Abstract
Transdermal administration of drugs is generally limited by the barrier function of the skin. Vesicular systems are one of the most controversial methods for transdermal delivery of active substances. The interest in designing transdermal delivery systems was relaunched after the discovery of elastic vesicles like transferosomes, ethosomes, cubosomes, phytosomes, etc. This paper presents the composition, mechanisms of penetration, manufacturing and characterization methods of transferosomes as transdermal delivery systems of active substances. For a drug to be absorbed and distributed into organs and tissues and eliminated from the body, it must pass through one or more biological membranes/barriers at various locations. Such a movement of drug across the membrane is called as drug transport. For the drugs to be delivered to the body, they should cross the membranous barrier. The concept of these delivery systems was designed in an attempt to concentrate the drug in the tissues of interest, while reducing the amount of drug in the remaining tissues. Hence, surrounding tissues are not affected by the drug. In addition, loss of drug does not happen due to localization of drug, leading to get maximum efficacy of the medication. Therefore, the phospholipid based carrier systems are of considerable interest in this era.
Keywords: Lecithin, stratum corneum, surfactant, transferosomes, vesicles
INTRODUCTION
Liposomes and niosomes are the vesicular carrier systems which have received a lot of attention over the last decades as a means of transdermal drug delivery. Researchers have understood the properties of vesicles structures for use in better drug delivery within their cavities, which would to tag the vesicles for cell specificity. The reason for using vesicles in transdermal drug delivery is based on the fact that they act as drug carriers to deliver entrapped drug molecules across the skin, as well as penetration enhancers because of their composition.[1] In addition, these vesicles serve as a depot for the sustained release of active compounds in the case of topical formulations, as well as rate limiting membrane barrier for the modulation of systemic absorption in the case of transdermal formulations. The vesicles have been well known for their importance in cellular communication and particle transportation for many years.
Liposomal formulations can be classified into two categories: Rigid vesicles liposomes and niosomes and elastic or ultra deformable vesicles-transferosomes and ethosomes.[2]
Disadvantages of liposomes and niosomes are the following:
They are not suitable for transdermal delivery because they cannot reach the deeper layers of the skin as they are trapped in the superior layers of stratum corneum.
Though vesicular systems assure targeted delivery, in most cases the liposomal or niosomal category vesicles do not achieve the desired transdermal penetration.[3–6]
A new vesicular derivative, the “transferosomes”, has paved the way to minimize the defective transdermal permeation of a number of low and high molecular weight drugs,[7] which has been found to be one of the major advancement in vesicle research.
Transferosomes are a special type of liposomes, consisting of phosphatidylcholine and an edge activator. They are soft malleable vesicles tailored for enhanced delivery of active agents.[8] They are registered by a German company IDEA AG and used by it to refer to its proprietary drug delivery technology. The name means ‘carrying body’ and is derived from the Latin word ‘transfere’ meaning ‘to carry across’ and the Greek word ‘soma’ for a ‘body’.
Figure 1 describes the structure of a transferosome. A transferosome carrier is an artificial vesicle designed to be like a cell vesicle or a cell engaged in exocytosis and thus suitable for controlled and potentially targeted drug delivery. Transferosome is a highly adaptable, stress responsive, complex aggregate. Its preferred form is an ultra-deformable vesicle possessing an aqueous core surrounded by the complex lipid bilayer. Interdependency of local composition and shape of the bilayer makes the vesicle both self regulating and self optimizing. This enables the transferosomes to cross various transport barriers efficiently and then act as a drug carrier for non-invasive targeted drug delivery and sustained release of therapeutic agents. One of the most controversial methods for drug transport across the skin is the use of vesicle formulation as skin delivery systems.
Figure 1.
