Abstract
Microcrystalline cellulose (MCCI) has been widely used as an excipient for direct compression due to its good flowability, compressibility, and compactibility. In this study, MCCI was obtained from agricultural by-products, such as corn cob, sugar cane bagasse, rice husk, and cotton by pursuing acid hydrolysis, neutralization, clarification, and drying steps. Further, infrared spectroscopy (IR), X-ray diffraction (XRD), optical microscopy, degree of polymerization (DP), and powder and tableting properties were evaluated and compared to those of Avicel PH101, Avicel PH102, and Avicel PH200. Except for the commercial products, all materials showed a DP from 55 to 97. Particles of commercial products and corn cob had an irregular shape, whereas bagasse particles were elongated and thick. Rice and cotton particles exhibited a flake-like and fiber-like shape, respectively. MCCI as obtained from rice husk and cotton was the most densified material, while that produced from corn cob and bagasse was bulky, porous, and more compressible. All products had a moisture content of less than 10% and yields from 7.4% to 60.4%. MCCI as obtained from bagasse was the most porous and compressible material among all materials. This product also showed the best tableting properties along with Avicel products. Likewise, all MCCI products obtained from the above-mentioned sources showed a more rapid disintegration time than that of Avicel products. These materials can be used as a potential source of MCCI in the production of solid dosage forms.
Keywords: Agricultural by-products, direct compression excipient, microcrystalline cellulose
INTRODUCTION
Cellulose is the most abundant polymer in nature formed by glucose units linked through a β-1,4 glycosidic linkage. The linear chains of this polymer are bound together forming microfibrils which structure the cell wall in most plants. Microcrystalline cellulose (MCCI) is produced by acid hydrolysis of wood pulp. During this process, the amorphous regions of the microfibrils are eliminated leaving the most crystalline parts intact.[1] The resulting product is washed and spray dried to get a powder of the desirable size, density, and moisture content.[2] MCCI is widely used as a pharmaceutical aid for direct compression, wet and dry granulation. It is also employed for the production of solid dosage forms due to its good compressibility, compactibility, and loading capacity of drugs.[3] Further, it renders tablets of good hardness without the need of using high compression forces[4] and these compacts usually show a low friability.[5]
Now-a-days, the impact of agricultural by-products on the environment and other threatened ecosystems is an issue to be resolved. This problem is more frequent in developing countries where most of these residues are either burned or dumped into rivers.[6] As a result, these by-products contribute to the greenhouse effect, soil erosion, and pollution of the atmosphere and water sources.[7] Since most of these residues are cellulose-based materials, they represent a potential source of inexpensive MCCI. Further, they are abundant and accessible, and the production process involved in the production of MCCI is simple and economical. Moreover, the availability of wood in the world is decreasing, along with a growing demand for wood pulp in developing countries of Asia, Africa, and Latin America. For this reason, the agricultural by-products, different from wood pulp sources, can be employed as an alternative source for MCCI.
The aim of this study was to evaluate the powder and tableting properties of MCCI obtained from rice husk, corn cob, sugar cane bagasse, and cotton, and compare those properties with commercial MCCIs named as Avicel PH101, Avicel PH102, and Avicel PH200. The reported cellulose content of these residues depends on the seasonal conditions and the source employed, ranging from 20% to 60%.[8–10]
MATERIALS AND METHODS
Materials
Sodium hydroxide (lot 58051305C), sodium metabisulfite (lot 09120342), and sodium hypochlorite (15% v/v, lot 093852) were obtained from Carlo Erba (Rodano, Italy) and Quimicos LM SA (Medellin, Columbia), respectively. Concentrated hydrochloric acid (37%, lot k40039517) and MCCI (Avicel PH101, lot 6N608C, Avicel PH102, lot C0909048, Avicel PH200, lot 70637) were purchased from Merck (Darmstadt, Germany) and FMC BioPolymer (Philadelphia, PA), respectively. The agricultural residues were obtained from local farmer markets.
