Abstract
Considerable evidence implicates glucocorticoid hormones in the regulation of memory consolidation and memory retrieval. The present experiments investigated whether the influence of these hormones on memory depends on the level of emotional arousal induced by the training experience. We investigated this issue in male Sprague–Dawley rats by examining the effects of immediate posttraining systemic injections of the glucocorticoid corticosterone on object recognition memory under two conditions that differed in their training-associated emotional arousal. In rats that were not previously habituated to the experimental context, corticosterone (0.3, 1.0, or 3.0 mg/kg, s.c.) administered immediately after a 3-min training trial enhanced 24-hr retention performance in an inverted-U shaped dose–response relationship. In contrast, corticosterone did not affect 24-hr retention of rats that received extensive prior habituation to the experimental context and, thus, had decreased novelty-induced emotional arousal during training. Additionally, immediate posttraining administration of corticosterone to nonhabituated rats, in doses that enhanced 24-hr retention, impaired object recognition performance at a 1-hr retention interval whereas corticosterone administered after training to well-habituated rats did not impair 1-hr retention. Thus, the present findings suggest that training-induced emotional arousal may be essential for glucocorticoid effects on object recognition memory.
Keywords: corticosterone, stress hormones, memory consolidation, memory retrieval
Emotionally arousing experiences activate the hypothalamic-pituitary-adrenocortical axis, resulting in elevated glucocorticoid levels (i.e., corticosterone and cortisol). Considerable evidence indicates that glucocorticoid hormones affect several aspects of cognitive functioning, including memory consolidation and memory retrieval (1–7). There is extensive evidence that glucocorticoids administered to animals or human subjects shortly before or immediately after a training experience dose-dependently enhance the consolidation of long-term memory (3, 6, 8–10). Other evidence indicates that glucocorticoids administered before retention testing impair retrieval of previously acquired information (11–14). Furthermore, glucocorticoids or a mild stressor administered shortly before or immediately after training impairs short-term retention performance (15, 16).
Recent findings have suggested that in human subjects stress hormones may not uniformly modulate the consolidation of all kinds of information but, rather, may selectively affect memory of emotionally arousing information (12). Buchanan and Lovallo (9) reported that cortisol administered shortly before training enhanced long-term memory of emotionally arousing, but not emotionally neutral, pictures. Studies investigating the effects on memory consolidation of posttraining administration of epinephrine (17) or cold pressor stress exposure, causing endogenous stress hormone activation (18), obtained similar results. However, another recent study (10) of memory in healthy human volunteers reported findings suggesting that cortisol enhances memory of emotionally neutral as well as emotionally arousing information.
In contrast to studies of memory in human subjects, animal experiments generally use emotionally arousing learning tasks. With the use of such experimental conditions, it is not possible to determine a possible role of emotional arousal in glucocorticoid influences on memory processes. The present study investigated this issue in rats trained on an object recognition task. This task, originally developed by Ennaceur and Delacour (19), is based on the tendency of rodents to explore a novel object more than a familiar one. Because no rewarding or aversive stimulation is used during training, the learning occurs under conditions of relatively low stress or arousal (19). However, placement of rats into an unfamiliar testing apparatus does evoke some degree of novelty-induced arousal, and repeated habituation of rats to the experimental context is known to reduce this arousal response (20, 21). Thus, rats habituated to the training apparatus would be expected to be less aroused by object recognition training than rats not given prior habituation training. To examine this implication, corticosterone was administered systemically immediately after training on an object recognition task to rats that were either nonhabituated or well-habituated to the experimental context. In a first experiment, retention was assessed 24 hr after the training trial to examine possible glucocorticoid-emotional arousal interactions on memory consolidation. As noted, other findings indicate that glucocorticoids can also impair memory retrieval (11) and that pre- or posttraining acute stress exposure impairs object recognition memory when tested after a short-term delay when corticosterone levels are still elevated (22, 23). To investigate whether glucocorticoid effects on short-term memory impairment might also depend on emotional arousal, a second experiment examined the effects of posttraining corticosterone administered to either nonhabituated or well-habituated rats on object recognition memory tested 1 hr after the training trial.
Materials and Methods
Animals. Male adult Sprague–Dawley rats (350–450 g at time of training) from Charles River Breeding Laboratories were kept individually in a temperature-controlled (22°C) vivarium room and maintained on a standard 12-hr/12-hr light/dark cycle (0700–1900 hours lights on). Food and water were available ad libitum. Training and testing were performed during the light phase of the cycle between 1000–1400 hours, at the rat nadir of the circadian cycle for corticosterone. All experimental procedures were in compliance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Object Recognition Task. The experimental apparatus used for the object recognition task was an open-field box (in cm, 40 wide × 40 deep × 40 high) made of gray-painted wood with a floor covered with sawdust, placed in a dimly illuminated room. The objects to be discriminated were white glass light bulbs (6-cm diameter and 11-cm length) and transparent glass vials (5.5-cm diameter and 5-cm height).
Approximately 2 weeks after arrival into the vivarium, the rats were handled twice per day for 1 min each for 7 days immediately preceding the training day. At the beginning of the handling period, the rats were divided into two groups. One group of rats was not habituated to the apparatus (WITHOUT-habituation condition), whereas the other group was excessively habituated to the experimental apparatus to decrease their novelty stress to the apparatus during the training trial (WITH-habituation condition). During habituation, the rats were allowed to freely explore the apparatus in the absence of objects twice per day for 3 min each for 7 days.