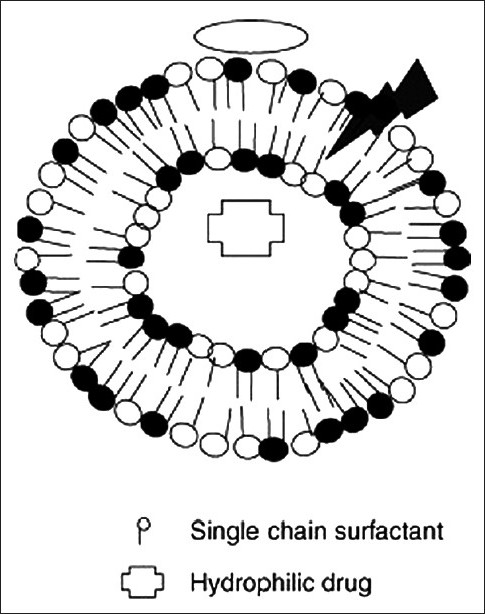
Ultra deformable vesicle
USES AND ADVANTAGES OF TRANSFEROSOMES
Transferosomes possess an infrastructure consisting of hydrophobic and hydrophilic moieties together and as a result can accommodate drug molecules with a wide range of solubility. Transferosomes can deform and pass through narrow constriction (from 5 to 10 times less than their own diameter) without measurable loss.[9,10] This high deformability gives better penetration of intact vesicles.
Transferosomes are self aggregates, with an ultra flexible membrane which delivers the drug reproducibly into or through the skin. These vesicular vesicles are several orders of magnitude more elastic than the standard liposomes. Transferosomes overcome the skin penetration difficulty by squeezing themselves along the intracellular sealing lipids of the stratum corneum.[10] The concept of transferosomes as a carrier for transdermal delivery was first developed by Cevc and coworkers, in 1992.[11] Since then, many investigations have been carried out on transferosomes and their possible application as drug carriers. Delivery of peptides by transferosomes provides a very successful means for the non-invasive therapeutic use of large molecular weight drugs like insulin on the skin. Hafer et al. studied the formulation of interleukin 2 and interferon α containing transferosomes for potential transdermal application.
Transferosomes for potential transdermal application, contain a mixture of lipids and biocompatible membrane softeners. The optimal mixture leads to flexibility of the elastic liposomal membranes and to the possibility of penetration through channels of the skin, which are opened by the carriers. Transferosome is a supramolecular entity that can pass through a permeability barrier and there by transport material from the application to the destination site. These are more elastic than the standard liposomes and therefore are used as a novel carrier for effective transdermal drug delivery.[10,11] They have easily deformable properties which make them easily squeeze out from the stratum corneum and the mechanism for penetration is the generation of ‘osmotic gradient’ due to the evaporation of water while applying the lipid suspension (transferosomes) on the skin surface.[10] Transferosomes penetrate the stratum corneum by either intracellular route or transcellular route.[7,9,10] With the excellent distribution properties of transferosomes, they have been widely used as a carrier for various proteins, anti cancer drugs, anti fungal drugs, analgesics, anaesthetics, corticosteroids, sex hormone, insulin, albumin etc.[5,10,12]
They are biocompatible and biodegradable as they are made from natural phospholipids similar to liposomes. They have high entrapment efficiency, which is nearly 90% in the case of lipophilic drug. They protect the encapsulated drug from metabolic degradation.[12,13] They act as depot, releasing their contents slowly and gradually. They can be used for both systemic as well as topical delivery of drugs. Thus, the complex lipid molecules, transferosomes, can increase the transdermal flux, prolong the release and improve the site specificity of bioactive molecules.
COMPOSITION AND MECHANISM
A transferosome is a self-adaptable and optimized mixed lipid aggregate.
The surfactant molecules act as “edge activators”, conferring ultra deformability on the transferosomes, which reportedly allows them to squeeze through channels in the stratum corneum that are less than one-tenth the diameter of the transferosome. According to their inventors, where liposomes are too large to pass through pores of less than 50 nm in size, transferosomes up to 500 nm can squeeze through to penetrate the stratum corneum barrier spontaneously.[14] They suggest that the driving force for penetration into the skin is the “transdermal gradient” caused by the difference in water content between the relatively dehydrated skin surface (approximately 20% water) and the aqueous viable epidermis (close to 100%).
Deformability of transferosomes is achieved by using surface active agent in the proper ratio. The concentration of surface active agent is crucial in the formulation of transferosomes because at sublytic concentration these agents provide flexibility to vesicle membranes and at higher concentration cause destruction of vesicles.[15] The resulting flexibility of transferosomal membrane minimizes the risk of complete vesicle rupture in the skin and allows the ultra deformable transferosomes to change their membrane composition locally and reversibly, when they are pressed against or attracted into a narrow pore. This dramatically lowers the energetic cost of membrane deformation and permits the resulting highly flexible particles first to enter and then pass through the pores rapidly and efficiently.
