Abstract
Immune responses can interfere with the effective use of therapeutic proteins to treat genetic deficiencies and have been challenging to manage. To address this problem, we adapted and studied methods of immune tolerance used in canine organ transplantation research to soluble protein therapeutics. A tolerization regimen was developed that prevents a strong antibody response to the enzyme α-l-iduronidase during enzyme replacement therapy of a canine model of the lysosomal storage disorder mucopolysaccharidosis I. The tolerizing regimen consists of a limited 60-day course of cyclosporin A and azathioprine combined with weekly i.v. infusions of low-dose recombinant human α-l-iduronidase. The canines tolerized with this regimen maintain a reduced immune response for up to 6 months despite weekly therapeutic doses of enzyme in the absence of immunosuppressive drugs. Successful tolerization depended on high plasma levels of cyclosporin A combined with azathioprine. In addition, the induction of tolerance may require mannose 6-phosphate receptor-mediated uptake because α-l-iduronidase and α-glucosidase induced tolerance with the drug regimen whereas ovalbumin and dephosphorylated α-l-iduronidase did not. This tolerization method should be applicable to the treatment of other lysosomal storage disorders and provides a strategy to consider for other nontoleragenic therapeutic proteins and autoimmune diseases.
The lysosomal storage disorder mucopolysaccharidosis I (MPS I) is caused by the genetic deficiency of the enzyme α-l-iduronidase (1) and can be treated by using enzyme replacement therapy with a recombinant human enzyme (2). In many MPS I patients treated to date with recombinant human α-l-iduronidase (rhIDU), anti-iduronidase antibodies are induced within weeks of exposure to rhIDU (2), (J. E. Wraith, L. A. Clarke, M. Beck, E. H. Kolodny, G. M. Pastores, J. Muenzer, S. Swiedler, E.K., T. Braakman, E. Chadbourne, et al., unpublished data). So far, there have been no discernible effects on efficacy or safety although changes in rhIDU pharmacokinetics have been noted (J. E. Wraith, L. A. Clarke, M. Beck, E. H. Kolodny, G. M. Pastores, J. Muenzer, S. Swiedler, E.K., T. Braakman, E. Chadbourne, et al., unpublished data). After long-term treatment over a 2-yr period, the immune responses in the first 10 MPS I patients treated have declined, suggesting that rhIDU is intrinsically tolerizing with continued exposure to the enzyme (3). Even so, as patients with null genotypes receive rhIDU treatment, stronger immune responses could occur and result in changes in efficacy and safety.
The adverse impact of antibodies on enzyme replacement therapy of lysosomal storage disorders has been documented in the canine model of MPS I. MPS I-affected canines (4) have a null genotype (5) and, during infusions with rhIDU, mount strong antibody responses that can lead to IgG-mediated complement activation and cause anaphylactoid reactions (6). Antibodies may also alter clearance from the circulation and may reduce the efficiency of enzyme uptake in tissues based on in vitro (unpublished data) and in vivo studies (7). Enzyme replacement studies in other animal models of MPS disorders, including MPS I, MPS VI, and MPS VII, have also shown the effects of antibodies on enzyme pharmacokinetics, stability, uptake, efficacy, and safety (8).
Antibodies can reduce the safety and efficacy of therapeutic proteins in patients with other severe deficiency disorders such as hemophilia A (9), adenosine deaminase deficiency (10), Gaucher disease (11, 12), and Pompe disease (13). As many new enzyme or protein replacement therapies are developed for different lysosomal disorders and other genetic diseases, patients with null genotypes may mount significant immune responses that could reduce safety and limit efficacy.
The induction of antigen-specific tolerance would be the most desirable way to manage the immune response to therapeutic proteins, but it has been difficult to achieve reliably in humans. Tolerance strategies based on antigen administration [e.g., oral tolerance (14), intrathymic antigen injection (15), and high, frequent doses of antigen (9)] may enhance the uptake and presentation of antigens by tolerizing antigen-presenting cells centrally in the thymus or peripherally (16). Alternatively, strategies based on the interference with T cell activation (e.g., costimulation blockade) block the normal T cell activation response to presented antigen, reducing the immune response based on mechanisms such as T cell deletion, anergy induction, or regulatory T cell production (17).
We undertook the development of an antigen-based approach to tolerance for soluble therapeutic proteins that is based on T cell-specific strategies and drugs studied in canine tissue transplantation rather than the B cell-specific drugs usually used for soluble protein tolerance (18–20). T cell responses fundamentally regulate B cell responses to complex antigens through T cell help and other mechanisms, and we reasoned that mechanisms that alter the T cell response to cell-associated antigens would be more effective at regulating T cell responses to soluble antigens. Surprisingly, after studying a variety of complex combinations used in canine transplantation studies, we discovered that a simple regimen consisting of infusions of lysosomal enzymes that carry the mannose 6-phosphate modification for high-affinity receptor-mediated endocytosis, combined with a 60-day course of adequate doses of the immunosuppressive drugs cyclosporin A (CsA) and azathioprine (Aza), prevented significant immune responses to these enzymes in canines.
Methods
MPS I and Normal Canines. The canine MPS I colony is composed of a mix of the original Plott hounds and beagles (4) and was bred and studied under approved treatment protocols at the C. W. Steers Biological Resource Center at Harbor-UCLA, an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility.