Preparation of Microcrystalline Cellulose Powders
Raw materials were dried in a convection oven (model ED 115 UL; Binder-USA, USA) until a moisture content of less than 5% was reached. The dried material was milled in a cutting mill (model 3, Wiley mill; Arthur H. Thomas Co., Philadelphia, PA, USA) to reach a particle size of less than 1 mm. Approximately, 400 g of the milled powder was placed on a 5 l round bottom flask and a 5% NaOH solution was added to eliminate the traces of lignin with heating at 74°C for 3 h. The powder-to-NaOH solution ratio was ~1:10. The dispersion was then cooled at room temperature and washed with distilled water until reaching a pH between 5 and 7. The resulting material was then treated with an excess of sodium hypochlorite (~700 ml) for 24 h at room temperature to eliminate low-molecular-weight carbohydrates such as pectins, proteins, hemicelluloses, and some mineral components. The resulting material was then washed and filtrated until reaching a pH between 5 and 7. The powder thus obtained was hydrolyzed with a 2 N HCl solution at 80°C for 1 h to remove the amorphous regions of cellulose followed by washing and filtration steps. A second NaClO treatment for 24 h was employed followed by washing and filtration steps. The resulting powder was dried on a convection oven (model ED 115 UL, Binder-USA) at 60°C for 12 h until reaching a moisture content less than 10%. The dry powder was then passed through an oscillating granulator (Riddhi Pharma Machinery, Gulabnagar, Gujarat, India) equipped with a 100 mesh screen.
The above procedure was repeated for rice husk except that a second NaOH treatment was conducted after the acid hydrolysis step. Further, cotton was not treated with NaOH since it did not possess lignin. However, this material required a treatment with ~100 g of sodium metabisulfite at 70°C for 2 h, a washing, and a final NaClO treatment for 24 h at room temperature to completely bleach the product.
Fourier Transform Infrared Spectroscopy Characterization
Approximately, 1 mg of the sample was mixed with 100 mg of KBr (both previously dried at 110°C for 4 h before being used) on an agate mortar and pestle. Pellets of these mixtures were then made on a portable press at a dwell time of 5 min and at a force of 10,000 pounds. The infrared spectra were collected between 650 and 4000/ cm on a Perkin Elmer IR Spectrometer (Spectrum BX, PerKin Elmer, CA, USA) equipped with the Ommic software (Nicolet Corp., Madison, WI, USA). The resolution, interval length, and number of scans employed were 16, 2, and 16/ cm, respectively.
Powder X-ray Diffraction Characterization
Powder X-ray diffractions were conducted over a 5-45° 2θ range using a Siemens diffractometer (model D5000; Siemens Energy and Automation, Inc., Madison, WI, USA), equipped with a monochromatic CuKα (α1 = 1.5460 Å, α2 = 1.54438 Å) X-ray radiation. The step width was 0.020° 2θ/min with a time constant of 0.5 s. The Difrac® plus Eva software, version 2.0 (Siemens Energy and Automatization, Inc.) was used to identify the crystalline peaks.
Powder Properties
The optical microphotographs were taken on an optical microscope (BM-180, Boeco, Germany) coupled with a digital camera (S8000fd, Fujifilm Corp., Japan). The true density was determined on a helium picnometer (AccuPyc II 1340, Micromeritics, USA) with ~2 g of the sample. The bulk density was determined by the ratio of 100 g of the sample divided by the measured volume in a graduated cylinder. The tap density was measured from the final volume of the tapped sample determined on the AUTO-TAP analyzer (AT-2; Quantachrome Instruments, USA). The Carr index was obtained from the following equation:
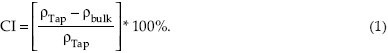
Porosity (ε) of the powder was determined from the following equation:
![]()
where ε, ρbulk, and ρtrue correspond to the porosity, bulk density, and true density of the powder, respectively. The Hausner ratio was determined from the bulk and tap densities according to the following relationship:
HR = ρtap/ρbulk, (3)
where ρtap and ρbulk are the tap and bulk densities, respectively.
The angle of repose was measured on 30 g of the sample and poured on a glass funnel (having the outer and inner diameters of 74.8 and 6.5 mm, respectively). The angle of repose was obtained from the arctan function of the quotient of the height with the radius of the cone formed after pouring the powder through the funnel.
Particle Size and Particle Size Distribution
Samples were fractionated on a ROTAP sieve shaker (RX29, WS Tyler Co., Mentor, OH, USA) using 400, 300, 250, 180, 120, 105, and 75 μm stainless steel sieves (Fisher Scientific Co., Pittsburgh, PA, USA), stacked together in the order written. Approximately 25 g of the sample was shaken for 30 min followed by weighing the fraction of the powder retained in each sieve.
