Abstract
A number of 3-benzyl-2-phenyl-4(5H)-(substituted phenylhydrazino)-1, 3-oxazolidines 6a-f derivatives were synthesized and their antihyperglycemic activity was evaluated by streptozotocin (STZ) and sucrose-loaded model. Many of oxazolidine derivatives as well as their corresponding substituted phenylhydrazine showed significant reduction in blood glucose level in STZ and sucrose-loaded rat models.
Keywords: Antihyperglycemic activity, oxazolidine, substituted phenylhydrazine
INTRODUCTION
Diabetes mellitus is a main threat to human health in the 21st century.[1] The incidence of the disease currently is estimated to reach 300 million by the year 2025.[2] Oxazolidine is an important group of heterocyclic compounds that plays a vital role in many biological activities in the areas of medicine and molecules containing oxazolidine ring systems and exhibits many activities such as anti-TB activity,[3] antibacterial activity,[4] mono-amine oxidase inhibition,[5] and GP IIb/IIIa antagonism[6] neuroleptic activity.[7] The information on oxadiazolidine as antihyperglycemic agents has been reported previously.[8] In view of the literature led to the conception that a novel series of 3-benzyl-2-phenyl-4(5H)-(substituted phenylhydrazino)-1, 3-oxazolidines 6a-f derivatives were synthesized and their chemical structure were confirmed by IR, 1H-NMR, mass spectral and elemental analyses. These compounds were screened for their antimicrobial activities.
MATERIALS AND METHODS
Materials
Synthetic starting material, reagents and solvents were of analytical reagent grade or of the highest quality that were commercially available and were purchased from Aldrich Chemical Co (Hyderabad, AndhraPradesh, India), Merck Chemical Co (Mumbai, Maharashtra, India) and Acros Organics (Mumbai, Maharashtra, India) and were dried when necessary.
The melting points were taken in open capillary tube and uncorrected. IR spectra were recorded with KBr pellets (ABB Bomem FT-IR spectrometer MB 104 ABB Limited, Bangaloru, India). Proton (1H) NMR spectra (Bruker 400 NMR spectrometer, Mumbai, India) were recorded with TMS as internal references. Mass spectral data were recorded with a quadrupole mass spectrometer (Shimadzu GC MS QP 5000, Chennai, India) and microanalyses were performed using a vario EL V300 elemental analyzer (Elemental Analysensysteme GmbH, Chennai, India). The purity of the compounds was checked by TLC on pre-coated SiO2 gel (HF254, 200 mesh) aluminium plates (E. Merck) using ethyl acetate:benzene ratio of 1:3 and visualized in UV chamber. IR, 1H-NMR, mass spectral data and elemental analyses were consistent with the assigned structures.
General Procedures
The target novel oxazolidine derivatives were synthesized by previously reported method.[9,10] Accordingly, benzylamine 1 was treated with an equimolar amount of benzaldehyde 2 and an hydroxyacetic acid 3 in dry toluene under reflux 24-48 h to give 3-benzyl-2-phenyl-1,3-oxazolidine-4(5H)-one 4, further its treat with thionyl chloride and DMF to get chloro derivative 5. 3-benzyl-2-phenyl-4(5H)-chloro-1,3-oxazolidine and then coupled with substituted phenyl hydrazine, in dimethyl sulfoxide at temperature 100 °C for 4-5 h and obtained 3-benzyl-2-phenyl-4(5H)-(substituted phenyl hydrazino)-1,3-oxazolidines 6a-f [Figure 1] were separated by filtration, vaccum dried and recrystallized from warm ethanol.
Figure 1.