The carrier aggregate is composed of at least one amphiphat (such as phosphatidylcholine)[16] which in aqueous solvent self resembles into lipid bilayer that closes into a simple lipid vesicle. By addition of at least one bilayer softening component (such as a biocompatible surfactant or an amphiphilic drug), lipid bilayer flexibility and permeation are greatly increased. Thus, by optimizing the resulting flexibility and permeability, the transferosome vesicles can adapt to their ambient shape easily and rapidly. Thus, they can also adjust the local concentration of each bilayer component to the local stress experienced by the bilayer. The basic organization of these vesicles is broadly similar to liposomes.[17] But the transferosomes differ from the conventional vesicles primarily by their softer, more deformable, and better adjustable artificial membrane.
Another beneficial consequence of strong bilayer deformability is the increased transferosome ability to bind and retain water. An ultradeformable and highly hydrophilic vesicle always seeks to avoid dehydration; this may involve a transport process related to, but not identical with, forward osmosis. For example, a transferosome vesicle applied on an open biological surface, such as non-occluded skin, tends to penetrate its barrier and migrate into the water-rich deeper strata to secure its adequate hydration. Barrier penetration involves reversible bilayer deformation, but must not compromise unacceptably either the vesicle integrity or the barrier properties for the underlying hydration affinity and gradient to remain in place. Since it is too large to diffuse through the skin, the transferosome needs to find and enforce its own route through the organ. The transferosomes usage in drug delivery consequently relies on the carrier's ability to widen and overcome the hydrophilic pores in the skin or some other barrier. The subsequent, gradual agent release from the drug carrier allows the drug molecules to diffuse and finally bind to their target. Drug transport to an intracellular action site may also involve the carrier's lipid bilayer fusion with the cell membrane, unless the vesicle is taken up actively by the cell in the process called endocytosis.[1,7,11]
Mechanism of Penetration of Transferosomes
Transferosomes, when applied under suitable condition, can transfer 0.1 mg of lipid per hour and square centimeter area across the intact skin. This value is substantially higher than that typically driven by the transdermal concentration gradients. The reason for this high flux rate is naturally occurring “transdermal osmotic gradients”, i.e. another much more prominent gradient is available across the skin. This osmotic gradient that is developed due to the skin penetration barrier prevents water loss through the skin and maintains a water activity difference in the viable part of the epidermis (75% water content) and nearly completely dry stratum corneum near to the skin surface (15% water content).[14,17,18] This gradient is very stable because ambient air is a perfect sink for the water molecule even when the transdermal water loss is unphysiologically high. All polar lipids attract some water. This is due to the energetically favorable interaction between the hydrophilic lipid residues and their proximal water. Thus, most lipid bilayers spontaneously resist an induced dehydration. Consequently, all lipid vesicles made from the polar lipid vesicles move from the rather dry location to the sites with a sufficiently high water concentration. So, when lipid suspension (transferosome) is placed on the skin surface that is partly dehydrated by the water evaporation loss, the lipid vesicles feel this “osmotic gradient” and try to escape complete drying by moving along this gradient. They can only achieve this if they are sufficiently deformable to pass through the narrow pores in the skin because transferosomes composed of surfactant have more suitable rheological and hydration properties than that responsible for their greater deformability; less deformable vesicles including standard liposomes are confined to the skin surface, where they dehydrate completely and fuse, so they have less penetration power than the transferosome. Transferosomes are optimized in this respect and thus attain maximum flexibility, so they can take full advantage of the transepidermal osmotic gradient (water concentration gradient). Transferosomes overcome the skin penetration difficulty by squeezing themselves along the intracellular sealing lipids of stratum corneum.[19]
At present, the mechanism of enhancing the delivery of active substances in and across the skin is not very well known. Two mechanisms of action have been proposed:[20]
Transferosomes act as drug vectors, remaining intact after entering the skin.
Transferosomes act as penetration enhancers, disrupting the highly organized intercellular lipids from stratum corneum, and therefore facilitating the drug molecule penetration in and across the stratum corneum.