On the training trial, the rat was placed in the experimental apparatus, facing the wall, at the opposite end from the objects. For 3 min, the rat was allowed to explore two identical objects (A1 and A2), which were placed in the back corners of the box. Then, the rat was removed from the apparatus and, after drug treatment, returned to its home cage. To avoid the presence of olfactory trails, sawdust was stirred and the objects were thoroughly cleaned with 70% ethanol after each rat. Rat's exploratory behavior of the experimental apparatus during the training trial was analyzed by the total number of rearings and locomotor activity. For locomotor activity, the floor of the apparatus was divided into four equal imaginary squares, and the total number of crossings between squares was determined.
Retention was tested either 1 or 24 hr after the training trial. Pilot data indicated that, in both experimental conditions, a 3-min training trial induces significant retention at a 1-hr, but not 24-hr, delay. On the retention test trial, one copy of the familiar object (A3) and a new object (B) was placed in the same location as stimuli during the training trial. All combinations and locations of objects were used in a balanced manner to reduce potential biases due to preference for particular locations or objects. The rat was placed in the experimental apparatus for 3 min, and its behavior was recorded by using a video camera mounted above the experimental apparatus. Tapes were analyzed off-line by a trained observer who was unaware of the treatment condition. The time spent exploring each object and the total time spent exploring both objects were recorded. Exploration of an object was defined as pointing the nose to the object at a distance of <1 cm and/or touching it with the nose. Turning around or sitting on an object was not considered as exploration. To analyze cognitive performance, a discrimination index was calculated as the difference in time exploring the novel and familiar object, expressed as the ratio of the total time spent exploring both objects, which made it possible to adjust for any differences in total exploration time (24). Rats showing a total exploration time of <10 s on either training or testing were removed from further analyses because pilot data indicated that such rats do not adequately acquire the task.
Drug Preparation and Administration. Corticosterone (0.3, 1.0, or 3.0 mg/kg; Sigma) was first dissolved in 100% ethanol and then diluted in 0.9% saline to reach its appropriate concentration. The final concentration of ethanol was 5%. The vehicle solution contained 5% ethanol in saline only. The doses of corticosterone were selected on the basis of previous experiments indicating that these doses induce plasma corticosterone levels resembling mild to moderately severe stress (11). Corticosterone or vehicle was administered s.c. immediately after the training trial in a volume of 2.0 ml/kg of body weight. All drug solutions were freshly prepared before each experiment.
Corticosterone Assay. Plasma corticosterone levels were determined in parallel groups of rats in the WITH-habituation and WITHOUT-habituation condition. Rats were decapitated 30 min after training and corticosterone administration. Trunk blood was collected in heparinized (500 units/ml) tubes and stored on ice. After centrifugation at 4,500 × g for 10 min, the supernatant was stored at –70°C until assay. Corticosterone plasma concentrations were determined in duplicate by a commercially available enzyme immunoassay kit by using 96-well microtiter plates coated with polyclonal antibody raised against corticosterone (Alpco, Windham, NH). The absorbance levels were measured with a photometric microplate reader (Thermo Labsystems, Helsinki) at 450 nm. The sensitivity was 0.023 μg/dl, and coefficients of variation within and between assays were <10%.
Statistics. All data were expressed as the mean ± SEM. Statistical analysis used one-way or two-way analysis of variance (ANOVA), followed by post hoc comparison tests or unpaired Student t tests. One-sample t tests were used to determine whether the discrimination index was different from zero. A probability level of <0.05 was accepted as statistical significance.
Results
Posttraining Corticosterone Enhanced Object Recognition Memory at a 24-hr Retention Test of Rats in the WITHOUT-Habituation but Not WITH-Habituation Condition. This experiment examined whether immediate posttraining injections of corticosterone enhanced long-term consolidation of object recognition memory and whether such glucocorticoid-induced memory enhancement was influenced by prior habituation to the experimental context. Training trial. Table 1 shows the total exploration time of the two identical objects on the training trial for rats in the two habituation conditions (WIT HOUT-habituation vs. WIT H-habituation). Two-way ANOVA for total object exploration time revealed a significant habituation effect (F1,87 = 18.53, P < 0.0001), but no differences between groups that later received posttraining drug treatment (F3,87 = 0.07, P = 0.97) or an interaction between habituation condition and posttraining drug treatment (F3,87 = 0.31, P = 0.82). Rats in the WITHOUT-habituation condition showed significantly less total exploration of the two objects than rats in the WITH-habituation condition (t93 = 4.44, P < 0.0001; Table 2). One-sample t tests, used to examine whether the discrimination index was different from zero (chance level), showed that all groups exhibited comparable time exploring each of the two identical objects on the training trial (P ≥ 0.45). Additionally, the several groups subsequently given posttraining drug treatment did not differ in their exploration of the two objects (F3,44 = 0.17, P = 0.92 for WITHOUT-habituation condition; F3,43 = 0.09, P = 0.97 for WITH-habituation condition).
Table 1. Total object exploration time.
