Abstract
In this work we devised a method to create smaller eye drops of the glaucoma medication timolol maleate by altering the dropper tip design and changing the physical properties of the formulation. Most ocular diseases are treated with topical application of eye drops. After instillation of an eye drop, typically, less than 5% of the applied drug penetrates the cornea and reaches the intraocular tissues; the major fraction of the instilled drug is absorbed and enters the systemic circulation. Ophthalmic solutions are available in multidose or single-dose glass/plastic dropper bottles that deliver drops with a volume that ranges from 25 μL to 70 μL (average 40 μL). Because of the low capacity of the precorneal area, the optimal drop volume is about 20 μL; with larger volumes there is the risk of adverse systemic effects due to absorption of the drug via the nasal mucosa. Thus, both from the biopharmaceutical and economic point of view, drops of only 5-15 mL volume should be instilled into the eye. In this present work we devised a method to reduce the size of the drop by inserting a glass capillary tube into the dropper tip and by changing the physical properties of the formulation (by altering the concentration of Tween 80™, i.e., 0.05% and 0.1% of Tween 80™). We measured the drop sizes of the different timolol eye drop formulations available in the market and estimated the yearly cost of the medications. Our timolol maleate formulation with 0.1% concentration of Tween 80™ delivered through the dropper tip with the inserted glass capillary was shown to be better than the other formulations available in the market in terms of ability to deliver smaller drops, meaning that each bottle would last longer and that the yearly cost of treatment would be lower.
Keywords: Drop size, eye drop, cost-effectiveness, timolol maleate
INTRODUCTION
Glaucoma is defined as state of raised intraocular pressure is not compatible with the normal health and function of the eye.[1] The normal range of intraocular pressure is 12.0-21.0 mmHg. Glauoma is a common cause of blindness in adults. With early recognition, it can be treated by medication and surgery.[2,3]
Pharmacokinetic studies have shown the maximal tear film concentration is achieved with a 20-μL eye drop.[4] Increasing the eye drop's size beyond this does not increase the drug concentration in the tear film. Therefore, reducing the eye drop's size from the presently available 50-70 μL to 20 μL should not reduce efficacy.[5,6] With smaller drop size, the number of bottles required per year will be less, and this may be particularly important for poor patients in whom compliance is often decided by the cost of treatment.[6–8] Besides the economic factors, there are important pharmacologic implications. The beta-blockade mechanism of timolol has resulted in a dramatic increase in serious complications due to eye drop-related timolol toxicity.[9] There is wide variability in the drop size of different products on the market and it would be worthwhile to devise a method to deliver an optimum drop size.[10,11] Alterations in the eye drop delivery system to reduce drop size, as well as alteration of the physical properties of the medication, can greatly diminish the cost of treatment and also improve the therapeutic index.
The ultimate aim of the present study was to reduce the cost of treatment by increasing the number of drops per bottle. We tried to accomplish this by increasing the surface active agents in the formulation and by changing the inner diameter of the dropper so that drop size was smaller.
MATERIALS AND METHODS
Preparation of Eye Drop Solutions of Timolol Maleate, Using 0.05%v/v, 0.1v/v, and 0.5% of tween 80
For preparation of the eye drop solution, 680 mg of timolol maleate was accurately weighed and, along with 10 mg of benzalkonium chloride, was dissolved in 100 mL of buffer solution (pH 7.4).
Determination of Surface Tension and Viscosity of the Formulation
Surface tension was determined by using the drop count method and viscosity was determined using the Ostwald viscometer.
Drop Count of Different Formulation and for the Medication Before Inserting the Capillary
The drops were counted as they fell into a 10 mL graduated cylinder. The total volume of medication was measured in each container. In all the cases blank was carried out with 0.9% v/v sodium chloride solution. The same graduated cylinder was used for all the trials. All the samples under each brand name were obtained from the same batch. The plastic dropper tips used were of the same batch, the dropper number used for both formulations and medication was as follows: LDPE Plastic eye dropper, 5 mL container.
Alteration of the Tip Dimension of the Dropper using a Capillary Tube
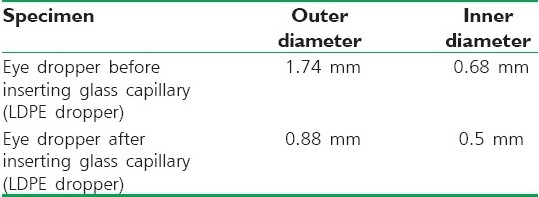
The inner and outer diameters of the droppers were measured. Then the glass capillary tube was inserted and fixed to the dropper and checked for leakage [Table 1].
Table 1.
Tip dimensions of eye dropper and capillary tube
Drop Count After Inserting the Glass Capillary Tube
The drops were counted as they fell into a 10-mL graduated measuring cylinder; the total volume of the medication was measured with 0.9%v/v sodium chloride solution. The same graduated cylinder was used for all the trials. All the samples name were obtained from the same batch under each branded name. The plastic dropper tips used were of the same batch, the dropper tip used were of the same batch, the dropper used for both formulations and medication was follows: LDPE dropper, 5 mL container [Table 2].