The geometric mean diameter, dg, and particle size distribution were determined from the log-normal distribution plot constructed between the mean diameter and the cumulative percent frequency using the Minitab software (v.15; Minitab, Inc., State College, PA, USA).
The moisture content was obtained by a gravimetric method on a convection oven (STM 80; Rigor Scientific, Inc., Chicago, IL, USA) employing ~5 g of the sample at 105°C for 3 h.
The degree of polymerization (DP) was obtained by the intrinsic viscosity method [η] at 25 ± 0.5°C using a Canon-Fenske capillary viscometer (cell size no. 50), and cupriethylenediamine hydroxide (CUEN) was used as a solvent.[11] The DP was found by the following relationship:
DP = 190*[η] (4)
The compressibility of the powder was obtained from the Kawakita equation:[12]
![]()
where N is the tap number, Vi the initial volume, Vn the volume after n taps, “a” is a constant related to the total volume reduction of the powder bed (compressibility index), and “b” is a constant related to the resistant forces (friction/cohesion) to compression.[13]
Tableting Properties
Preparation of compacts
Compacts weighting ~500 mg were made on a hydraulic press (Compac 060804; Indemec Ltd., Itagüí, Columbia) coupled with a 13 mm concave punches and die set. The pressure range used was between 0 and 270 MPa.
Disintegration time
The USP/NP method was employed.[14] Briefly, a Hanson disintegrator (39-133-115; Hanson Research Corporation, Northridge, CA, USA) was used and operated at 30 strokes/ min. The medium employed was distilled water maintained at 37 ± 2°C.
Compact tensile strength
It was determined on a Vankel hardness tester (UK 200; Vankel, Manasquan, NJ, USA). Each compact was placed between the platens and the crushing force was then measured. The radial tensile strength (TS) values were obtained according to the Pitt et al. equation:[15]
![]()
where F is the breaking force (N) needed to break the compact into two halves, D is the diameter of the compact (mm), t is the compact total thickness (mm), and w is the central cylinder thickness.
Compact friability test
A Vankel Friabilator apparatus was employed (FAB-25; Logan Instruments Corp., NJ, USA) at 25 rpm. The friability test was performed according to the USP specifications.[15] Briefly, 13 compacts, each weighing about 500 mg and made at ~180 psi, were placed in the rotating drum and operated for 100 cycles. Compacts were then dedusted and reweighed. The friability percentage value was calculated according to the following equation:
![]()
where Wi and Wf are the initial and final weight, respectively.
RESULTS AND DISCUSSION
Production and Characterization of MCCI
As seen in Figure 1, MCCI samples showed the following characteristic vibration peaks of cellulose: 3445/cm corresponding to intramolecular OH stretching, including hydrogen bonds; 2898/cm due to CH and CH2 stretching; 1650/cm corresponding to OH from absorbed water; 1430/cm due to CH2 symmetric bending; 1375/cm due to CH bending; 1330/cm due to OH in-plane bending; 1161/cm due to C-O-C asymmetric stretching (β-glucosidic linkage); 1061/cm due to C-O/C-C stretching; and 898/cm corresponding to the asymmetric (rocking) C-1 (β-glycosidic linkage) out-of-plane stretching vibrations.[16,17]
Figure 1.
FTIR spectra of MCCI produced from different sources
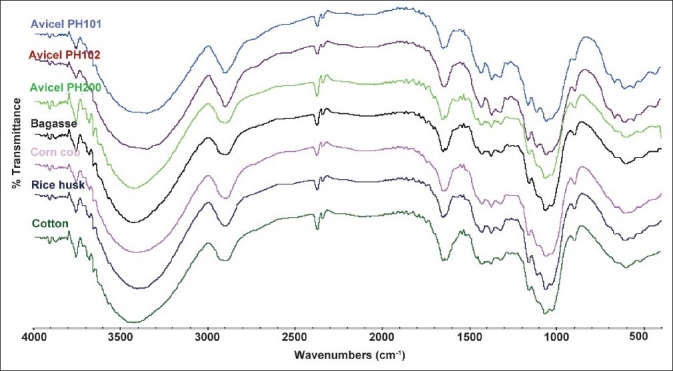
The powder XRD of MCCI obtained from the different sources and the commercial Avicel products is shown in Figure 2. It is well known that cellulose I exhibits a parallel arrangement of the chains.[18] This arrangement gives the following characteristic diffraction peaks at 14.8, 16.3, and 22.4° 2θ, confirming the presence of the cellulose I lattice corresponding to the 1 ī 0, 110, and 200 reflections, respectively.[19] A shoulder at 20.4° 2θ has also been identified in some cellulose I excipients.[20]
Figure 2.