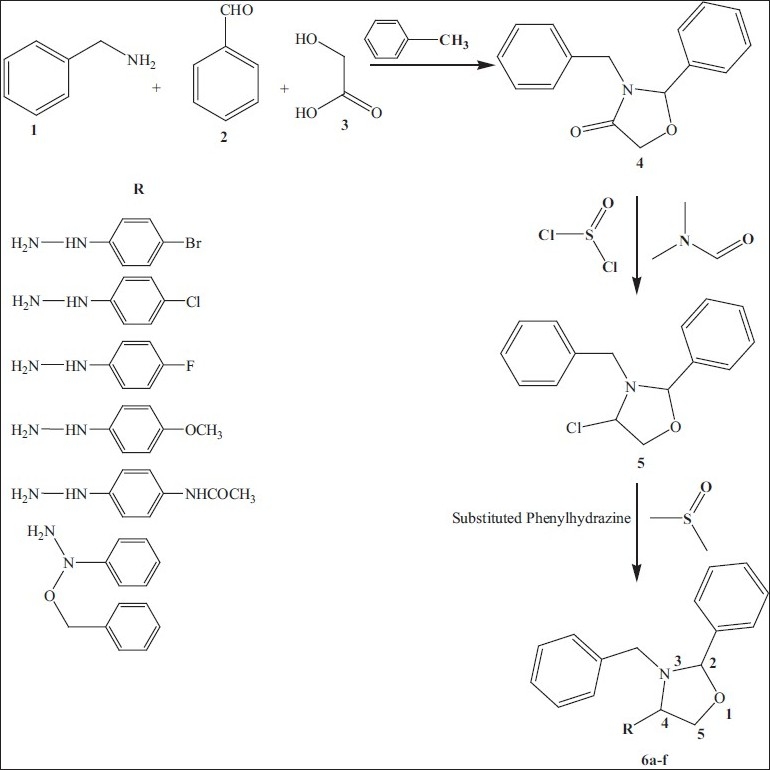
3-benzyl-2-phenyl-4(5H)-(substituted phenyl hydrazino)-1, 3-oxazolidines
The in vivo antihyperglycemic activity of synthesized compounds was evaluated using male Sprague–Dawley rats in two different models.
Streptozotocin model
A solution of streptozotocin (STZ) (60 mg/kg) in 100 mM citrate buffer, pH 4.5 was prepared and calculated amount of the fresh solution was dosed to overnight fasted rats (60 mg/kg) intraperitoneally. The blood sugar level was measured after 48 h by glucometer. Animals showing 200-400 mg/dL were selected for antidiabetic screening. The diabetic animals were divided into groups of six animals each. Rats of experimental group were administered a suspension of the desired test sample (prepared in 1% gum acacia) orally (100 mg/kg body weight). Controlled group animals were also fed with 1% gum acacia. The blood glucose levels were measured at 1-, 2-, 3-, 4-, 5-, 6-, 7- and 24-h intervals. The % fall in blood glucose from 1 to 24 h by test sample was calculated according to the area under curve (AUC) method. The average fall in AUC in experimental group compared to control group provided % antihyperglycemic activity.
Sucrose-loaded (SLM) model
Overnight-fasted male Sprague-Dawley rats were used for sucrose-loaded experiment. Blood was collected initially and there after test compounds were given to the test group consisting of five rats by oral gavage at a dose of 100 mg/kg body weight. After half an hour post-test treatment, a sucrose load of 10 gm/kg body weight was given to each rat. Blood was collected at 30, 60, 90 and 120 min post-sucrose load. The % fall in blood glucose level was calculated according to the AUC method.
RESULTS AND DISCUSSION
Chemistry
The synthesized series of heterocycles, 6a-f by the reaction of 5 with appropriate substituted phenyl hydrazine in the presence of anhydrous sodium acetate and glacial acetic acid as presented in Scheme. The IR, 1H-NMR, mass spectroscopy and elemental analysis for the new compound is in accordance with the assigned structures. The IR spectra of compounds 4 showed stretching bands of keto group at 1728 cm-1. In 5, stretching bands of chloro group at 749 cm-1 is evidence to conversion of oxazolidinone. The title compounds 6a-f stretching and bending NH bands appear at 3300-3400 cm-1 and 1300-1350 cm-1, respectively. The recorded IR spectrum of representative compounds 6a-f showed missing of chloro group bands. This clearly envisages that the chloro group of 5 is converted into secondary NH. The proton magnetic resonance spectra of oxazolidine and their corresponding derivatives have been recorded in CDCl3. In this 6a-f NH signal of 3-benzyl-2-phenyl-4(5H)-(4”-substituted phenylhydrazino)-1, 3-oxazolidines moiety appear at 7.49 (s), 7.51 (s), 7.54 (s), 7.48 (s), 7.29 (s), 7.31 (s) ppm, respectively. The position and presence of NH signal in the 1H-NMR spectra of final compounds conforms the secondary NH proton in oxazolidine moiety. This clearly envisages that oxazolidine-4(5H)-one moiety involve in 4(5H)-chloro-1,3-oxazolidine and further (4”- substituted phenyl hydrazino)-1,3-oxazolidines formation. All these observed facts clearly demonstrate that the 4th position of keto group in oxazolidine ring is converted into secondary amino group as indicated in scheme 1 and conforms the proposed structure (6a-f).