| Prior habituation condition | Drug | Training | Retention |
|---|---|---|---|
| 24-hr retention interval | |||
| WITHOUT | Vehicle | 22.9 ± 2.3 | 19.5 ± 2.1 |
| 0.3 mg/kg | 23.6 ± 1.6 | 19.7 ± 2.0 | |
| 1.0 mg/kg | 25.9 ± 1.8 | 22.4 ± 2.4 | |
| 3.0 mg/kg | 24.7 ± 2.5 | 22.8 ± 1.9 | |
| WITH | Vehicle | 32.9 ± 2.5 | 27.6 ± 4.3 |
| 0.3 mg/kg | 33.8 ± 4.0 | 24.2 ± 2.5 | |
| 1.0 mg/kg | 31.6 ± 3.1 | 21.1 ± 2.1 | |
| 3.0 mg/kg | 33.7 ± 3.7 | 21.2 ± 1.4 | |
| 1-hr retention interval | |||
| WITHOUT | Vehicle | 24.5 ± 1.4 | 19.5 ± 2.1 |
| 0.3 mg/kg | 24.2 ± 2.1 | 19.7 ± 2.0 | |
| 1.0 mg/kg | 25.1 ± 2.7 | 22.4 ± 2.4 | |
| 3.0 mg/kg | 24.6 ± 1.7 | 22.8 ± 1.9 | |
| WITH | Vehicle | 33.7 ± 3.3 | 27.5 ± 4.4 |
| 0.3 mg/kg | 36.1 ± 2.6 | 22.9 ± 1.4 | |
| 1.0 mg/kg | 35.2 ± 2.9 | 28.7 ± 2.5 | |
| 3.0 mg/kg | 32.1 ± 1.8 | 21.7 ± 2.3 |
Total time spent exploring the two objects (two identical objects for the training trial, and a familiar and a novel object for the test trial), expressed as mean ± SEM in seconds. The statistical analysis is described in Results (n = 10-14 per group).
Table 2. Exploration behavior of rats in the WITHOUT-habituation and WITH-habituation condition on the training trial.
| Prior habituation condition | No. of crossings | No. of rearings | Total object exploration time |
|---|---|---|---|
| WITHOUT | 23.1 ± 1.0 | 15.6 ± 0.7 | 24.3 ± 1.0 |
| WITH | 17.4 ± 1.1* | 7.9 ± 0.8* | 32.9 ± 1.7* |
The number of crossings, the number of rearings, and the total time spent exploring the two objects on the training trial of all groups in the WITHOUT-habituation and WITH-habituation condition. Results are expressed as mean ± SEM in seconds. *, Significantly different compared with rats in the WITHOUT-habituation group. See Results for statistical analysis.
Examination of rats' exploratory behavior of the training apparatus during the training trial indicated that rats in the WITHOUT-habituation condition spent more time exploring the experimental apparatus than rats in the WITH-habituation condition (Table 2). The number of crossings and rearings were significantly higher in rats in the WITHOUT-habituation condition than in rats in the WITH-habituation condition (t93 = –3.90, P < 0.0002 for crossings; t93 = –7.53, P < 0.0001 for rearings).
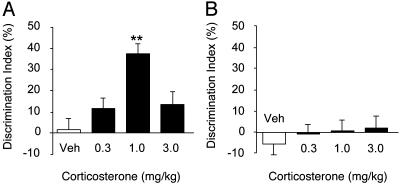
Retention trial. One-sample t tests revealed no preference for the novel object in vehicle-treated rats in both the WITHOUT-habituation (t12 = 0.29, P = 0.78) and the WITH-habituation condition (t10 = –0.95, P = 0.36). These findings indicate that rats of both vehicle groups did not express retention of the familiar object. As shown in Fig. 1, corticosterone dose-dependently enhanced retention performance of rats in the WITHOUT-habituation condition (F3,44 = 9.26, P < 0.0001; Fig. 1 A) but failed to affect retention of rats in the WITH-habituation condition (F3,43 = 0.37, P = 0.77; Fig. 1B). Post hoc analysis of the WITHOUT-habituation condition revealed that the 1.0 mg/kg dose of corticosterone, but not lower or higher doses, significantly increased the discrimination index as compared with that of vehicle-treated rats (P < 0.0001), indicating a stronger preference for the novel object. Also, one-sample t tests indicated that rats treated with the 0.3 and 1.0 mg/kg doses of corticosterone exhibited a significant exploration preference for the novel object (t10 = 2.34, P = 0.041; t11 = 9.19, P < 0.0001, respectively). Moderate, but not significant, exploration preference for the novel object was observed in rats in the WITHOUT-habituation condition treated with the 3.0 mg/kg dose (t11 = 2.18; P = 0.052). In contrast, one-sample t tests of the WITH-habituation condition indicated that corticosterone did not increase the preference for the novel object (P ≥ 0.72).
Fig. 1.
Posttraining administration of corticosterone enhanced 24-hr object recognition performance of rats in the WITHOUT-habituation (A) but not the WITH-habituation (B) condition. Rats received a single injection of corticosterone or vehicle immediately after the 3-min training trial. Corticosterone administered in a dose of 1.0 mg/kg significantly enhanced 24-hr object recognition memory of rats in the WITHOUT-habituation condition but failed to affect memory of rats in the WITH-habituation condition. **, P < 0.0001 compared with the corresponding vehicle control group (n = 11–13 per group).
A two-way ANOVA for total exploration time of the two objects during the retention trial revealed no habituation condition effect (F1,87 = 2.04, P = 0.16), no drug treatment effect (F3,87 = 0.25, P = 0.86), and no interaction between both factors (F3,87 = 1.89, P = 0.14; Table 1). These findings indicate that neither the experimental condition nor the posttraining administration of corticosterone influenced the total amount of time exploring the two objects on the retention test trial.
Posttraining Corticosterone Impaired Object Recognition Memory at a 1-hr Retention Test of Rats in the WITHOUT-Habituation but Not WITH-Habituation Condition. This experiment examined whether immediate posttraining injections of corticosterone impaired short-term performance on an object recognition task and whether such glucocorticoid-induced impairment depends on emotional arousal.