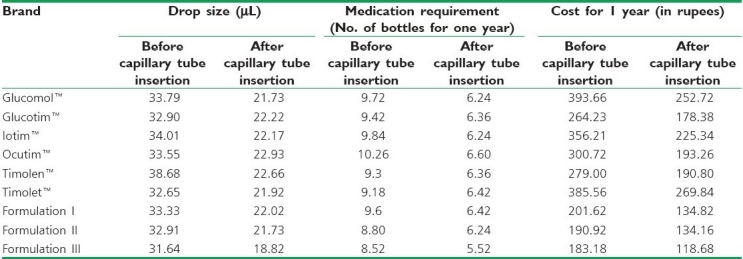
Table 2.
Drop size, medicament required, cost for 1 year of timolol maleate for before and after inserting the capillary tube
RESULTS
DISCUSSION AND CONCLUSION
A drop size greater than 25 μL will cause wastage of the medication and unnecessary expenses to the patient. Pharmacokinetic studies have shown that the maximal tear film concentration can be achieved with a drop of 20 μL. While increasing the eye drop's size beyond this does not increase efficacy, reducing the eye drop's size will decrease wastage.
Before the capillary tube was inserted, the drop size varies, from 31.64 μL (formulation III) to 33.33 μL (formulation I). Drop size for the commercial preparations varies from 32.65 μL (Timolet™) to 38.68 μL (Timolen™). The medication requirement ranges from 8.52 bottles (formulation III) to 9.6 (formulation I); for the marketed formulations the range was from 9.18 bottles (Timolet™) to 10.26 (Ocutim™). The cost for one year ranges from Rs. 183.18 (formulation III) to Rs. 201.62 (formulation I) and, for the commercial preparations, from Rs. 264.23 (Glucotim™) to Rs. 393.66 (Glucomol™). After inserting the capillary, the drop size varies from 18.82 μL (formulation III) to 22.02 μL (formulation I) and, for the commercial prepatations, from 21.73 μL (Glucomol™) to 22.93 μL (Ocutim™). The medication requirement ranges from 5.52 bottles (formulation III) to 6.42 bottles (formulation I); for the commercial preparations the range was from 6.24 bottles (Glucomol™ and Iotim™) to 6.60 bottles (Ocutim™). The cost for 1 year ranges from Rs. 118.68 (formulation III) to Rs. 134.82 (formulation I) and, for the commercial preparations, from Rs. 178.38 (Glucotim™) to Rs. 269.84 (Timolet™).
Thus, alteration of the eye drop delivery system and medication properties can produce smaller drops and can eatly decrease the cost of topical therapy in glaucoma and at the same time improve the therapeutic index.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Lederer CM, Harold RE. Drop size of commercial glaucoma medications. Am J Ophthalmol. 1986;101:691–4. doi: 10.1016/0002-9394(86)90771-3. [DOI] [PubMed] [Google Scholar]
- 2.Brown RH, Hotchkiss ML, Davis EB. Ocular absorption following topical delivery. Adv Drug Del Rev. 1985;16:3–19. [Google Scholar]
- 3.Jain MR. Delhi: Japee brothers Medical Publishers Pvt. Ltd; 1991. Text Book of Glaucoma; pp. 1–6. [Google Scholar]
- 4.Nagataki S, Mishima S. Pharmacokinetics of instilled drugs in the human eye. Int Ophthalol Clin. 1980;20:33–7. doi: 10.1097/00004397-198002030-00006. [DOI] [PubMed] [Google Scholar]
- 5.Maurice DM. Factors influencing the preparation of topically applied drugs. Int Ophthalmol Clin. 1980;20:21–5. doi: 10.1097/00004397-198002030-00005. [DOI] [PubMed] [Google Scholar]
- 6.File RR, Patton TF. Topically applied pilocarpine.Human pupillary response as a function of drop size. Arch Ophthamol. 1980;98:112–5. doi: 10.1001/archopht.1980.01020030114010. [DOI] [PubMed] [Google Scholar]
- 7.Leopold IH. Ocular cholinesterase and cholinesterase inhibitors. Am J Ophthalmol. 1961;51:855–60. doi: 10.1016/0002-9394(61)91776-7. [DOI] [PubMed] [Google Scholar]
- 8.Maurice DM. Factors influencing the penetration of topically applied drugs. Int Ophthalmol Clin. 1980;20:21–5. doi: 10.1097/00004397-198002030-00005. [DOI] [PubMed] [Google Scholar]
- 9.Sklubalova Z, Zatloukal Z. Classification of plastic eye dropper tips using Harkin's and Brown's factor. Pharmazie. 2007;62:750–5. [PubMed] [Google Scholar]
- 10.Sklubalova Z, Zatloukal Z. A comparison of capillary and rotational viscometry of aqueous solutions of hypromellose. Pharmazie. 2007;62:779–81. [PubMed] [Google Scholar]
- 11.Petrusson G, Cole R, Hanna C. Treatment of Glaucoma using minidrops clonidine. Arch Ophthalmol. 1984;102:1180–1. doi: 10.1001/archopht.1984.01040030958024. [DOI] [PubMed] [Google Scholar]