XRD difractograms of MCCI produced by different sources
The morphology of different MCCI materials is shown in Figure 3. The commercial powders were formed by particles of an irregular shape. They did not exhibit a fibrous morphology since the spray drying process used in their manufacture allowed for the formation of particle aggregates. Likewise, particles obtained from corn cob showed aggregates of an irregular and smooth shape. Conversely, bagasse particles were elongated and thick, whereas, rice and cotton particles showed a flake-like and a thin and fibrous shape, respectively.
Figure 3.

MCCI microphotographies obtained from (a) bagasse, (b) corn cob, (c) rice husk, (d) cotton, (e) Avicel PH101, (f) Avicel PH102, (g) Avicel PH200
Powder Properties
Table 1 shows the powder properties of the materials evaluated. MCCI obtained from cotton rendered the highest yield (60.4%), whereas that obtained from sugar cane bagasse had the lowest yield (7.4%). It has been reported that bagasse and cotton have a cellulose content of ~70% and 95%, respectively.[21] The MCCI content is expected to be lower than that reported in the literature since during the hydrolysis and washing steps, the abundant amorphous regions are solubilized and eliminated. The DP was strongly dependant on the source employed. The DP of MCCI obtained from cob, bagasse, and cotton was ~95. This indicates that the hydrolysis process with 2 N HCl removed the amorphous regions of cellulose leaving only the crystalline chains of about 95 unit length. In contrast, MCCI as obtained from rice husk rendered the lowest DP, indicating a short average length of the chains. The commercial Avicel samples had the largest DP. It is well known that Avicel products have a large DP since they are obtained from soft- and hardwoods which have originally a larger chain length (>1000).[22]
Table 1.
Powder properties of MCCI obtained from different sources
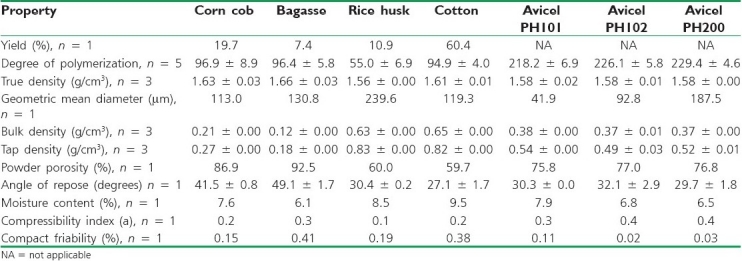
The true density of the products was ~1.6 g/cm3 and was slightly lower for rice husk, probably due to the lower DP presented. The geometric mean diameter of MCCI obtained from corn cob, bagasse, and cotton ranged from 113 to 131 μm. In contrast, MCCI as obtained from rice husk had the largest geometric mean (~240 μm). The particle size of all these materials was mainly dependant on the final sieving of the powder through a 100 mesh screen. Conversely, the particle size of Avicel PH101, Avicel PH102, and Avicel PH200 was close to 42, 93, and 188 μm, respectively.[22]
MCCI as obtained from rice husk and cotton presented the largest bulk and tap densities (~0.63 and 0.83 g/cm3, respectively). As a result, these two materials presented the fastest flow and lowest powder porosity values (0.63 and 0.65 g/cm3 and 60% and 59.7%, respectively). The possible effect of moisture is expected to be negligible for MCCI materials since in all cases, the moisture content was below 10%.
The compressibility index or volume reduction tendency is shown in Table 1. Results show that MCCI produced from bagasse and Avicel products were the most compressible materials. This indicates that these materials are desirable to be used as a fillers or diluents of large-dose active ingredients. In contrast, MCCI as obtained from rice husk had the lowest volume reduction making it appropriate to formulate only low-dose active ingredients. This is due to the large particle size, high degree of densification, and low porosity exhibited by this material.