Training trial. Table 1 shows the total time spent exploring the two objects on the training trial for rats in the two habituation conditions. The pattern of effects was highly comparable with that observed in the first experiment. Two-way ANOVA revealed a significant habituation effect (F1,94 = 32.24, P < 0.0001), but no differences between posttraining drug groups (F3,94 = 0.26, P = 0.85) or an interaction between habituation condition and later drug treatment (F3,94 = 0.29, P = 0.83). Furthermore, all groups exhibited comparable amounts of time exploring each of the two identical objects on the training trial (P ≥ 0.31). There were also no differences in the discrimination index among groups on the training trial before the drug treatment (F3,50 = 0.13, P = 0.94 for WITHOUT-habituation condition; F3,44 = 1.04, P = 0.38 for WITH-habituation condition). Also, as was found in the first experiment, rats in the WITHOUT-habituation group showed significantly more exploration of the experimental apparatus during the training trial than did rats in the WITH-habituation group (t100 = –5.01, P < 0.0001 for crossings; t100 = –10.30, P < 0.0001 for rearings; data not shown).
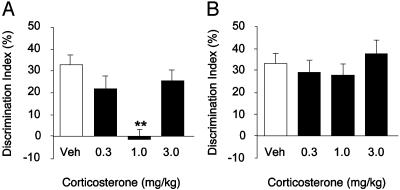
Retention trial. In contrast to the findings after a 24-hr retention interval, the discrimination index of vehicle-treated animals was significantly different from zero under both the WITHOUT-habituation (t12 = 6.91, P < 0.0001) and WITH-habituation condition (t12 = 7.00, P < 0.0001), indicating that rats in both experimental conditions readily discriminated the novel object at the 1-hr retention test. Furthermore, the discrimination index of vehicle-treated rats in the two habituation conditions did not differ from each other (t24 = –0.13, P = 0.90). As shown in Fig. 2, corticosterone treatment immediately after training dose-dependently impaired retention performance of rats in the WITHOUT-habituation condition, but not of rats in the WITH-habituation condition. Fig. 2 A shows the effect of corticosterone (0.3, 1.0, or 3.0 mg/kg, s.c.) of rats in the WITHOUT-habituation condition. One-way ANOVA for discrimination index indicated that corticosterone impaired retention performance (F3,44 = 8.66, P = 0.0001). Post hoc analysis revealed that the 1.0 mg/kg dose of corticosterone, but not 0.3 or 3.0 mg/kg, significantly decreased the discrimination index compared with vehicle (P < 0.0001). One-sample t tests indicated that only rats treated with the 1.0 mg/kg dose of corticosterone did not show a significant exploration preference for the novel object (t11 = –2.53, P = 0.80). Fig. 2B shows the corticosterone effect of rats in the WITH-habituation condition. In this condition, corticosterone failed to impair object recognition memory (F3,50 = 0.65, P = 0.59), and all groups of rats showed a similar strong preference for the novel object.
Fig. 2.
Posttraining administration of corticosterone impaired 1-hr object recognition performance of rats in the WITHOUT-habituation (A) but not the WITH-habituation condition (B). Rats received a single injection of corticosterone (0.3, 1.0, or 3.0 mg/kg, s.c.) or vehicle immediately after the 3-min training trial. Corticosterone administered in a dose of 1.0 mg/kg significantly impaired 1-hr object recognition memory of rats in the WITHOUT-habituation condition but failed to affect memory of rats in the WITH-habituation condition. **, P < 0.0001 compared with the corresponding vehicle control group (n = 10–14 per group).
A two-way ANOVA for total exploration time of the two objects during the retention trial revealed a habituation condition effect (F1,94 = 7.31, P = 0.008), a drug treatment effect (F3,94 = 2.82, P = 0.04), but no interaction between both factors (F3,94 = 0.20, P = 0.90; Table 1).
Corticosterone Levels of Rats in the WITHOUT-Habituation and WITH-Habituation Condition. Table 3 shows plasma corticosterone levels of parallel groups of trained rats in the WITHOUT-habituation and WITH-habituation condition, as assessed 30 min after the training trial and corticosterone injection. Plasma corticosterone levels of rats in the WITHOUT-habituation condition treated with vehicle were slightly higher, but not significantly different, compared with those of vehicle-injected rats in the WITH-habituation condition (P = 0.22). Corticosterone injection induced dose-dependent increases in plasma corticosterone levels of rats in both habituation conditions. Two-way ANOVA showed a significant corticosterone administration effect (F3,56 = 107.29, P < 0.0001), but no habituation condition effect (F1,56 = 2.29, P = 0.14) or an interaction between both factors (F3,56 = 0.05, P = 0.98). Post hoc analyses revealed that injection of 1.0 and 3.0 mg/kg, but not 0.3 mg/kg, of corticosterone significantly elevated plasma corticosterone levels of rats in both habituation conditions.
Table 3. Plasma corticosterone levels of rats in the WITHOUT-habituation and WITH-habituation condition.
| WITHOUT-habituation | WITH-habituation | |
|---|---|---|
| Vehicle | 10.0 ± 3.2 | 5.8 ± 0.9 |
| 0.3 mg/kg | 17.2 ± 1.5 | 14.2 ± 2.1 |
| 1.0 mg/kg | 35.5 ± 1.2** | 33.5 ± 2.8** |
| 3.0 mg/kg | 60.3 ± 3.9** | 56.4 ± 5.8** |
Plasma corticosterone levels (mean ± SEM) in μg/dl as assessed 30 min after the training trial and drug injection. **, P < 0.0001 compared with corresponding vehicle control group (n = 8 per group).