3-benzyl-2-phenyl-1, 3-oxazolidine-4(5H)-one (4)
Yellow Solid; Yield: 82%; Rf value:0.47; mp. 172-174°C, IR: 3096 (Ar-CH), 1728 (C=O), 1468 (C=C) cm-1; 1H-NMR (CDCl3): δ 6.96-7.54 (m,10H, Ar-H), 6.67 (s, 1H, -CH), 4.12-4.62 (m, 4H, 2 × CH2); EI-MS (m/z, %): 253 [M]+; (Calcd for C16H15NO2; 253.3). Anal. Calcd for C16H15NO2, C, 75.87; H, 5.97; N, 5.53; Found: C, 75.46; H, 5.61; N, 5.36.
3-benzyl-2-phenyl-4(5H) chloro-1, 3-oxazolidine (5)
Pale Yellow Solid; Yield: 71%; Rf value:0.43; mp. 146-148°C, IR: 3084 (Ar-CH), 827 (C-Cl), 1412 (C=C) cm-1; 1H-NMR (CDCl3): δ 6.18-7.42 (m, 10H, Ar-H), 4.98-5.27 (s, 2H, -CH), 3.46-4.22 (m, 4H, 2 × CH2); EI-MS (m/z, %): 273 [M]+; (Calcd for C16H16ClNO; 273.09). Anal. Calcd for C16H16ClNO, C, 70.20; H, 5.89; N, 5.12; Found: C, 70.41; H, 5.96; N, 5.72.
3-benzyl-2-phenyl-4(5H)-4’bromophenyl hydrazino-1, 3-oxazolidine (6a)
Yellow Crystals; Yield: 74%; Rf value:0.33; mp. 171-173°C, IR: 3031 (Ar-CH), 813 (C-Br), 1496 (C=C), 1309 (N-H bending), 3389 (N-H stretching) cm-1; 1H-NMR (CDCl3): δ 6.89-7.49 (m, 14H, Ar-H), 5.19 (s, 2H, -CH), 7.49 (s, 2H, N-H), 3.56-3.69 (m, 4H, 2 × CH2); EI-MS (m/z, %): [M+2] 425; (Calcd for C22H22BrN3O; 425.33). Anal. Calcd for C22H22BrN3O; C, 62.27; H, 5.23; N, 9.90; Found: C, 62.11; H, 5.22; N, 9.74.
3-benzyl-2-phenyl-4(5H)-4’chlorophenyl hydrazino-1, 3-oxazolidine (6b)
Pale Yellow Solid; Yield: 78%; Rf value:0.41; mp. 175-177°C, IR: 3029 (Ar-CH), 829 (C-Cl), 1484 (C=C), 1316 (N-H bending), 3391 (N-H stretching) cm-1; 1H-NMR (CDCl3): δ 6.87-7.42 (m, 14H, Ar-H), 5.22 (s, 2H, -CH), 7.51 (s, 2H, N-H), 3.49-3.57 (m, 4H, 2 × CH2); EI-MS (m/z, %): [M+2] 381; (Calcd for C22H22ClN3O; 381.15). Anal. Calcd for C22H22ClN3O; C, 69.56; H, 5.84; N, 11.06; Found: C, 69.51; H, 5.96; N, 11.12.