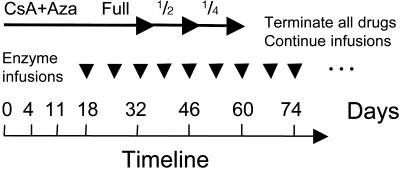
Tolerance Regimens. The effective tolerance-inducing regimen consisted of the immunosuppressive drugs CsA (Neoral, Novartis Pharmaceuticals, East Hanover, NJ) by mouth at 25 mg/kg every day (divided into two doses every 12 h) and Aza (Imuran, Glaxo Wellcome) by mouth at 5 mg/kg every other day (qod) from day 0 to day 32. Weekly antigen/toleragen infusions began at day 18. After 2 weeks of antigen administration, CsA and Aza doses were halved at day 32, and halved again to one-quarter dose at day 46 before ending at day 60 (Fig. 1). Regimens that failed to induce tolerance included the immunosuppressive drugs, intrathymic injection (ITI) of iduronidase (1 mg/kg rhIDU on day 4), and anti-T cell monoclonal antibody infusions, in various combinations [anti-canine CD3, T cell antigen receptor (TCR), and Thy-1 monoclonal antibodies from hybridomas CA17.6B3, CA15.9D5, and CA1.4G8] from day 5 to day 9. CsA was administered at 25 mg/kg every other day, rather than every day, and the dose was halved beginning at day 18, followed by titration every 2 weeks to one-fourth and one-eighth doses.
Fig. 1.
Immune tolerance induction regimen. The tolerance regimen is diagrammed including CsA plus Aza dosing and rhIDU infusions. On day 0, full-dose CsA plus Aza are initiated (CsA, 25 mg/kg per day divided two times per day; Aza, 5 mg/kg every other day) and are later tapered to one-half the initial dose on day 32 and one-fourth the initial dose on day 46 and terminated on day 60. Low-dose rhIDU (0.056 mg/kg) infusions begin as indicated on day 18 and continue weekly thereafter.
Clinical Laboratory Assessments. CsA blood levels were assessed at 12-hr trough samples taken 1 week after beginning CsA administration. A clinical contract laboratory performed complete blood cell counts, leukocyte differentials, blood chemistry assessments, and total Ig assays.
Antigen Administration. Canine serum for antibody determinations was collected before each infusion, and the canines were treated prophylactically with 2.2 mg/kg diphenhydramine and sedated with butorphanol and acepromazine. Doses of rhIDU [prepared in the lab (21) or donated by BioMarin Pharmaceutical], ovalbumin (Sigma), acid α-glucosidase (high uptake, K uptake ≈1 nM, donated by BioMarin Pharmaceutical), and dephosphorylated rhIDU were diluted to 50 ml in 0.9% saline, 10 mM sodium phosphate, and 1 mg/ml canine albumin (not added for ovalbumin or α-glucosidase) and infused into a cephalic vein over 2 or 3 h. Canines were administered 0.056 mg/kg antigen i.v. weekly and were monitored clinically during infusions for adverse reactions. To ramp to full therapeutic dose, canines were administered 0.125, 0.25, and 0.58 mg/kg antigen per wk in subsequent weeks, then continued at 0.58 mg/kg weekly.
Determination of Specific Antibody ELISA Titers in Canine Serum. Standard ELISAs were used, and each plate contained positive, negative, and internal quality controls for signal strength. Antigen was bound in 96-well plates by incubation overnight at 4°C with 50 μl per well of 4 μg/ml rhIDU or 1 mg/ml ovalbumin diluted in acidic PBS (10 mM sodium phosphate/150 mM sodium chloride, pH 5.8). The remaining steps were carried out at room temperature, including blocking with 3% BSA (Sigma) in PBS (pH 5.8) for 2 h, binding primary antiserum dilutions for 1 h, and detection with goat anti-canine IgG (Southern Biotechnology Associates), IgM, IgA, or IgE (Bethyl Laboratories, Montgomery, TX), conjugated to alkaline phosphatase. Alkaline phosphatase activity was developed for 1 h with 50 μl per well of 1 mg/ml p-nitrophenyl phosphate chromogenic substrate (Sigma), and the developing reaction was stopped with 50 μl per well of 0.1 M EDTA (pH 8.0). Absorbance at 405 nm was detected as OD units by using a SpectraMAX 340 (Molecular Devices) spectrophotometer. For α-glucosidase, the method was similar except that high protein-binding plates were used, coating occurred at 37°C, blocking was with 2% BSA containing 0.05% polysorbate 20, and anti-canine IgG conjugated to horseradish peroxidase (HRP) were secondary antibodies. HRP was detected with the chromogenic substrate TMB (Bio-Rad), followed by acidification with H2SO4 as a stop solution and measurement of the absorbance at 450 nm. The calculated OD/μl for any sample is taken from dilutions within the linear signal range.
Dephosphorylation of Iduronidase. Potato acid phosphatase (Roche Diagnostics Corporation, Indianapolis), 10 ml of 36 mg/ml, was covalently immobilized onto 25 ml of Affi-Gel 10 agarose beads (Bio-Rad) and incubated with 7 mg of rhIDU in Specra/Por 4 dialysis membrane (molecular mass cutoff of 12–14 kDa), with slow rotation for 24 h at 25°C. Inhibitory phosphate was removed simultaneously during the reaction by dialyzing against 280 vol of 0.1 M sodium acetate, 150 mM sodium chloride (pH 5.2), with three changes. Dephosphorylated iduronidase was confirmed to be dephosphorylated and did not have significant uptake into MPS I fibroblasts in culture at up to 80 nM concentration (21). Canine WI and XO were treated similarly with dephosphorylated rhIDU and CsA plus Aza but were challenged with either 0.58 mg/kg rhIDU weekly beginning at week 12 (WI) or a ramp of increasing rhIDU dose in weeks 13, 14, and 15 of 0.125, 0.25, and 0.58 mg/kg per week (XO).