Discussion
These experiments examined the effects of posttraining corticosterone administration on object recognition memory in rats that either had received no previous habituation to the experimental context or who had reduced novelty stress/arousal because of extensive prior habituation. Corticosterone enhanced 24-hr retention performance of rats that were not previously habituated to the experimental context but failed to enhance memory of rats that were well habituated before the training. Furthermore, corticosterone selectively impaired short-term (1 hr) memory performance in rats that were not previously habituated to the experimental context. The findings support the hypothesis that glucocorticoids have opposing effects on memory consolidation and memory retrieval (6) and that the effects of posttraining glucocorticoid administration on memory consolidation and short-term memory retrieval depend on the level of emotional arousal associated with initial encoding. These findings are consistent with those of several studies of human subjects indicating that glucocorticoids (and other stress-related compounds) interact with the degree of emotional arousal at initial encoding to modulate memory processes (9, 12, 17, 18, 25). Considerable evidence indicates that emotionally arousing stimuli activate noradrenergic mechanisms in the amygdala, and that this noradrenergic activation is critically involved in modulating memory processes (5, 26–28). The selective influence of glucocorticoids in modulating memory for emotionally arousing information observed in this study fits well with extensive evidence that glucocorticoid effects on memory processes require noradrenergic activation in the amygdala (3, 6).
Most previous studies examining object recognition in mice or rats have investigated the effects of brain lesions, pretraining drug treatments, or genetic manipulations (29–32). Such treatments can affect retention performance by influencing attentional, motivational, motor, or sensory-perceptual mechanisms at training or retention testing. The use of posttraining drug administration obviously avoids such influences. The present findings that corticosterone administered immediately after training influences the consolidation of object recognition memory are consistent with previous evidence that glucocorticoids produce dose-dependent enhancement of memory consolidation on a wide variety of emotionally arousing learning tasks, including discrimination learning (33), inhibitory avoidance (34–36), water-maze spatial training (37), and contextual and auditory fear conditioning (38–40). Corticosterone can bind to two subtypes of adrenal steroid receptors that differ in their affinity for corticosterone: the low-affinity glucocorticoid receptors that become activated during high levels of circulating glucocorticoids and the high-affinity mineralocorticoid receptors that are almost saturated during basal levels of corticosterone (41). The present findings that stress-level glucocorticoid administration enhanced memory consolidation fits well with extensive evidence indicating that the effects of corticosterone on memory consolidation are selectively mediated by an activation of glucocorticoid receptors (8, 42–45).
Corticosterone administration did not enhance memory consolidation of rats given prior habituation to the experimental context. Because vehicle-control rats in both habituation conditions showed a similar strong preference for the novel object at a 1-hr retention interval, and, additionally, expressed no evidence of memory of the training trial at a 24-hr interval, it is unlikely that a difference in acquisition (i.e., total exploration of the objects) between both groups underlies the selective influence of corticosterone on memory in nonhabituated rats. Moreover, habituated rats actually showed significantly more exploration of the objects during the training trial than did nonhabituated rats. It seems more likely that the difference between the habituated and nonhabituated animals in the level of emotional arousal during the training trial was critical. The findings that nonhabituated rats expressed significantly higher levels of locomotion and rearing behavior during the training trial than habituated rats is consistent with previous evidence that, in rats, exposure to novel contexts induces changes in behavioral responses, including hyperlocomotion and increased rearing behavior (46, 47). Novelty-induced arousal also activates stress hormone systems, including glucocorticoids (48, 49) and epinephrine (50–52). In the present study, levels of corticosterone, assessed 30 min after the training trial, were slightly, but nonsignificantly, elevated in vehicle-injected rats in the nonhabituated group. Because the effects of novelty stress on plasma corticosterone are short lasting, usually peaking after 15 min and declining within 45 min after stress exposure (20), corticosterone levels assessed at 30 min after object recognition training probably reflected a decline from peak levels.
The finding that posttraining administration of corticosterone impaired 1-hr retention performance of nonhabituated rats is consistent with previous evidence indicating that restraint stress or exposure of rats to a predator odor after object recognition training elevates plasma corticosterone levels and disrupts shortterm retention performance (22, 23). Importantly, the subjects used in the study by Morrow et al. (22) were unhandled and not habituated to the experimental context; thus, the degree of arousal of those rats may have been comparable to that of nonhabituated rats in the present study. Moreover, the present findings are in accord with those of previous studies reporting that exposure of rats to stress or glucocorticoid injection impairs short-term memory on radial-arm and water-maze spatial tasks (53–55) and that, in human subjects, stress and/or glucocorticoid exposure impairs short-term retention on declarative learning tasks (15). These results are also similar to findings indicating that glucocorticoids administered to rats or human subjects shortly before retention testing impair retrieval of long-term memory (11, 12) and provide additional evidence suggesting that elevated glucocorticoid levels directly influence retention performance (6). Our finding that corticosterone administration selectively impaired short-term memory performance of nonhabituated rats suggests that glucocorticoids may also interact with emotional arousal at encoding in influencing this memory process (56). Alternatively, it is possible that the nonhabituated rats maintained increased levels of emotional arousal during retention testing. This result seems unlikely in view of our finding that in nonhabituated rats the corticosterone levels assessed 30 min after training were not significantly elevated. Interestingly, the same dose of corticosterone that impaired short-term memory performance enhanced long-term memory performance. Therefore, it is possible that such a temporary impairment of short-term memory retrieval induced by the posttraining corticosterone administration may be linked and perhaps be critical for inducing enhancement of long-term memory consolidation (6). For example, it is possible that corticosterone-induced short-term memory impairment may inhibit retroactive interference, resulting in enhanced and more accurate memory consolidation.