Cevc and coworkers proposed the first mechanism, suggesting that deformable liposomes penetrate the stratum corneum because of the transdermal hydration gradient normally existing in the skin, and then cross the epidermis, and enter the systemic circulation. The recent studies propose that the penetration and permeation of the vesicles across the skin are due to the combination of the two mechanisms. Depending on the nature of the active substance (lipophilic or hydrophilic) and the composition of the transferosomes, one of the two mechanisms prevails.[21]
METHOD OF PREPARATION
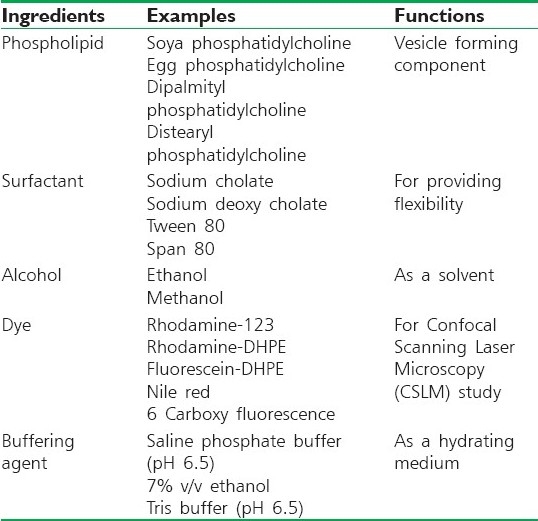
Phospholipids, surfactants and the drug are dissolved in alcohol. The organic solvent is then removed by rotary evaporation[22,23] under reduced pressure at 40°C.[24,25] Final traces of solvent are removed under vacuum. The deposited lipid film is hydrated with the appropriate buffer by rotation at 60 rpm for 1 hour at room temperature. The resulting vesicles are swollen for 2 hours at room temperature. The multilamellar lipid vesicles (MLV) are then sonicated at room temperature to get small vesicles [Table 1].[16,18,26]
Table 1.
Different additives used in the formulation of transferosomes
STABILITY OF TRANSFEROSOMES
Transferosomes are chemically unstable because of their predisposition to oxidative degradation. Purity of natural phospholipids is another criterion militating against adoption of transferosomes as drug delivery vehicles. Transferosome formulations are expensive.
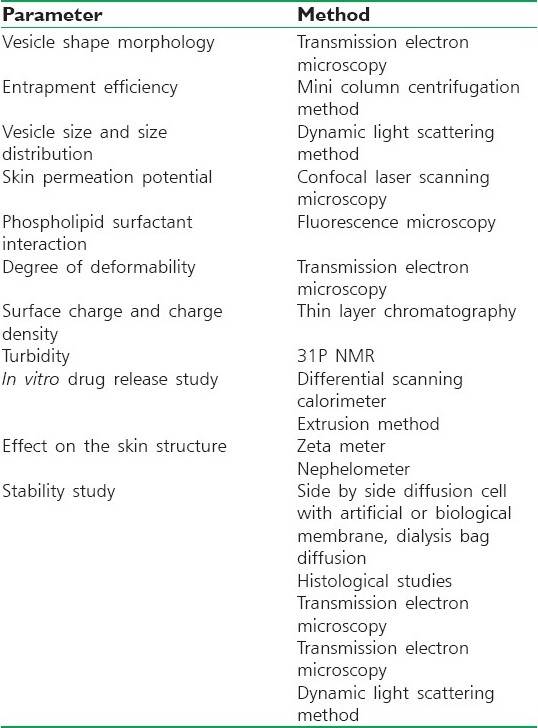
CHARACTERIZATION
Visualization of transferosomes can be performed using transmission electron microscopy (TEM) and scanning electron microscopy (SEM).[23]
Particle size and size distribution can be determined by dynamic light scattering (DLS) and photon correlation spectroscopy (PCS).[23,24]
The drug entrapment efficiency by transferosomes can be measured by the ultracentrifugation technique.
Vesicle stability can be determined by assessing the size and structure of the vesicles over time, and drug content can be quantified by high performance liquid chromatography (HPLC) or Spectrophotometric methods.
In vitro drug release can be measured using a diffusion cell or a dialysis method [Table 2].[27,28]
Propensity of penetration[29]
The magnitude of the transport driving force, of course, also plays an important role:
Table 2.
Methods for the characterization of transferosomes
Flow = Area × (Barrier) Permeability × (Trans-barrier) force.
Therefore, the chemically driven lipid flow across the skin always decreases dramatically when lipid solution is replaced by some amount of lipids in a suspension.