Results
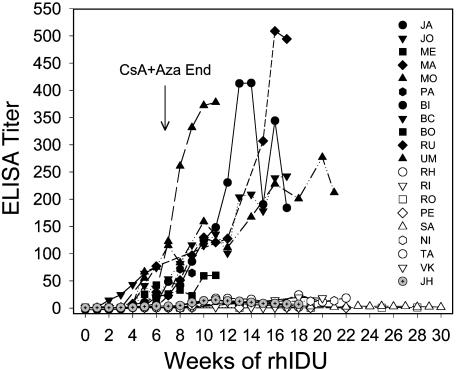
A Tolerance Induction Regimen Combining Low-Dose Enzyme Infusions with the Immunosuppressive Drugs CsA and Aza. We initially studied a series of 60-day regimens that combined methods used in canine tissue transplantation models including ITI of antigen, T cell-specific immunosuppressive drugs, and monoclonal antibodies that deplete mature T cells (19) as shown in Table 1. The antibody response was measured by a direct ELISA for anti-rhIDU IgG antibodies. After a number of experiments, we determined that a simple 60-day regimen (Fig. 1) using high doses of CsA plus Aza and low-dose infusions of rhIDU was sufficient to prevent a strong immune response to subsequent rhIDU infusions in all canines treated (Fig. 2). The key early discovery underlying the successful regimen was the requirement for a high CsA level as suggested by the early data on canine JH who became relatively tolerant and had higher levels of CsA on the original lower dose regimen (Fig. 2, gray symbols).
Table 1. Immune response to rhIDU infusions by normal and MPS I canines.
| Canine | Treatment | Diagnosis | Strong immune response? | CsA trough, ng/ml | Pretreatment ELISA, OD/μl | Posttreatment (week 12) ELISA, OD/μl | Post-full-dose challenge |
|---|---|---|---|---|---|---|---|
| Nontolerant canines | |||||||
| JA | CsA qod + Aza + ITI + TCR mAb | Normal | Yes | 63 | 0.0 | 230.7 | ND |
| JO | CsA qod + Aza + ITI + TCR mAb | Normal | Yes | 342 | 2.6 | 101.2 | ND |
| ME | CsA qod + Aza + ITI + TCR mAb | Normal | Yes | 60 | 0.3 | 60.2 | ND |
| MA | CsA qod + Aza + ITI + CD3/Thy1/TCR mAb | Normal | Yes | 120 | 1.5 | 120.8 | ND |
| MO | CsA qod + Aza + ITI + CD3/Thy1/TCR mAb | Normal | Yes | 110 | 0.0 | 377.9 | ND |
| PA | CsA qd × 48 days, No Aza | Normal | Yes | 490 | 0.3 | 64.4 | ND |
| RU | No drug control | MPS I | Yes | NA | 1.4 | 127.7 | 494.4 |
| UM | No drug control | MPS I | Yes | NA | 0.0 | 111.2 | 212.1 |
| Mean | 139 | 0.8 | 149 | 353 | |||
| Tolerant canines | |||||||
| JH | CsA qod + Aza + ITI | Normal | No | 570 | 0.7 | 12.7 | ND |
| RH | CsA qd + Aza + ITI | Normal | No | 520 | 0.4 | 8.8 | ND |
| RI | CsA qd + Aza + ITI | Normal | No | 450 | 0.8 | 0.8 | ND |
| RO | CsA qd + Aza | Normal | No | 680 | 0.4 | 0.6 | 0.9 |
| PE | CsA qd + Aza | MPS I | No | 470 | 0.4 | 0.5 | 0.4 |
| SA | CsA qd + Aza | MPS I | No | 520 | 0.3 | 10.4 | 2.5 |
| NI | CsA qd + Aza | MPS I | No | 540 | 0.5 | 12.4 | 13.4 |
| VK | CsA qd + Aza | MPS I | No | 390 | 0.4 | 10.2 | 13.1 |
| TA | CsA qd + Aza | MPS I | No | 350 | 0.6 | 13.8 | 18.5 |
| Mean | 490 | 0.5 | 7.2 | 8.1 |
The identity, treatment, diagnosis, and anti-iduronidase ELISA titer are shown for canines treated with various regimens before, during, and after 12 weeks of weekly infusions of rhIDU. The upper section contains data from nontolerant canines treated with control or ineffective regimens. The lower section contains data from canines treated with the effective regimen and canine JH, the single canine with a reduced immune response using the every-other-day dosing of CsA. The mean titers for canines with 12 weeks of low-dose rhIDU exposure and for 6 weeks of high-dose exposure are shown in bold. In the lower section, all of the canines except JH are included in the mean values. The CsA trough average does not include PA on the full-dose CsA. CsA qod, 25 mg/kg every other day; CsA qd, 25 mg/kg every day. CsA divided into two dailydoses. ND, not done; NA, not applicable.
Fig. 2.