Tableting Properties
The variation of compact tensile strength with compression pressure is shown in Figure 4. Independent of the compression pressure used, Avicel products and MCCI as obtained from bagasse formed compacts with the highest tensile strength. In contrast, MCCI as obtained from rice husk, cotton, and corn cobs rendered compacts of comparable tensile strength. Results indicate that the source of MCCI had a major effect on the resulting compact tensile strength. For example, Avicel products which are produced from softwood followed by spray drying always rendered the strongest compacts. It has been reported that different natural sources of cellulose might affect the mechanical properties of the isolated fibers.[1] A compact with a high tensile strength is desirable when a formulation of a solid dosage form with a poorly compressible drug is desirable.
Figure 4.
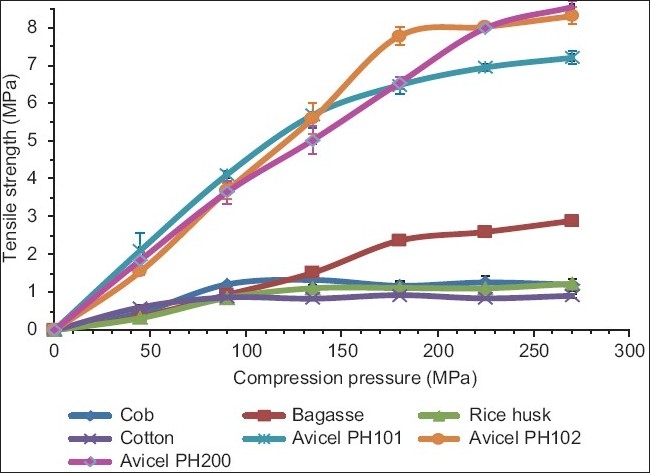
Tensile strength of compacts made of MCCI obtained from different sources
The disintegration times of the compacts are shown in Figure 5. Independent of the compression pressure used, in all cases, MCCI materials produced from the agricultural by-products sources rendered compacts with lower disintegration times than those shown by Avicel products. Since Avicel materials rendered the strongest compacts, it is not surprising that these products showed the slowest disintegrating compacts. A short disintegration time is desirable when a formulation of a fast disintegrating compact is needed.
Figure 5.
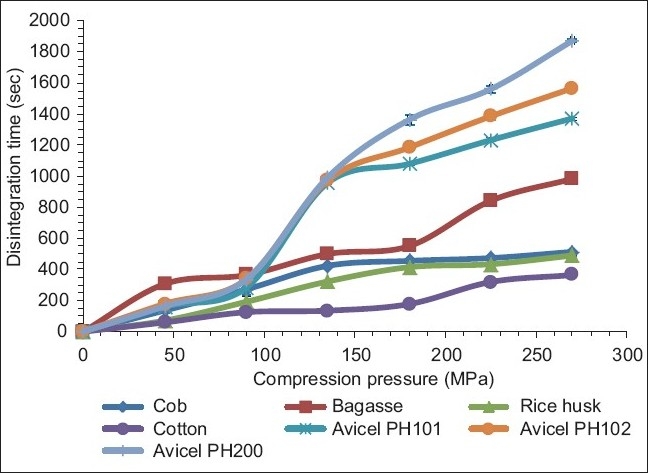
Disintegration time of compacts made of MCCI obtained from different sources
CONCLUSION
MCCI can be obtained from rice husk, sugar cane bagasse, corn cob, and cotton having a potential use as a direct compression agent. MCCI as produced from rice husk and cotton were the densest and least porous materials having the fastest flow and lowest compressibility. In contrast, MCCI as obtained from bagasse was the least dense and the most porous material and it was as compressible as Avicel products rendering compacts of good strength. All MCCI materials formed compacts of faster disintegration times than those of Avicel products. Results indicated that these sources offer an inexpensive and a simple method to produce MCCI for use in the manufacture of solid dosage forms.
