Abstract
Insects possess an elaborate tracheal system that enables transport of gaseous oxygen from the atmosphere directly to the inner organs. Therefore, the presence of specialized oxygen-transport proteins in the circulatory system of insects has been considered generally unnecessary. Here, we show for the first time, to our knowledge, the presence of an ancestral and functional hemocyanin (Hc) in an insect. In the hemolymph of nymphs and adults of the stonefly Perla marginata, a hexameric Hc was identified, which consists of two distinct subunit types of 659 and 655 amino acids. P. marginata Hc displays cooperative oxygen binding with a moderately high oxygen affinity [(half-saturation pressure, P50 ≈8 torr (1 torr = 133 Pa)]. No evidence was found for the presence of Hcs in the more evolutionarily advanced holometabolan insects, suggesting that this type of respiratory protein was lost later in insect evolution. However, our results demonstrate that, in contrast to the accepted paradigm, certain basal insects have retained an ancestral blood-based mechanism of gas exchange.
Insects are the world's dominant life form, yet there remain uncertainties regarding their key evolutionary transitions. The leading phylogenetic hypothesis indicates a close relationship between insects and crustaceans (1, 2), suggesting that fundamental changes in the mechanisms of respiration must have accompanied the diversification of insects from crustaceans and their invasion of terrestrial and aerial environments. Gas exchange in extant insects is usually mediated through tracheae and tracheoles, tubular structures that connect the inner organs with the air, thus enabling diffusion of oxygen to the metabolically active tissues (3, 4). The insect tracheal system is so well developed that respiratory proteins, which transport oxygen (O2) in the hemolymph (i.e., blood), have been regarded as unnecessary (5–7). Only a few species that live in a temporarily hypoxic environment, such as the aquatic larvae of the chironomid midges, some aquatic Hemiptera, or larvae of the horse botfly Gasterophilus intestinalis, harbor hemoglobins either in the hemolymph or in specialized tissues (8).
Other arthropod subphyla (Crustacea, Chelicerata, Myriapoda, and Onychophora), however, usually use hemocyanins (Hcs) for convective oxygen transport in the hemolymph (9–13). Arthropod Hcs are large hexameric or oligohexameric proteins composed of similar or identical subunits in the range of 75 kDa (9, 11). Each subunit can bind to an O2 molecule through two copper ions that are coordinated by six histidine residues. Although investigated in detail in other arthropods, a functional Hc in the circulatory system of insects has not previously been demonstrated. Hc-like larval storage proteins (hexamerins) are present in all insect taxa studied, but they lack functional copper-binding sites and thus do not transport O2 (14, 15). The six-histidine copper-binding sites are present in another Hc-like protein identified from the embryonic hemolymph of the grasshopper Schistocerca americana (16). However, the function of this protein is uncertain because neither O2 binding nor its expression in later developmental stages could be demonstrated. Thus, it is unlikely that it acts as a respiratory protein in nymphs or adults.
Stoneflies (Plecoptera) arose near the base of one of the two main branches of winged insects and have retained many ancestral morphological and behavioral traits (17–19). Thus, we reasoned that stoneflies might be an especially likely group to have retained partial reliance on an ancestral form of gas exchange and use Hcs for oxygen transport.
Materials and Methods
Cloning of Hc cDNA. Approximately 5 micrograms of purified poly(A)+ RNA extracted from 3-year-old female nymphs of Perla marginata were used for the construction of a directionally cloned cDNA expression Lambda ZAP library (Stratagene). Various degenerated oligonucleotide primers were designed according to conserved amino acid sequences of the crustacean Hcs (20) and the embryonic hemolymph protein of S. americana (16). These primers were tested in RT-PCR experiments with the total RNA from P. marginata nymphs, applying the One Step kit according to the manufacturer's instructions (Qiagen, Valencia, CA). Two primers, 5′-CCNCCNCCNTAYGARRTCTACCC-3′ and 5′-TCGTACTTGGGTCCNAGGAAGAC-3′, resulted in the expected fragment of ≈1,000 base pairs. The fragment was sequenced, labeled with digoxigenin (Roche PCR labeling kit, Roche, Mannheim, Germany), and used to screen the cDNA library. Positive phage clones were converted to pBK-cytomegalovirus vectors by using the material provided by Stratagene and sequenced on both strands by a commercial service (Genterprise, Mainz, Germany).