Immune response to rhIDU in canines. The ELISA titer (OD units/μlof serum) of anti-rhIDU antibodies are plotted vs. weeks of rhIDU infusions for canines treated with the tolerance regimen (open symbols) and for those treated without the tolerance regimen (filled symbols) as indicated in Table 1. The data show that 8 canines treated with the optimum regimen have low anti-rhIDU titers for up to 6 months whereas 11 canines infused with rhIDU, with inadequate or no tolerance regimen, mounted substantial immune responses. Preimmune serum was drawn at week 0, 18 days after starting the CsA plus Aza drug regimen and before the first infusion was administered. Canine JH was treated with the every-other-day CsA regimen and has gray symbols to distinguish it from the other tolerant canines. The nontolerant canines BI, BC, and BO are included for completeness although they received 5 weeks or less of rhIDU and are not included in Table 1 for this reason.
Canines treated with control or ineffective tolerance regimens (Table 1) and infused weekly with rhIDU have rising anti-rhIDU ELISA titers by week 4–6, reaching >100 OD/μl by week 12 (Fig. 2, filled symbols). Canines treated with the successful CsA plus Aza tolerance regimen had titers <15 OD/μl even after termination of all immunosuppressive drugs by week 7 and continuation of weekly enzyme infusions for as long as 6 months (Fig. 2, open symbols). The normal and MPS I canines treated with a control or ineffective regimen had a mean ELISA titer at week 12 of 149 OD/μl, compared with an ELISA titer of 7.2 OD/μl for canines receiving the successful tolerance regimen, a 20-fold difference (Table 1). The reduced IgG immune response observed with this regimen has not been observed in over 10 yr of studies of the immune response to rhIDU replacement therapy in this colony (6, 22) (data not shown).
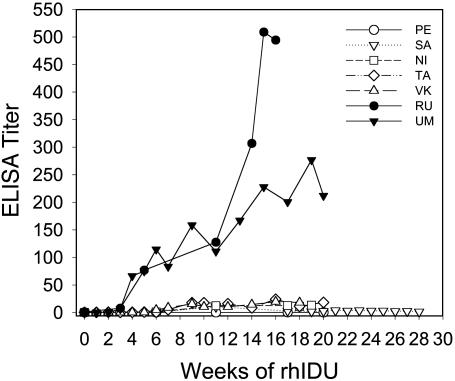
Immune Tolerance to Therapeutic Doses of rhIDU in MPS I-Affected Canines. Among the canines studied, five MPS I-affected canines (NI, PE, SA, TA, and VK) received the 60-day drug regimen and weekly infusions of 0.056 mg/kg rhIDU, and their mean titer reached 9.5 OD/μL serum at 12 weeks (Table 1 and Fig. 3). Two parallel control MPS I canines (RU and UM) developed a high mean antibody titer of 119 OD/μl to even this low dose of enzyme (Table 1 and Fig. 3).
Fig. 3.
Immune tolerance to full-dose rhIDU in treated MPS I canines. Seven MPS I canines were treated with rhIDU infusions, two with no tolerance regimen (filled symbols), and five with the tolerance regimen (open symbols) for the first 60 days ending at week 7. Through week 12, the five treated MPS I canines had low anti-rhIDU titers compared with the two untreated canines. After this initial tolerization period at week 13, the dose of rhIDU was doubled each week and finally reached 0.58 mg/kg each week (full therapeutic dose) at week 15. The tolerant MPS I canines (open symbols) did not respond to the increased rhIDU dose whereas the control canines had increasing anti-rhIDU titers.
Having shown that the responses to lower than therapeutic doses of rhIDU were decreased, we wanted to determine whether the state of reduced immune response in these MPS I canines was sufficiently robust to prevent an increased immune response to the 10-fold higher therapeutic dose of 0.58 mg/kg rhIDU used in human clinical trials of enzyme replacement therapy (2). The weekly dose of rhIDU was ramped up during weeks 12–14, reaching the full therapeutic dose of 0.58 mg/kg rhIDU per week at week 15 for the five tolerant MPS I canines (NI, PE, SA, TA, and VK) and two control canines (RU and UM). After receiving six weekly infusions of the higher therapeutic dose of rhIDU, the control canines had an increase in ELISA titer of 2- to 3-fold and reached a mean of 353 OD units/μl whereas the immune response in the five tolerized MPS I canines barely changed from a mean of 9.5 OD/μl titer to a mean of 9.6 OD/μl (Table 1 and Fig. 3). MPS I canines treated for 14 weeks of weekly therapeutic doses of 0.58 mg/kg did not have a significant increase in titer, unlike that observed in the control canines, RU and UM, and all other MPS I canines treated with 0.1–0.5 mg/kg rhIDU for 12 weeks or longer in previous studies (data not shown). For example, canines RT and RS were previously treated in other enzyme therapy studies with the same rhIDU dose of 0.58 mg/kg per week but no tolerance regimen and reached titers of 1,820 and 2,040 OD/μl by week 14.
Immune Tolerance Observed for Other Ig Isotypes. To confirm that the reduced IgG immune response to rhIDU was also observed for other Ig isotypes, the immune sera of nontolerant and tolerant MPS I canines were analyzed with secondary antibody reagents specific for canine IgM, IgA, and IgE. Nontolerant MPS I canines, RU and UM, had a significant increase in ELISA signal in IgA- and IgE-specific anti-iduronidase ELISAs (between 3- and 10-fold over preimmune background), but three tolerant MPS I canines studied, PE, SA and NI, did not (<3-fold over preimmune background; data not shown). Studies of IgM titers showed a significant (>3-fold) increase at week 4 in the nontolerant canines that was not observed in the tolerant canines (data not shown). The ELISA titers for other Ig isotypes therefore parallel the results observed for IgGs and indicate a general effect of the regimen on reducing antibody production.