3-benzyl-2-phenyl-4(5H)-4’fluorophenyl hydrazino-1, 3-oxazolidine (6c)
Pale Brown Solid; Yield: 71%; Rf value:0.37; mp. 170-172°C, IR: 3021 (Ar-CH), 810 (C-F), 1481 (C=C), 1326 (N-H bending), 3397 (N-H stretching) cm-1; 1H-NMR (CDCl3): δ 6.81-7.43 (m, 14H, Ar-H), 5.25 (s, 2H, -CH), 7.54 (s, 2H, N-H), 3.42-3.52 (m, 4H, 2 × CH2); EI-MS (m/z, %): [M] 381; (Calcd for C22H22FN3O; 363.17). Anal. Calcd for C22H22FN3O; C, 72.71; H, 6.10; N, 11.56; Found: C, 72.62; H, 6.19; N, 11.42.
3-benzyl-2-phenyl-4(5H)-4’methoxyphenyl hydrazino-1, 3-oxazolidine (6d)
Pale Solid; Yield: 71%; Rf value:0.32; mp. 181-183°C, IR: 3012 (Ar-CH), 1426 (C=C), 1331 (N-H bending), 3326 (N-H stretching) cm-1; 1H-NMR (CDCl3): δ 6.83-7.47 (m, 14H, Ar-H), 5.27 (s, 2H, -CH), 7.48 (s, 2H, N-H), 3.16 (s, 3H, -OCH3) 3.36-4.85 (m, 4H, 2 × CH2); EI-MS (m/z, %): [M] 375; (Calcd for C23H25N3O2; 375.19). Anal. Calcd for C23H25N3O2; C, 73.57; H, 6.71; N, 11.19; Found: C, 73.43; H, 6.76; N, 11.21.
3-benzyl-2-phenyl-4(5H)-4’acetamidophenyl hydrazino-1, 3-oxazolidine (6e)
Pale Brown Solid; Yield: 76%; Rf value:0.33; mp. 176-178°C, IR: 3024 (Ar-CH), 1423 (C=C), 1731 (C=O), 1337 (N-H bending), 3316 (N-H stretching) cm-1; 1H-NMR (CDCl3): δ 6.76-7.32 (m, 14H, Ar-H), 5.29 (s, 2H, -CH), 7.29 (s, 3H, N-H), 3.19 (s, 3H, -CH3) 3.31-4.82 (m, 4H, 2 × CH2); EI-MS (m/z, %): [M] 402; (Calcd for C24H26N4O2; 402.21). Anal. Calcd for C24H26N4O2; C, 71.62; H, 6.51; N, 13.92; Found: C, 71.52; H, 6.59; N, 13.82.
3-benzyl-2-phenyl-4(5H)-benzyloxyphenyl hydrazino-1, 3-oxazolidine (6f)
Pale White Solid; Yield: 82%; Rf value:0.35; mp. 182-184°C, IR: 3036 (Ar-CH), 1534 (C=C), 1326 (N-H bending), 3329 (N-H stretching) cm-1; 1H-NMR (CDCl3): δ 6.72-7.32 (m, 20H, Ar-H), 6.24 (s, 2H, -CH), 7.31 (s, 1H, N-H), 3.44-3.84 (m, 6H, 3 × CH2); EI-MS (m/z, %): [M]+ 451; (Calcd for C29H29N3O2; 451.23). Anal. Calcd for C29H29N3O2; C, 77.13; H, 6.74; N, 9.31; Found: C, 77.28; H, 6.62; N, 9.37.
