and chow.
5. von Frey testing (baseline, and from the day after surgery)
Place the mice in red colored plastic cylinders placed on a wire mesh table two days and one day prior to surgery. Habituate for 15 min in cylinders prior to testing. The red color of the cylinders helps the mice to quickly relax as they cannot see each other and are in a darker area.
- Verify that the mice are calm and apply von Frey filaments to the lateral part of the paw: Starting with the 0.02 g filament, first apply force to the left paw five times over a total period of 30 seconds (approximately 2 seconds per stimulus) and gauge the mouse's reaction after each application. Repeat with the same filament and the left paw of any other mice, and then start over again with the right paw of the first mouse before moving on to the next filament, e.g.:
- Mouse 1: left paw 5 times with filament 0.02 g
- Mouse 2: left paw 5 times with filament 0.02 g etc.
- Mouse 1: right paw 5 times with filament 0.02 g
- Mouse 2: right paw 5 times with filament 0.02 g etc.
- Mouse 1: left paw 5 times with filament 0.04 g
- Mouse 2: left paw 5 times with filament 0.04 g etc.
Response in three out of five stimuli is regarded as a positive reaction. Filaments above threshold can be applied to verify the threshold level. Response = sudden paw withdrawal, sudden flinching, sudden paw licking.
6. Representative results
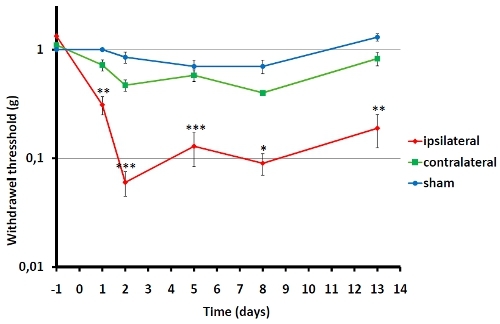 Figure 1. Von Frey testing on C57BL/6 mice age 8-10 weeks old was performed 1 day prior to operation and repeatedly following surgery. 4-8 animals were included in each group. Von Frey testing on the operated side is represented by ipsilateral and the non-operated control side is represented by contralateral. The day after surgery the animals develop significant hypersensitivity on the operated paw while the non-operated is only slightly affected relative to sham operated mice. (* <0.05, **<0.01, ***<0.001, ipsilateral relative to contralateral).
Figure 1. Von Frey testing on C57BL/6 mice age 8-10 weeks old was performed 1 day prior to operation and repeatedly following surgery. 4-8 animals were included in each group. Von Frey testing on the operated side is represented by ipsilateral and the non-operated control side is represented by contralateral. The day after surgery the animals develop significant hypersensitivity on the operated paw while the non-operated is only slightly affected relative to sham operated mice. (* <0.05, **<0.01, ***<0.001, ipsilateral relative to contralateral).
Discussion
Critical steps
Damage to the sural nerve should be avoided in order to study pathological changes in the intact sural nerve following injury to the tibial and common peroneal nerves. Collateral damage to the sural nerve may lead to paralysis and can thus be visualized as dragging of the operated hind limb.
Only the lateral side of the paw is innervated by the spared sural nerve and, hence, only this area develops neuropathy. Testing other areas, innervated by the transected nerves, can strongly bias the evaluation of altered mechanical threshold.
Possible modifications
Other types of ligations may be supplementary in terms of studying pathological pain conditions following peripheral nerve injury, such as chronic constriction injury [5] or partial nerve ligation [6]. Each experimental procedure results in distinct phenotypic changes which should be considered prior to post-surgery testing.
Furthermore, other sensory tests such as thermal hyperalgesia may be applied [7], although this phenotype is less robust following SNI.
Future applications
This technique can be used for testing of drugs altering the development or maintenance of mechanical allodynia [8]. Analysis of the sciatic nerve, the dorsal root ganglia (DRG) and/or the lumbar spinal cord allow research of the molecular mechanisms responsible for the induced phenotype.
Animal experimentation was performed according to good laboratory practice in full compliance with Danish and European regulations. All experiments were approved by the Danish Animal Experiments Inspectorate under the Ministry of Justice (permission number 2006/561-1206).
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by the Lundbeck Foundation, the Carlsberg Foundation and Dagmar Marshall Foundation.
References
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Bourquin A-F, Süveges M, Pertin M, Gilliar N, Sardy S, Davidson AC, Spahn DR, Decosterd I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14.e1–14.e14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie Y-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutanous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Erichsen HK, Blackburn-Munro G. Pharmacological characterization of the spared nerve injury model of neuropathic pain. Pain. 2002;98:151–161. doi: 10.1016/s0304-3959(02)00039-8. [DOI] [PubMed] [Google Scholar]