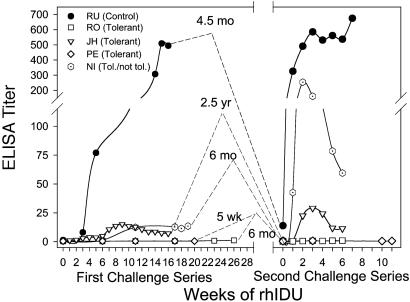
Maintenance of the Tolerant State in the Absence of Antigen Exposure. To explore the durability of the tolerant state in the absence of rhIDU exposure, we challenged previously tolerized canines with a new set of therapeutic rhIDU infusions after varying periods without rhIDU exposure. We studied four tolerant canines (JH, NI, PE, and RO) and compared them with one control canine (RU) previously responding to rhIDU (Table 1 and Fig. 3). When the control canine RU was reexposed to full-dose (0.58 mg/kg) rhIDU infusions after 4.5 months without exposure, the titer rose sharply from <25 to >300 OD/μl after a single dose in a typical anamnestic response (Fig. 4). In contrast, the tolerant MPS I canine PE had no significant response when challenged with full-dose rhIDU after 5 weeks of hiatus (Fig. 4). The tolerant normal canine RO also had no response when challenged with full-dose enzyme after a 6-month hiatus (Fig. 4). Finally, challenge of the tolerant normal canine JH after 2.5 yr of hiatus resulted in a minimal induction of anti-rhIDU antibodies with a titer below 30 OD/μl serum (Fig. 4). The antibody levels declined rapidly thereafter with ongoing weekly exposures, indicating that he was largely unresponsive. In contrast, however, one previously tolerant MPS I canine, NI, had a strong anamnestic response after a 6-month hiatus from weekly rhIDU exposure, with a titer reaching >200 OD/μl serum (Fig. 4). The result indicates that this canine retained the potential to mount a strong IgG titer response and that his initial limited immune response to rhIDU was not due to an intrinsic defect in his immune system. The overall results demonstrate that the tolerant state was maintained over a period of weeks to years in the absence of antigen exposure in three of four canines.
Fig. 4.
Immune response to rhIDU after a hiatus from antigen exposure. Canines PE, RO, NI, and JH (open symbols) were previously tolerized with rhIDU infusions and the tolerance regimen and were then rechallenged with rhIDU at 5 weeks, 6 months, 6 months, and 2.5 yr, respectively, after the last exposure to the enzyme. All but NI showed an absent or muted response to enzyme after a hiatus from exposure. The control canine RU showed a typical anamnestic response after a hiatus from enzyme exposure, rising from a titer of <25 to >300 after a single dose. The data show that the tolerant state is retained for weeks to months without continuous antigen exposure.
Antigen-Specific Tolerance and Overall Immune Status in Tolerant Canines. To test whether the reduced immune response was rhIDU-specific, we challenged one rhIDU-tolerant canine, NI, with ovalbumin and found a significant response to ovalbumin (Fig. 5a, filled circles). In addition, none of the tolerant canines had evidence of a generalized immune deficiency at 12 weeks (6 weeks off immunosuppressive drugs). The canines were maintained in a normal animal care environment and did not develop infections after the cessation of immunosuppressive drugs although respiratory infections did transiently occur during the immunosuppressive drug regimen in several canines. Complete blood counts and leukocyte differential counts were comparable between nontolerant and tolerant canines, and there was no evidence of a persistent generalized immune suppression or cytopenia after the completion of the regimen. Total serum IgG, IgM, and IgA were in the normal or near-normal range in tolerant MPS I canines and did not differ from nontolerant canines (data not shown).
Fig. 5.
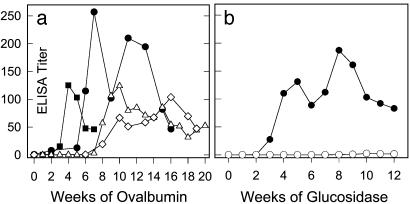
Immune responses to other antigens. (a) Immune response to ovalbumin. Canines SM (open triangles) and TR (open diamonds) were treated with the effective tolerance regimen by using ovalbumin as the toleragen; canine VS (filled squares) was the control. A strong immune response is induced in all canines regardless of the drug regimen. Canine NI (filled circles) received ovalbumin as a second antigen without the tolerance regimen and showed a similar degree of immune response. The data show that ovalbumin is not a tolerizing antigen with the regimen, unlike rhIDU. (b) Immune responses to α-glucosidase. Canine SC (open circles) was treated with the CsA plus Aza regimen by using α-glucosidase as the toleragen and showed no significant immune response (<1 OD/μl). Canine ST (filled circles) was given the same enzyme without the regimen and showed a >100 OD/μl response to the enzyme. The data suggest that α-glucosidase is a tolerizing antigen with the regimen although the data are limited to one canine.