Antihyperglycemic activity
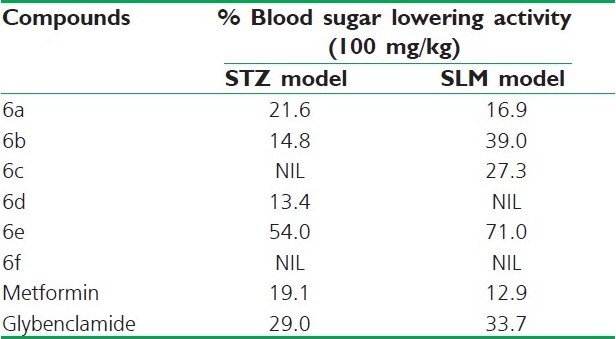
Among the six screened compounds only 6e, 6a, 6b exhibit antihyperglycemic activity in STZ model and SLM model. Compound 6c displayed 27.3% blood glucose lowering activity at 100 mg/kg dose in SLM model and 6d showed 13.4% blood glucose lowering activity at 100 mg/kg dose in STZ model. In these compounds, 3-benzyl-2-phenyl-4(5H)-4’acetamidophenyl hydrazino-1, 3-oxazolidine 6e reduced blood glucose level to 54 and 71% in STZ and SLM models, respectively. It is evident from Table 1 that, the presence of benzyloxy (6f) caused a complete loss of antihyperglycemic activity. A closer inspection of data from this table indicates that compounds acetamido, bromo and chloro phenylhydrazino substitution of oxazolidine having excellent antihyperglycemic activity compared with standard. The transformation order of screened compounds is 6e>6a>6b>6c>6d>6f. Metformin and glybenclamide were used as standard antidiabetic drug in both the models.
Table 1.
In vivo antihyperglycemic activity of various 3-benzyl-2-phenyl-4(5H)-(substituted phenylhydrazino)-1, 3-oxazolidines 6a-f derivatives in streptozotocin (STZ) and sucroseloaded (SLM) rat models
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Zimmet P. Globalization, coca-colonization and the chronic disease epidemic: Can the Doomsday scenario be averted? J Int Med. 2000;247:301–10. doi: 10.1046/j.1365-2796.2000.00625.x. [DOI] [PubMed] [Google Scholar]
- 2.King H, Aubert R, Herman W. Global burden of diabetes. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Wallace RJ, Brown-Elliott BA, Ward SC, Crist CJ, Mann LB, Wilson RW. Activities of linezolid against rapidly growing mycobacteria. Antimicrob Agents Chemother. 2001;4:764. doi: 10.1128/AAC.45.3.764-767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbachyn MR, Ford CW. Oxazolidinone structure-activity relationships leading to linezolid angew. Chem Int Ed. 2003;42:2010. doi: 10.1002/anie.200200528. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay RR, Gravestock MB. Monoamine oxidases: To inhibit or not to inhibit. Mini Rev Med Chem. 2009;3:129. doi: 10.2174/1389557033405287. [DOI] [PubMed] [Google Scholar]
- 6.Gante J, Juraszyk H, Raddatz P, Wurziger H, Bernotat-Danielowski S, Melzer G, et al. Aminimides as peptidomimetics. Lett Pep Sci. 1995;2:135. [Google Scholar]
- 7.Pruecher H, Gottschlich R, Haase A, Stohrer M, Seyfried C. (5S)-3-aryl-5-(1-piperidinylmethyl)-2-oxazolidinones, a new class of potential neuroleptics with a high affinity for sigma receptors. Bioorg Med Chem Lett. 1992;2:165–70. [Google Scholar]
- 8.Goldstein SW, McDermott RE, Gibbs EM, Stevenson RW. Hydroxyurea derivatives as hypoglycemic agents. J Med Chem. 1993;36:2238–40. doi: 10.1021/jm00067a023. [DOI] [PubMed] [Google Scholar]
- 9.Zarghi A, Najafnia L, Daraie B, Dadrass OG, Hedayati M. Synthesis of 2,3-diaryl-1,3-thiazolidine-4-one derivatives as selective cyclooxygenase (COX-2) inhibitor. Bioorg Med Chem Lett. 2007;1:5634–7. doi: 10.1016/j.bmcl.2007.07.084. [DOI] [PubMed] [Google Scholar]
- 10.Mahesh TC, Mitesh HJ. Design, synthesis and antimycobacterial activity of some novel imidazo[1,2-c]pyrimidines. Eur J Med Chem. 2009;44:3837–44. doi: 10.1016/j.ejmech.2009.04.002. [DOI] [PubMed] [Google Scholar]