ACKNOWLEDGMENTS
The authors thank professors Jorge Arango, Gloria Valencia, Isabel Henao, and Oscar Florez for providing us with the equipments and access to the laboratories needed to carry out this project. The authors Also thank CODI and the pharmacy department for sponsoring this project.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.El-Sakhawy M, Hassan ML. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohyd Polym. 2007;67:1–10. [Google Scholar]
- 2.Bhimte NA, Tayade PT. Evaluation of microcrystalline cellulose prepared from sisal fibers as a tablet excipient: A technical note. AAPS PharmSciTech. 2007;8:E1–7. doi: 10.1208/pt0801008. [DOI] [PubMed] [Google Scholar]
- 3.Foster AA, Ibrahim MM, El-Zawawy WK. Coupled acid and enzyme mediated production of microcrystalline cellulose from corn cob and cotton gin waste. Cellulose. 2007;14:247–56. [Google Scholar]
- 4.Ohwoavworhua FO, Adelakun TA, Okhamafe AO. Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: Extraction methods and characterization. Int J Green Pharm. 2009;3:97–104. [Google Scholar]
- 5.Wang DS, Shang ZQ. Evaluation of microcrystalline cellulose prepared from kenaf fibers. J Ind Eng Chem. 2010;16:152–6. [Google Scholar]
- 6.Ramírez JJ, Martínez JD, Petro SL. Basic design of a fluidized bed gasifier for rice husk on a pilot scale. Latin Am Appl Res. 2007;37:299–306. [Google Scholar]
- 7.Chungsangunsit T, Gheewala SH, Patumsawad S. Emission assessment of rice husk combustion for power production. World Acad Sci Eng Technol. 2009;53:1070–5. [Google Scholar]
- 8.Faria LF, Barboza JC, Serra AA, Castro DE. Preparation of bleached cellulose from sugar cane bagasse for chemical processing. Cell Chem Technol. 1998;32:441–55. [Google Scholar]
- 9.Valverde A, Sarria B, Monteagudo JP. Análisis comparativo de las características fisicoquímicas de la cascarilla de arroz. Scientia et Technica. 2007;13:255–60. [Google Scholar]
- 10.Uhumwangho MU, Okor RS. Effect of humidity on the disintegrant property of α-cellulose, Part II: A technical note. AAPS PharmSciTech. 2005;06:E31–4. doi: 10.1208/pt060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer V. West Conshhocken, PA: ASTM International; 2006. Annual book of ASTM STM. Paints related Coatings and Aromatics. [Google Scholar]
- 12.Kawakita K, Ludde KH. Some considerations on powder compression equations. Powder Technol. 1971;4:61–8. [Google Scholar]
- 13.Patel S, Kaushal AM, Bansal AK. Effect of particle size and compression force on compaction behavior and derived mathematical parameters of compressibility. Pharm Res. 2007;24:111–24. doi: 10.1007/s11095-006-9129-8. [DOI] [PubMed] [Google Scholar]
- 14.USP 32/NF 27: U.S. Pharmacopeia the Standard of Quality. New York, USA: United States Pharmacopeial Convention; 2009. Pharmacopoeial Convention and the National Formulary. [Google Scholar]
- 15.Pitt KG, Newton JM, Richardson R, Stanley P. The material tensile strength of convex-faced aspirin tablets. J Pharm Pharmacol. 1989;41:289–92. doi: 10.1111/j.2042-7158.1989.tb06458.x. [DOI] [PubMed] [Google Scholar]
- 16.Carrillo F, Colom X, Suñol JJ, Saurina J. Structural FTIR analysis and thermal characterization of lyocel and viscose-type fibers. Eur Polym J. 2004;40:2229–34. [Google Scholar]
- 17.Zhbankov RG. New York: Consultants Bureau; 1964. Infrared Spectra of Cellulose and its Derivatives. [Google Scholar]
- 18.Krassig H. Amsterdam: Gordon and Breach; 1996. Cellulose, structure, accessibility and reactivity, science. [Google Scholar]
- 19.Klemm D, Philipp B, Heinze T, Heinze U. 2nd ed. New York: John Wiley and Sons; 1998. Comprehensive cellulose chemistry: Functionalization of cellulose. [Google Scholar]
- 20.Kothari SH, Kumar V, Banker GS. Compression, compaction, and disintegration properties of low crystallinity celluloses produced using different agitation rates during their regeneration from phosphoric acid solutions. Int J Pharm. 2002;232:69–80. doi: 10.1208/pt020207. [DOI] [PubMed] [Google Scholar]
- 21.Ilindra A, Dhake JD. Microcrystalline cellulose from bagasse and rice straw. Indian J Chem Technol. 2008;15:497–9. [Google Scholar]
- 22.Jonat S, Hasenzahl S, Drechsler M, Alberts P, Wagner KG, Schmidt PC. Investigation of compacted hydrophilic and hydrophobic colloidal silicon dioxides as glidants for pharmaceutical excipients. Powder Technol. 2004;141:31–43. [Google Scholar]