Successful Tolerance Requires High CsA Plasma Levels. The critical nature of the CsA level was originally suggested by the data from the canine JH. This canine received the less frequent dosing regimen of 25 mg/kg CsA every other day and still had a CsA trough level of 570 ng/ml, possibly due to a slower rate of CsA metabolism, and became tolerant to rhIDU infusions (Table 1). By providing CsA at 25 mg/kg per day divided into two doses every 12 h, we raised minimum CsA trough levels in treated canines, and these canines became tolerant to rhIDU infusions, with trough CsA levels of at least 350 and preferably >400 ng/ml (Table 1). An adequate CsA level alone did not induce tolerance because administration of adequate doses of CsA without Aza did not induce tolerance as observed in canine PA (Table 1).
Successful Tolerance May Require Mannose 6-Phosphate Receptor-Mediated Uptake. To determine whether the induction of immune tolerance depended on specific features of the toleragen, we studied the immune response with other antigens in combination with the tolerance regimen. We first studied normal canines treated with recombinant human α-glucosidase and the tolerance regimen. Like α-l-iduronidase, this preparation of recombinant human α-glucosidase contains high levels of mannose 6-phosphate on its N-linked carbohydrates. Two normal canines received weekly infusions of 0.056 mg/kg α-glucosidase, one with (SC) and one without (ST) the CsA plus Aza regimen (Fig. 5b). The canine SC treated with the regimen had a minimal immune response to α-glucosidase infusion after termination of the drugs and reached a titer of <1 OD/μl serum. In comparison, the control canine ST, which received recombinant human α-glucosidase alone, reached a titer of >100 OD/μl in the same 12-week time frame (Fig. 5b). These data on a single canine suggest that lysosomal acid α-glucosidase is also a tolerizing antigen under the CsA and Aza regimen.
To determine whether the use of high-uptake lysosomal enzymes as tolerizing antigens was essential for tolerization, we also studied the effect of the tolerance regimen on the immune response to ovalbumin, a glycoprotein lacking mannose 6-phosphate modifications. Despite the use of the tolerance regimen and low-dose ovalbumin infusions, two canines (SM and TR) developed significant immune responses with antibody titers >100 OD/μl (Fig. 5a). The anti-ovalbumin titer was comparable with that achieved when we infused ovalbumin without the tolerance regimen to a control canine (VS) or as a second test antigen to the iduronidase-tolerant canine, NI (Fig. 5a). The data suggest that lysosomal enzymes can induce tolerance whereas ovalbumin cannot tolerize with the same regimen.
Because the two lysosomal enzymes with mannose 6-phosphate were toleragenic but ovalbumin was not, mannose 6-phosphate-dependent uptake could be a key feature of the toleragenic proteins, permitting efficient uptake and presentation by cells that can limit the immune response. To test further this possibility, we enzymatically dephosphorylated rhIDU (see Methods) and studied this enzyme in normal canines.
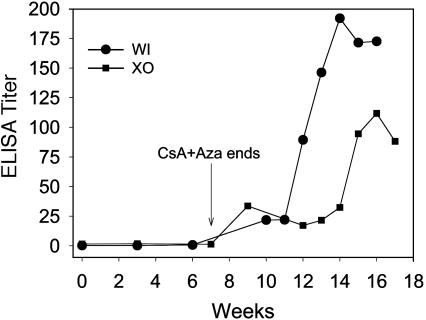
When we administered the dephosphorylated rhIDU with the drug regimen to two canines (WI and XO), a slowly increasing immune response was observed over the first 12-week period (Fig. 6). When we challenged the canines with weekly infusions of the full therapeutic dose of rhIDU, the antibody titers climbed to >100 OD/μl (Fig. 6). The data suggest that dephosphorylated rhIDU is not toleragenic with the CsA plus Aza regimen and is consistent although not definitive proof that tolerance induction requires efficient uptake of the toleragen via the mannose 6-phosphate receptor.
Fig. 6.
Dephosphorylated rhIDU is ineffective as toleragen. Dephosphorylated rhIDU was administered to canine WI and XO by weekly infusions at a dose of 0.056 mg/kg per wk with the CsA plus Aza tolerance regimen. A low but significant titer was observed in both canines. After week 12, the canines were then challenged weekly with either a full dose of 0.58 mg/kg rhIDU immediately (WO) or a ramped dose of rhIDU (XO), and both showed a strong immune response, reaching a titer of >100 OD/μl. The data suggest that dephosphorylated rhIDU is not tolerizing to the same degree as native rhIDU, consistent with a role for the mannose 6-phosphate receptor in the tolerizing uptake of the antigen.
Discussion
A strong antibody response during enzyme replacement therapy of lysosomal disorders and other deficiency disorders could potentially impede successful treatment. These studies show that low-dose infusions of lysosomal enzymes can be combined with a simple immunosuppressive regimen consisting of CsA and Aza to induce a state of immune tolerance to enzyme infusions characterized by reduced antigen-specific antibodies in the absence of long-term immune suppression. The reduced antibody responsive state is consistent with antigen-specific immune tolerance to the enzyme although the cellular aspects of the immune response were not studied and cannot readily be studied in an out-bred canine model.
The reduced immune response observed in this study seems to depend on two important features of the method: (i) profound T cell suppression based on the requirement for high plasma levels of CsA and inclusion of Aza, and (ii) the use of antigens with mannose 6-phosphate modifications. The T cell suppressive drugs CsA plus Aza are often used in transplantation studies (19) but have been used rarely in the study of the immune response to soluble proteins (23). The use of CsA may be counterintuitive based on some studies of costimulation blockade that suggest that CsA may prevent tolerance induction (24), but, in this regimen, CsA may play a different role in preventing T cell activation and immune response during the toleragen uptake period rather than a directly tolerizing role. The value of Aza in the tolerance regimen may relate to recent data suggesting that Aza blocks CD28-dependent costimulatory signaling through Rac1 (25).