Zhu et al. (57, 58) investigated c-Fos expression in brain areas of rats that were trained on an object recognition task under conditions that were comparable to our WITHOUT-habituation and WITH-habituation conditions and found that exposure to novel objects induced activation of perirhinal cortical neurons whereas a novel environment activated hippocampal neurons. Several findings of electrophysiological and behavioral studies implicate the perirhinal cortex and hippocampus in object recognition memory (59–61). Such results may suggest that the degree of emotional arousal may determine which brain regions are activated during training and that the perirhinal cortex and hippocampus may be involved, respectively, in memory formed under WITH-habituation and WITHOUT-habituation conditions. Such an involvement of the hippocampus in object recognition memory formed under emotionally arousing conditions fits well with the evidence that the hippocampus expresses a high density of glucocorticoid receptors (41, 62) and is a major target structure in regulating glucocorticoid effects on memory (14, 63–65).
Glucocorticoid effects on hippocampal neuroplasticity and memory depend on an intact amygdala (64, 66–73). Lesions or pharmacological inactivation of the basolateral amygdala block the enhancing effects of glucocorticoids administered either systemically or directly into the hippocampus on memory consolidation as well as glucocorticoid-induced impairment of memory retrieval (14, 35, 64, 71). It should be noted, however, that the amygdala interacts with many other brain regions as well in regulating emotional arousal effects on memory functions (74). Because extensive evidence indicates that the amygdala is activated during emotionally arousing experiences (75–77), training-induced amygdala activation may be a critical link between emotional arousal and memory processes (78, 79). Furthermore, considerable evidence indicates that emotionally arousing experiences induce norepinephrine release in the basolateral amygdala (26–28) and that a blockade of β-adrenoceptors within the basolateral amygdala prevents glucocorticoid effects on both memory consolidation and memory retrieval (refs. 68, 80, and 81; and B.R., E. Hahn, S. V. Nathan, D. J.-F. de Quervain, and J.L.M., unpublished observation). Such findings suggest that glucocorticoid effects on memory processes may depend on emotional arousal because of critical interactions of these hormones with training-induced noradrenergic activation of the amygdala.
In summary, the results reported here add to the evidence that adrenal stress hormones influence memory consolidation and short-term memory retrieval in various animal and human memory tasks. The present findings strongly suggest that glucocorticoids modulate memory only for information acquired under emotionally arousing conditions. Such evidence supports the view that endogenously released stress hormones normally play a role in modulating the consolidation of memory for emotionally arousing experiences that induce their release (3, 5).
Acknowledgments
We thank Angélica Córdova for assistance with the behavioral analysis. Research was supported by U.S. Public Health Service Grant MH12526 (to J.L.M.).
References
- 1.Lupien, S. J. & McEwen, B. S. (1997) Brain Res. Rev. 24, 1–27. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet, E. R., Oitzl, M. S. & Joëls, M. (1999) Trends Neurosci. 22, 422–426. [DOI] [PubMed] [Google Scholar]
- 3.Roozendaal, B. (2000) Psychoneuroendocrinology 25, 213–238. [DOI] [PubMed] [Google Scholar]
- 4.Kim, J. J. & Diamond, D. M. (2002) Nat. Rev. Neurosci. 3, 453–462. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh, J. L. & Roozendaal, B. (2002) Curr. Opin. Neurobiol. 12, 205–210. [DOI] [PubMed] [Google Scholar]
- 6.Roozendaal, B. (2002) Neurobiol. Learn. Mem. 78, 578–595. [DOI] [PubMed] [Google Scholar]
- 7.Wolf, O. T. (2003) Best Pract. Res. Clin. Endocrinol. Metab. 17, 287–299. [DOI] [PubMed] [Google Scholar]
- 8.Sandi, C. & Rose, S. P. R. (1994) Brain Res. 647, 106–112. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan, T. W. & Lovallo, W. R. (2001) Psychoneuroendocrinology 26, 307–317. [DOI] [PubMed] [Google Scholar]
- 10.Abercrombie, H. C., Kalin, N. H., Thurow, M. E., Rosenkranz, M. A. & Davidson, R. J. (2003) Behav. Neurosci. 117, 505–516. [DOI] [PubMed] [Google Scholar]
- 11.de Quervain, D. J.-F., Roozendaal, B. & McGaugh, J. L. (1998) Nature 394, 787–790. [DOI] [PubMed] [Google Scholar]