Protein Biochemistry. The hemolymph of living P. marginata nymphs and adults was withdrawn with a syringe, immediately diluted with an equal volume of 100 mM Tris·HCl, pH 7.8/5mM MgCl2/5 mM CaCl2 and centrifuged for 5 min at 10,000 × g. Oxygen-binding curves were obtained in the same buffer by using the polarographic fluorometric method (21). For antibody production, a fragment of PmaHc1, covering amino acid positions 158–422, was amplified by PCR and cloned into the KpnI and SpeI sites of the pQE30 vector (Qiagen). The peptide was recombinantly expressed in the M15 Escherichia coli strain, was purified by using the His tag according to the instructions provided with the Qiagen pQE protocol, and was used to raise a polyclonal antiserum in rabbits. SDS/PAGE was performed on a 7.5% polyacrylamide gel. For Western blotting, the proteins were transferred to nitrocellulose. Nonspecific binding sites were blocked overnight by 5% nonfat dry milk inTris-buffered saline/Tween (TBST) (10 mM Tris·HCl, pH 7.4/140 mM NaCl/0.3% Tween 20). Incubation with anti-Hc antiserum, diluted 1:20,000 in 5% nonfat dry milk/TBST, was carried out for 2 h at room temperature. The filters were washed in TBST, were incubated for 1 h with the goat-anti-rabbit IgGs conjugated with alkaline phosphatase (Dianova, Hamburg, Germany), and were diluted in 5% nonfat dry milk/TBST. The membranes were washed and detection was carried out with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate.
Phylogenetic Analyses. An amino acid alignment of 33 selected arthropod Hcs, three pseudo-Hcs, and 10 insect hexamerins was constructed as described (13, 20). The data set comprises the complete alignment excluding N- and C-terminal extensions (768-aa positions). Bayesian phylogenetic analyses (22) were performed by mrbayes 3.0beta4 with the WAG matrix (23) with gamma distribution of substitution rates. Prior probabilities for all trees and for amino acid replacement models were equal, starting trees were random. Metropolis-coupled Markov chain Monte Carlo sampling was performed with one cold chain and three heated chains that were run for 100,000 generations. Trees were sampled every tenth generation. Posterior probabilities were estimated on 2,000 trees (burnin = 8,000).
Results and Discussions
Cloning of Two Hc Subunits from the Stonefly. We investigated the occurrence of Hc cDNA sequence in the stonefly P. marginata by generating specific oligonucleotide primers according to conserved arthropod Hc sequences (21). A 1,048-bp Hc fragment was amplified from the RNA of 3-year-old P. marginata nymphs by RT-PCR. This fragment was used to screen a cDNA-library constructed from the RNA. Two cDNA clones of 2,601 and 2,367 base pairs were obtained that cover the complete coding regions of two distinct Hc subunits, plus the 5′ and 3′ untranslated regions (Figs. 5 and 6, which are published as supporting information on the PNAS web site). The deduced amino acid sequences of the Hc subunits comprise 678 and 671 amino acids, respectively, and are 48.3% identical. Sequence comparisons show that both sequences display the highest identity with the embryonic hemolymph protein of S. americana (65.3% and 47.3% identity), whereas lower similarity scores were observed with other Hcs or with insect hexamerins (Fig. 1). Both P. marginata Hcs contain N-terminal signal sequences required for translocation through the endoplasmic reticulum (see Figs. 5 and 6), resulting in native polypeptides of 659 and 655 amino acids with predicted molecular masses of 77.2 and 76.3 kDa, respectively. The amino acid residues required for reversible oxygen binding and subunit cooperativity are present in both sequences, suggesting that they are able to carry out respiratory functions (9–13, 24). These conserved residues include the six copper-binding site histidines in the central second domain, as well as the three phenylalanines that stabilize the binding of O2 in the first and second domain. The phenylalanine found in the first domain of the two subunits, at positions 79 and 76, respectively, is implied to be a key residue in regulation of oxygen binding in most Hcs (24).
Fig. 1.
Comparison of Hc subunit sequences from the stonefly P. marginata (PmaHc1 and PmaHc2) with the sequences of the Hc-like embryonic hemolymph protein of S. americana (SamEHP, GenBank accession no. AAC16760) and the Hc subunit a from the spiny lobster Panulirus interruptus (PinHcA, GenBank accession no. P04254). (Upper) The copper-binding sites (CuA and CuB) are indicated. The copper-binding histidines are shaded in black and other conserved residues are shaded in gray. The asterisks denote the phenylananines that stabilize the O2 binding.
Hcs Are Present Throughout the Life Cycle of the Stonefly. The presence of the Hc in P. marginata hemolymph was established by Western blotting employing a polyclonal antibody raised against a recombinant peptide that covers amino acids 158–422 of Hc subunit 1. This antibody recognizes two distinct bands in the hemolymph of 1-, 2-, and 3-year-old nymphs, as well as in adult specimens (Fig. 2). The molecular masses of the Hcs are in the range of 75 kDa and thus are in agreement with those predicted from the translated cDNA sequences. In 3-year-old nymphs and in the adult stonefly, the Hc makes up ≈25% of the total hemolymph proteins. Another highly expressed polypeptide with a slightly higher molecular mass was identified in the hemolymph of 3-year-old nymphs and adults by Coomassie staining, which likely represents an insect storage hexamerin (S.H.-H. and T.B., unpublished data). Electron microscopic studies and size exclusion chromatography show that both Hcs and hexamerins are hexameric, although these studies do not allow the discrimination between these proteins (data not shown).
Fig. 2.