The second important feature of the regimen is the use of antigens that have the mannose 6-phosphate modification on their N-linked carbohydrates, which permits efficient receptor-mediated uptake by a wide variety of cell types (26). The low-uptake antigens ovalbumin and dephosphorylated α-l-iduronidase without mannose 6-phosphate did not prevent the strong immune response to infusions of enzyme whereas the high-uptake lysosomal enzymes α-l-iduronidase and acid α-glucosidase did. These data are not definitive due to the limited number of animals studied, but the combination of data from two tolerizing and two nontolerizing antigens is supportive of this conclusion. The significance of the efficient uptake feature of rhIDU may also account for the intrinsic tolerizing activity of rhIDU in MPS I patients on enzyme replacement therapy for 2 yr (3). Further work is needed to verify the importance of mannose 6-phosphate uptake on rhIDU and other model antigens.
Antigen uptake by tolerizing immature dendritic cells (27) and other tolerizing antigen-presenting cells such as liver sinusoidal endothelial cells (28) or thymic medullary endothelial cells (29) may be critically important for peripheral tolerance (16, 30). Studies of the DEC205 receptor show that ovalbumin can be tolerizing rather than immunizing if targeted for uptake into immature dendritic cells by conjugation to anti-DEC205 antibodies (31). DEC205 delivers antigens to the late endocytic compartment (32) just as the large cation-independent mannose 6-phosphate receptor does (33). The mannose 6-phosphate receptor is widely expressed and is likely present on tolerizing cells such as immature dendritic cells (33).
The importance of antigen uptake by specific cell types in the induction of tolerance has also been suggested by other studies (16). Other antigen-focused tolerance methods such as oral tolerance (34), intraportal vein injection (35), or ITI (15) may depend on the placement of large quantities of antigen in proximity to tolerizing antigen-presenting cells to compensate for the low uptake efficiency of the antigen.
The canine immune system is a better model of the human immune system and a more rigorous test system than inbred mouse strains (36), and therefore this tolerance regimen is more likely to translate successfully to human use. The detailed immunological mechanisms that may be operating in these outbred canines cannot be analyzed as easily as inbred mouse strains for many reasons. Even without detailed knowledge of the underlying mechanisms, the synthesis and administration of toleragenic high-uptake forms of therapeutic proteins or antigens, combined with the drug regimen described here, may allow the prevention of strong antibody responses during chronic protein replacement therapies or the reduction in antibodies associated with autoimmune disease.
Acknowledgments
We thank Rita Esquivel, Dan Garner, Dave Tokuda, Lin Chen, Her-Shyang Chiang, and Diana Welsh for technical assistance and P. Moore for the hybridomas making monoclonal antibodies to canine TCR, Thy-1, and CD3. We thank J. Bluestone and E. Neufeld for reviewing the manuscript. Funding was provided by National Institutes of Health Grant DK54566, the Ryan Foundation, and BioMarin Pharmaceutical Inc.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: rhIDU, recombinant human α-l-iduronidase; MPS I, mucopolysaccharidosis I; CsA, cyclosporin A; Aza, azathioprine; TCR, T cell antigen receptor; ITI, intrathymic injection.
References
- 1.Neufeld, E. F. & Muenzer, J. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C., Beaudet, A. L., Valle, D. & Sly, W. (McGraw–Hill, New York), pp. 3421–3452.
- 2.Kakkis, E. D., Meunzer, J., Tiller, G., Waber, L., Belmont, J., Passage, M., Izykowski, B., Phillips, J., Doroshow, R., Walot, I., et al. (2001) N. Engl. J. Med. 344, 182–188. [DOI] [PubMed] [Google Scholar]
- 3.Kakavanos, R., Turner, C. T., Hopwood, J. J., Kakkis, E. D. & Brooks, D. A. (2003) Lancet 361, 1608–1613. [DOI] [PubMed] [Google Scholar]
- 4.Spellacy, E., Shull, R. M., Costantopoulos, G. & Neufeld, E. F. (1983) Proc. Natl. Acad. Sci. USA 80, 6091–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon, K. P., Tieu, P. T. & Neufeld, E. F. (1992) Genomics 14, 763–768. [DOI] [PubMed] [Google Scholar]
- 6.Shull, R. M., Kakkis, E. D., McEntee, M. F., Kania, S. A., Jonas, A. J. & Neufeld, E. F. (1994) Proc. Natl. Acad. Sci. USA 91, 12937–12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner, C. T., Hopwood, J. J. & Brooks, D. A. (2000) Mol. Genet. Metab. 69, 277–285. [DOI] [PubMed] [Google Scholar]