- 12.de Quervain, D. J.-F., Roozendaal, B., Nitsch, R. M., McGaugh, J. L. & Hock, C. (2000) Nat. Neurosci. 3, 313–314. [DOI] [PubMed] [Google Scholar]
- 13.Wolf, O. T., Convit, A., McHugh, P. F., Kandil, E., Thorn, E. L., de Santi, S., McEwen B. S. & de Leon, M. J. (2001) Behav. Neurosci. 115, 1002–1011. [DOI] [PubMed] [Google Scholar]
- 14.Roozendaal, B., Griffith, Q. K., Buranday, J., de Quervain, D. J.-F. & McGaugh, J. L. (2003) Proc. Natl. Acad. Sci. USA 100, 1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirschbaum, C., Wolf, O. T., May, M., Wippich, W. & Hellhammer, D. H. (1996) Life Sci. 58, 1475–1483. [DOI] [PubMed] [Google Scholar]
- 16.Bats, S., Thoumas, J. L., Lordi, B., Tonon, M. C., Lalonde, R. & Caston, J. (2001) Behav. Brain Res. 118, 11–15. [DOI] [PubMed] [Google Scholar]
- 17.Cahill, L. & Alkire, M. T. (2003) Neurobiol. Learn. Mem. 79, 194–198. [DOI] [PubMed] [Google Scholar]
- 18.Cahill, L., Gorski, L. & Le, K. (2003) Learn. Mem. 10, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ennaceur, A. & Delacour, J. (1988) Behav. Brain Res. 31, 47–59. [DOI] [PubMed] [Google Scholar]
- 20.de Boer, S. F., Koopmans, S. J., Slangen, J. L. & van der Gugten, J. (1990) Physiol. Behav. 47, 1117–1124. [DOI] [PubMed] [Google Scholar]
- 21.Cerbone, A. & Sadile, A. G. (1994) Neurosci. Biobehav. Rev. 18, 497–518. [DOI] [PubMed] [Google Scholar]
- 22.Morrow, B. A., Roth, R. H. & Elsworth, J. D. (2000) Brain Res. Bull. 52, 519–523. [DOI] [PubMed] [Google Scholar]
- 23.Baker, K. B. & Kim, J. J. (2002) Learn. Mem. 9, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mostafa, R. M., Mostafa, Y. M. & Ennaceur, A. (2002) Phsiol. Behav. 76, 589–595. [DOI] [PubMed] [Google Scholar]
- 25.Messier, C., Pierre, J., Desrochers, A. & Gravel, M. (1998) Cogn. Brain Res. 7, 221–233. [DOI] [PubMed] [Google Scholar]
- 26.Galvez, R., Mesches, M. H. & McGaugh, J. L. (1996) Neurobiol. Learn. Mem. 66, 253–257. [DOI] [PubMed] [Google Scholar]
- 27.Quirarte, G. L., Galvez, R., Roozendaal, B. & McGaugh, J. L. (1998) Brain Res. 808, 134–140. [DOI] [PubMed] [Google Scholar]
- 28.McIntyre, C. K., Hatfield, T. & McGaugh, J. L. (2002) Eur. J. Neurosci. 16, 1223–1226. [DOI] [PubMed] [Google Scholar]
- 29.Lebrun, C., Pillière, E. & Lestage, P. (2000) Eur. J. Pharmacol. 401, 205–212. [DOI] [PubMed] [Google Scholar]
- 30.Mumby, D. G., Gaskin, S., Glenn, M. J., Schramek, T. E. & Lehmann, H. (2002) Learn. Mem. 9, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obinu, M. C., Reibaud, M., Miquet, J. M., Pasquet, M. & Rooney, T. (2002) Prog. Neuropsychopharmacol. Biol. Psychiatry 26, 913–918. [DOI] [PubMed] [Google Scholar]
- 32.Bourtchouladze, R., Lidge, R., Catapano, R., Stanley, J., Gossweiler, S., Romashko, D., Scott, R. & Tully, T. (2003) Proc. Natl. Acad. Sci. USA 100, 10518–10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flood, J. F., Vidal, D., Bennett, E. L., Orme, A. E., Vasquez, S. & Jarvik, M. E. (1978) Pharmacol. Biochem. Behav. 8, 81–87. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs, G. L., Telegdy, G. & Lissak, K. (1977) Horm. Behav. 8, 155–165. [DOI] [PubMed] [Google Scholar]
- 35.Roozendaal, B. & McGaugh, J. L. (1996) Neurobiol. Learn. Mem. 65, 1–8. [DOI] [PubMed] [Google Scholar]
- 36.Roozendaal, B., Williams, C. L. & McGaugh, J. L. (1999) Eur. J. Neurosci. 11, 1317–1323. [DOI] [PubMed] [Google Scholar]
- 37.Sandi, C., Loscertales, M. & Guaza, C. (1997) Eur. J. Neurosci. 9, 637–642. [DOI] [PubMed] [Google Scholar]
- 38.Pugh, C. R., Tremblay, D., Fleshner, M. & Rudy, J. W. (1997) Behav. Neurosci. 111, 503–511. [PubMed] [Google Scholar]
- 39.Cordero, M. I. & Sandi, C. (1998) Brain Res. 786, 11–17. [DOI] [PubMed] [Google Scholar]
- 40.Hui, G. K., Figueroa, I. R., Poytress, B. S., Roozendaal, B., McGaugh, J. L. & Weinberger, N. M. (2004) Neurobiol. Learn. Mem. 81, 67–74. [DOI] [PubMed] [Google Scholar]
- 41.Reul, J. M. H. M. & de Kloet, E. R. (1985) Endocrinology 117, 2505–2511. [DOI] [PubMed] [Google Scholar]
- 42.Oitzl, M. S. & de Kloet, E. R. (1992) Behav. Neurosci. 106, 62–71. [DOI] [PubMed] [Google Scholar]
- 43.Roozendaal, B., Portillo-Marquez, G. & McGaugh, J. L. (1996) Behav. Neurosci. 110, 1074–1083. [DOI] [PubMed] [Google Scholar]
- 44.Conrad, C. D., Lupien, S. J. & McEwen, B. S. (1999) Neurobiol. Learn. Mem. 72, 39–46. [DOI] [PubMed] [Google Scholar]
- 45.Oitzl, M. S., Reichardt, H. M., Joëls, M. & de Kloet, E. R. (2001) Proc. Natl. Acad. Sci. USA 98, 12790–12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borella, A., Sumangali, R., Ko, J. & Whitaker-Azmitia, P. M. (2003) Behav. Brain Res. 141, 229–236. [DOI] [PubMed] [Google Scholar]