Encapsulation efficiency is expressed as the percent of drug trapped:[27]
![]()
SAFETY CONSIDERATIONS
Phospholipid suspensions comprising liposomes have been reported to be harmless and non-irritating to the skin after repeated epicutaneous administration; they may even have additional advantageous cosmetic effect. The main component of transferosomes is typically soya phosphatidylcholine of greater than 95% purity, which is generally regarded as safe because it has been already in use as an emulsifier in Microemulsion for the parenteral nutrition and is also used in injectable drug information.[28] In light of these data, one can expect the transferosome product to be very safe from the carrier point of view.[28]
CONCLUSION
The use of the transdermal route has been well established in the past, and because of its inherent advantages, new methods for transdermal delivery are continuously being developed. The introduction of ultradeformable vesicles, transferosomes, will thus surely become an important step in relaunching the researches regarding the use of vesicles as transdermal drug delivery systems. In comparison to other transdermal delivery systems, the use of elastic vesicles has certain advantages: They allow enhanced permeation of drug through skin; their composition is safe and the components are approved for pharmaceutical and cosmetic use; they can increase the transdermal flux, prolonging the release and improving the site specificity of bioactive molecules; they can accommodate drug molecules with a wide range of solubility.
Hence, enhanced delivery of bioactive molecules through the skin by means of an ultradeformable vesicular carrier opens new challenges and opportunities for the development of novel improved therapies. Thus, it could be concluded that the new ultra flexible drug carrier (transferosome) can overcome all the problems associated with the transdermal delivery as transferosomes itself are specially optimized vesicles having the capability of responding to an external stress by rapid and energetically inexpensive shape transformations.
FUTURE PERSPECTIVES
The high tolerability and efficiency of these vesicular systems open vast potential therapeutic uses. These nanocarriers might offer advanced local and systemic new therapies with agents that are unable to efficiently penetrate the stratum corneum via passive diffusion. The non steroidal anti-inflammatory drug (NSAID), ketoprofen, in a transferosome formulation gained marketing approval by the Swiss regulatory agency (SwissMedic). The product is expected to be marketed under the trademark Diractin. Further therapeutic products based on the transferosome technology, according to IDEA AG, are in clinical development.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Vyas SP, Khar RK. 1st Ed. Delhi: CBS Publishers; 2002. Targeted and controlled drug delivery; pp. 219–43. [Google Scholar]
- 2.Jain S, Umamaheshwari RB, Bhadra D, Jain NK. Ethosomes: A novel vesicular carrier for enhanced transdermal delivery of an anti-HIV agent. Indian J Pharm Sci. 2004;66:72–81. [Google Scholar]
- 3.Kumar R, Philip A. Modified Transdermal Technologies: Breaking the barriers of drug permeation via skin. Trop J Pharm Res. 2007;6:633–44. [Google Scholar]
- 4.Chien YW. Logics of transdermal controlled drug administration. Drug Dev Ind Pharm. 1983;9:497–520. [Google Scholar]
- 5.Nandha A, Nandha S, Dhall M, Rao R. Transferosomes: A novel Ultra deformable vesicular carrier for transdermal drug delivery. Pharma Info Net. 2005 Oct;:395. [Google Scholar]
- 6.Cevc G. Drug delivery across the skin. Exp Opin Investig Drugs. 1997;6:1887–973. doi: 10.1517/13543784.6.12.1887. [DOI] [PubMed] [Google Scholar]
- 7.Langer R. Transdermal drug delivery: Past progress, current status, and future prospects. Adv Drug Deliv Rev. 2004;56:557–8. doi: 10.1016/j.addr.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Cevc G, Blume G. New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultra-deformable drug carriers. Transfersomes Biochem Biophys Acta. 2001;1514:191–205. doi: 10.1016/s0005-2736(01)00369-8. [DOI] [PubMed] [Google Scholar]