- 8.Brooks, D. A. (1999) Mol. Genet. Metab. 68, 268–275. [DOI] [PubMed] [Google Scholar]
- 9.Aledort, L. (1994) Am. J. Hematol. 47, 208–217. [DOI] [PubMed] [Google Scholar]
- 10.Chaffee, S., Mary, A., Stiehm, E. R., Girault, D., Fischer, A. & Hershfield, M. S. (1993) J. Clin. Invest. 89, 1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards, S. M., Olson, T. A. & McPherson, J. M. (1993) Blood 82, 1402–1409. [PubMed] [Google Scholar]
- 12.Rosenberg, M., Kingma, W., Fitzpatrick, M. A. & Richards, S. M. (1999) Blood 93, 2081–2088. [PubMed] [Google Scholar]
- 13.Amalfitano, A., Bengur, A. R., Morse, R. P., Majure, J. M., Case, L. E., Veerling, D. L., Mackey, J., Kishnani, P., Smith, W., McVie-Wylie, A., et al. (2001) Genet. Med. 3, 132–138. [DOI] [PubMed] [Google Scholar]
- 14.Garside, P. & Mowat, A. M. (2001) Semin. Immunol. 13, 177–185. [DOI] [PubMed] [Google Scholar]
- 15.Chen, W., Issazadeh, S., Sayegh, M. H. & Khoury, S. J. (1997) Cell. Immunol. 179, 165–173. [DOI] [PubMed] [Google Scholar]
- 16.Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. (2002) Annu. Rev. Immunol. 20, 621–667. [DOI] [PubMed] [Google Scholar]
- 17.Sebille, F., Vanhove, B. & Soulillou, J. P. (2001) Philos. Trans. R. Soc. London B Biol. Sci. 356, 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson, C. J., Davies, H. F., Cobbold, S. P., Rasmussen, A., Rebello, P. R., Thiru, S., Waldmann, H., Calne, R. Y. & Metcalfe, S. M. (1995) Transplant. Proc. 27, 123–124. [PubMed] [Google Scholar]
- 19.Watson, C. J. E., Cobbold, S. P., Davies, H. S., Rebello, P. R. U. B., Thiru, S., McNair, R., Rasmussen, A., Waldmann, H., Calne, R. Y. & Metcalfe, S. M. (1994) Tissue Antigens 43, 155–162. [DOI] [PubMed] [Google Scholar]
- 20.Davies, H. S., Cobbold, S. P., Watson, C. J., Metcalfe, S. M., Rebello, P. R., Thiru, S., McNair, R., Rasmussen, A., Waldmann, H. & Calne, R. Y. (1994) Transplant. Proc. 26, 1941–1942. [PubMed] [Google Scholar]
- 21.Kakkis, E. D., Matynia, A., Jonas, A. J. & Neufeld, E. F. (1994) Protein Expression Purif. 5, 225–232. [DOI] [PubMed] [Google Scholar]
- 22.Kakkis, E. D., McEntee, M. F., Schmidtchen, A., Neufeld, E. F., Ward, D. A., Gompf, R. E., Kania, S., Bedolla, C., Chien, S. L. & Shull, R. M. (1996) Biochem. Mol. Med. 58, 156–167. [DOI] [PubMed] [Google Scholar]
- 23.Agerso, H., Wilken, M., Drustrup, J., Haahr, P. M. & Jorgensen, K. D. (1999) J. Pharmacol. Toxicol. Methods 41, 1–8. [DOI] [PubMed] [Google Scholar]
- 24.Adams, A. B., Pearson, T. C. & Larsen, C. P. (2001) Philos. Trans. R. Soc. London B Biol. Sci. 356, 703–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiede, I., Fritz, G., Strand, S., Poppe, D., Dvorsky, R., Strand, D., Lehr, H. A., Wirtz, S., Becker, C., Atreya, R., et al. (2003) J. Clin. Invest. 111, 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahms, N. M., Lobel, P. & Kornfeld, S. (1989) J. Biol. Chem. 264, 12115–12118. [PubMed] [Google Scholar]
- 27.Steinman, R. M., Hawiger, D., Liu, K., Bonifaz, L., Bonnyay, D., Mahnke, K., Iyoda, T., Ravetch, J., Dhodapkar, M., Inaba, K., et al. (2003) Ann. N.Y. Acad. Sci. 987, 15–25. [DOI] [PubMed] [Google Scholar]
- 28.Knolle, P. A. & Limmer, A. (2001) Trends Immunol. 22, 432–437. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann, M. W., Allison, J. & Miller, J. F. (1992) Proc. Natl. Acad. Sci. USA 89, 2526–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. (2003) Annu. Rev. Immunol. 21, 685–711. [DOI] [PubMed] [Google Scholar]
- 31.Bonifaz, L., Bonnyay, D., Mahnke, K., Rivera, M., Nussenzweig, M. C. & Steinman, R. M. (2002) J. Exp. Med. 196, 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinman, R. M. & Nussenzweig, M. C. (2002) Proc. Natl. Acad. Sci. USA 99, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornfeld, S. (1992) Annu. Rev. Biochem. 61, 307–330. [DOI] [PubMed] [Google Scholar]
- 34.Weiner, H. L., Friedman, A., Miller, A., Khoury, S. J., Al-Sabbagh, A., Santos, L., Sayegh, M., Nussenblatt, R. B., Trentham, D. E. & Hafler, D. A. (1994) Annu. Rev. Immunol. 12, 809–837. [DOI] [PubMed] [Google Scholar]
- 35.Wrenshall, L. E., Ansite, J. D., Eckman, P. M., Heilman, M. J., Stevens, R. B. & Sutherland, D. E. (2001) Transplantation 71, 841–850. [DOI] [PubMed] [Google Scholar]
- 36.Felsburg, P. J. (2002) Hum. Exp. Toxicol. 21, 487–492. [DOI] [PubMed] [Google Scholar]