- 47.van den Buuse, M., van Acker. S. A. B. E., Fluttert, M. F. J. & de Kloet, E. R. (2002) Pysiol. Behav. 75, 207–215. [DOI] [PubMed] [Google Scholar]
- 48.Emmert, M. H. & Herman, J. P. (1999) Brain Res. 845, 60–67. [DOI] [PubMed] [Google Scholar]
- 49.Handa, R. J., Nunley, K. M., Lorens, S. A., Louie, J. P., McGivern, R. F. & Bollnow, M. R. (1994) Physiol. Behav. 55, 117–124. [DOI] [PubMed] [Google Scholar]
- 50.McQuade, R., Creton, D. & Stanford, S. C. (1999) Psychopharmacology 145, 393–400. [DOI] [PubMed] [Google Scholar]
- 51.Feenstra, M. G. P., Botterblom, M. H. A. & Mastenbroek, S. (2000) Neuroscience 100, 741–748. [DOI] [PubMed] [Google Scholar]
- 52.Ihalainen, J. A., Riekkinen, P., Jr., & Feenstra, M. G. P. (1999) Neurosci. Lett. 277, 71–74. [DOI] [PubMed] [Google Scholar]
- 53.Diamond, D. M., Fleshner, M., Ingersoll, N. & Rose, G. M. (1996) Behav. Neurosci. 110, 661–672. [DOI] [PubMed] [Google Scholar]
- 54.Diamond, D. M., Park, C. R., Heman, K. L. & Rose, G. M. (1999) Hippocampus 9, 542–552. [DOI] [PubMed] [Google Scholar]
- 55.Woodson, J. C., Macintosh, D., Fleshner, M. & Diamond, D. M. (2003) Learn. Mem. 10, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Honk, J., Kessels, R. P. C., Putman, P., Jager, G., Koppeschaar, H. P. F. & Postma, A. (2003) Psychoneuroendocrinology 28, 941–948. [DOI] [PubMed] [Google Scholar]
- 57.Zhu, X. O., Brown, M. W., McCabe, B. J. & Aggleton, J. P. (1995) Neuroscience 69, 821–829. [DOI] [PubMed] [Google Scholar]
- 58.Zhu, X. O., McCabe, B. J., Aggleton, J. P. & Brown, M. W. (1997) Neurosci. Lett. 229, 141–143. [DOI] [PubMed] [Google Scholar]
- 59.Zhu, X. O., Brown, M. W. & Aggleton, J. P. (1995) Eur. J. Neurosci. 7, 753–765. [DOI] [PubMed] [Google Scholar]
- 60.Ennaceur A., Neave, N. & Aggleton, J. P. (1996) Behav. Brain Res. 8, 9–25. [DOI] [PubMed] [Google Scholar]
- 61.Clark, R. E., Zola, S. M. & Squire, L. R. (2000) J. Neurosci. 20, 8853–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McEwen, B. S., Weiss, J. M. & Schwartz, L. S. (1969) Brain Res. 16, 227–241. [DOI] [PubMed] [Google Scholar]
- 63.McEwen, B. S. & Sapolsky, R. M. (1995) Curr. Opin. Neurobiol. 5, 205–216. [DOI] [PubMed] [Google Scholar]
- 64.Roozendaal, B. & McGaugh, J. L. (1997) Eur. J. Neurosci. 9, 76–83. [DOI] [PubMed] [Google Scholar]
- 65.Lupien, S. J. & Lepage, M. (2001) Behav. Brain Res. 127, 137–158. [DOI] [PubMed] [Google Scholar]
- 66.Ikegaya, Y., Saito, H. & Abe, K. (1994) Brain Res. 656, 157–164. [DOI] [PubMed] [Google Scholar]
- 67.Ikegaya, Y., Saito, H. & Abe, K. (1995) Brain Res. 671, 351–354. [DOI] [PubMed] [Google Scholar]
- 68.Roozendaal, B., Nguyen, B. T., Power, A. E. & McGaugh, J. L. (1999) Proc. Natl. Acad. Sci. USA 96, 11642–11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akirav, I. & Richter-Levin, G. (1999) J. Neurosci. 19, 10530–10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akirav, I. & Richter-Levin, G. (2002) J. Neurosci. 22, 9912–9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim, J. J., Lee. H. J., Han, J. S. & Packard, M. G. (2001) J. Neurosci. 21, 5222–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frey, S., Bergado-Rosado, J., Seidenbecher, T., Pape, H. C. & Frey, J. U. (2001) J. Neurosci. 21, 3697–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Almaguer-Melian, W., Martinez-Marti, L., Frey, J. U. & Bergado, J. A. (2003) Neuroscience 119, 319–322. [DOI] [PubMed] [Google Scholar]
- 74.McGaugh, J. L. (2000) Science 287, 248–251. [DOI] [PubMed] [Google Scholar]
- 75.Campeau, S., Hayward, M. D., Hope, B. T., Rosen, J. B., Nestler, E. J. & Davis, M. (1991) Brain Res. 565, 349–352. [DOI] [PubMed] [Google Scholar]
- 76.Adolphs, R & Tranel, D. (2000) in The Amygdala, ed. Aggleton, J. P. (Oxford Univ. Press, Oxford), pp. 587–630.
- 77.Dolan, R. J. (2000) in The Amygdala, ed. Aggleton, J. P. (Oxford Univ. Press, Oxford), pp. 631–653.
- 78.Cahill, L., Haier, R. J., Fallon, J., Alkire, M. T., Tang, C., Keator, D., Wu, J. & McGaugh, J. L. (1996) Proc. Natl. Acad. Sci. USA 93, 8016–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamann, S. B., Ely, T. D., Grafton, S. T. & Kilts, C. D. (1999) Nat. Neurosci. 2, 289–293. [DOI] [PubMed] [Google Scholar]
- 80.Quirarte, G. L., Roozendaal, B. & McGaugh, J. L. (1997) Proc. Natl. Acad. Sci. USA 94, 14048–14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roozendaal, B., Quirarte, G. L. & McGaugh, J. L. (2002) Eur. J. Neurosci. 15, 553–560. [DOI] [PubMed] [Google Scholar]