- 9.Jain NK. Advances in controlled and novel drug delivery. 1:426–51. [Google Scholar]
- 10.Verma P, Ram A, Jha AK, Mishra A, Thakur A. Phosphatidylcholine: A revolution in drug delivery technology. Int J Pharm Sci Res. 2010 [Google Scholar]
- 11.Jain CP, Vyas SP, Dixit VK. Niosomal system for delivery of rifampicin to lymphatics. Int J Pharma Sci. 2006;68:5758. [Google Scholar]
- 12.Chien YW. New York: Marcel Decker Inc; 1982. Novel drug delivery systems; pp. 149–215. [Google Scholar]
- 13.Nanda A, Nanda S, Dhall M, Rao R. Transferosomes - A novel ultra-deformable vesicular carrier for transdermal. Drug Deliv. 2005;5:395. [Google Scholar]
- 14.Chuanping N. College of pharmacy liaoning medical university. preparation and study on properties of ibuprofen transferosomes. J Math Med. 2010:2. [Google Scholar]
- 15.Shen Y, Zhang Y, Liao Ming. Preparation and quality evaluation of drug loading transferosomes. Med J Chin People's Liberation Army. 2007:10. [Google Scholar]
- 16.Duangjit S, Opanasopit P, Rojanarata T, Ngawhirunpat T. Characterization and In vitro Skin Permeation of Meloxicam- loaded liposomes versus Transferosomes. J Drug Deliv. 2011;5:207–14. doi: 10.1155/2011/418316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochem Biophys Acta. 1992;1104:226–32. doi: 10.1016/0005-2736(92)90154-e. [DOI] [PubMed] [Google Scholar]
- 18.Jain S, Jain P, Umamaheshwari RB, Jain NK. Transferosomes: A novel vesicular carrier for enhanced transdermal delivery: Development, characterization and performance evaluation. Drug Dev Ind Pharm. 2003;29:1013–26. doi: 10.1081/ddc-120025458. [DOI] [PubMed] [Google Scholar]
- 19.Benson HA. Transdermal drug delivery: Penetration enhancement techniques. Curr Drug Deliv. 2005;2:23–33. doi: 10.2174/1567201052772915. [DOI] [PubMed] [Google Scholar]
- 20.Maurya SD, Agarwal S, Tilak VK, Dhakar RC, Singh A, Maurya G. Enhanced transdermal delivery of indinavir Sulfate via transferosomes. Int J Compr Pharm. 2010;1:1–7. [Google Scholar]
- 21.Sheo DM, Shwetha A, Ram CD, Ghanshyam M, Girish K, Sunil KP. Transferosomes - A novel vesicular carrier for enhanced Transdermal delivery of stavudine: Development, characterization and performance evaluation. J Sci Speculations Resea. 2010:130–6. [Google Scholar]
- 22.Patel R, Singh, Singh S, Sheth NK, Gendle R. Development and characterization of curcumin loaded transferosomes for transdermal Delivery. J Pharm Sci Res. 2009;1:271–80. [Google Scholar]
- 23.Pandey S, Goyani M, Dev MV, Fakir J. Transferosomes: A novel approach of transdermal drug. Deliv Sch Res Libr. 2009;1:143–50. [Google Scholar]
- 24.Boinpally RR, Zhou SL, Poondru S, Devraj G, Jasti BR. Lecithin vesicles for topical delivery of diclofenac. Eur J Pharm Biopharm. 2003;56:389–92. doi: 10.1016/s0939-6411(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 25.Cevc G. Material transport across permeability barriers by means of lipid vesicles. In: Lipowsky R., Sackmann E, editors. Handbook of physics of biological systems. Vol. 1. Amsterdam: Elsevier; 1995. pp. 441–64. [Google Scholar]
- 26.Singodia D, Gupta GK, Verma A, Singh V, Shukla P, Mishra P, et al. Development and performance evaluation of amphotericin B transferosomes against resistant and sensitive clinical isolates of visceral leishmaniasis. J Biomed Nanotechnol. 2010;6:293–302. doi: 10.1166/jbn.2010.1121. [DOI] [PubMed] [Google Scholar]
- 27.Jain S, Umamaheshwari RB, Bhadra D, Tripathi P, Jain P, Jain NK. Ultra deformable liposomes: A recent tool for 78okffective transdermal drug delivery. Indian J Pharm Sci. 2003;65:223–31. [Google Scholar]
- 28.Barry B. Transdermal drug delivery. In: Aulton EM, editor. Pharmaceutics, The science of dosage forms design. 2nd ed. Churchill Livingstone, Newyork: Harcourt Publishers; 2002. pp. 499–33. [Google Scholar]
- 29.Cevc G. Transfersomes, liposomes and other lipid suspensions on the skin: Permeation enhancement, vesicle penetration, and transdermal drug delivery. Crit Rev Ther Drug Career Syst. 1996;13:257–388. doi: 10.1615/critrevtherdrugcarriersyst.v13.i3-4.30. [DOI] [PubMed] [Google Scholar]