Identification of Hc in P. marginata nymph and adult hemolymph. Ten micrograms of total hemolymph proteins of 1-, 2-, and 3-year-old female nymphs (1, 2, 3) and adults (A) were separated by SDS/PAGE and were stained with Coomassie brilliant blue (lanes 1–4). The Hc subunits were detected by an antibody raised against a recombinant Hc peptide (lanes 5–8). On the right side, the positions of the hexamerins (Hx) and hemocyanins (Hc) are indicated. The molecular mass standard is given on the left side.
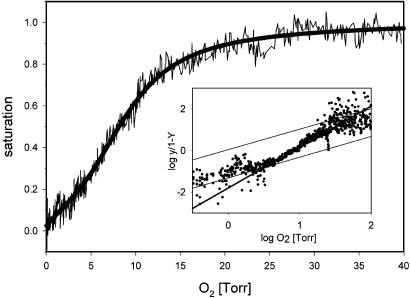
Stonefly Hc Binds Oxygen Reversibly. Oxygen-binding properties of the stonefly hemolymph, and thus, the function of the Hc as a respiratory protein, were measured by using a polarographic fluorometric method (21). Reversible binding of O2 was demonstrated in hemolymph from both 3-year-old nymphs and adults (Fig. 7, which is published as supporting information on the PNAS web site) with essentially identical O2 binding curves: P. marginata Hc has a half-saturation pressure (P50) of ≈8 ± 0.3 torr with cooperative ligand binding (Hill coefficient: nmax = 2; Fig. 3). The O2 affinity constants of the Hc T and R state were determined to be 16 and 1.6 torr, respectively. These equilibrium characteristics agree with the proposed hexameric structure and are within the range observed for other arthropod Hcs (9, 25). After freezing, the Hc dissociates into apparently two distinct species with different oxygen affinities of 0.063 and 0.023 torr. These two species most likely correspond to the two distinct Hc subunit types. This rather large difference in oxygen affinities correlates with the structural differences of the two Hc subunits, which are only 48.3% identical and are phylogentically highly diverged (see below).
Fig. 3.
Oxygen-binding properties of the P. marginata Hc. Equilibrium oxygen binding of larval hemolymph was determined by the polarographic fluorometric method. Protein concentration was 1.2 mg/ml and the temperature was 20°C, pH 7.8. The hemolymph shows cooperative oxygen binding with a half-saturation pressure of P50 = 8. (Inset) The Hill plot.
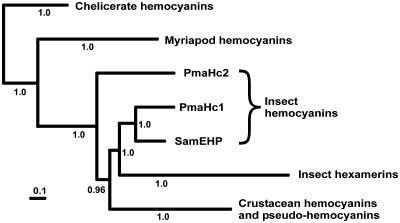
Ancient Origin and Crustacean Affinities of Insect Hcs. The amino acid sequences of the insect Hcs were added to an alignment of arthropod Hcs and related proteins (refs. 13 and 20 and Fig. 8, which is published as supporting information on the PNAS web site). Phylogenetic analysis employing a Bayesian approach shows that the insect Hcs (including the S. americana embryonic hemolymph protein) are basal to the insect hexamerins (Fig. 4, and Fig. 9, which is published as supporting information on the PNAS web site), suggesting that the insect storage proteins evolved only after the separation of the Hexapoda from a putative crustacean ancestor (1, 2), which may have occurred roughly 450 million years ago. The stonefly Hc subunit 2 appears to be of more ancient phylogenetic origin, diverging even before the time the Crustacea and Hexapoda split, and thus rendering the insect Hcs paraphyletic.
Fig. 4.
Simplified phylogeny of arthropod Hcs and hexamerins. The Bayesian phylogenetic tree (22) was deduced from the analyses of an amino acid alignment (Fig. 8). The complete tree is shown in Fig. 9.
Loss of Hcs During Hexapod Evolution. The presence of a Hc in the circulatory system of stoneflies is surprising because no specialized respiratory protein was considered necessary to support the diffusive O2 transport in the tracheal system of the insects. Additional experiments using anti-Hc antibodies and oligonucleotide primers show that Hcs are present in Zygentoma, but not in Ephemeroptera, Odonata, Saltatoria, and various other neopteran insect orders (S.H.-H. and T.B., unpublished data). This observation agrees with previous studies (6, 14) that found no evidence for a Hc from a large variety of higher insects, although their hemolymph proteins have thoroughly been investigated. Accordingly, no Hc gene was found in the complete genomic sequences of the fruitfly Drosophila melanogaster (26) or of the mosquito Anopheles gambiae (27). Thus, higher Neoptera have lost any Hc-based oxygen carrier. This notion is supported by the observation that certain aquatic Hemiptera and Diptera, which require a respiratory protein due to their hypoxic environment, use hemoglobins (8) that most likely evolved independent from an intracellular globin common to the insects (28, 29).
Stonefly Oxygen Transport as Functional Intermediate Between Crustaceans and Insects? Our results indicate that functional Hcs were most likely present in early insects, are still present in certain extant taxa, and were inherited from the common ancestor of insects and crustaceans. Why stoneflies in particular have retained this oxygen transport molecule in their blood remains an interesting question. Although the Plecoptera possess what appears to be a typically developed insect tracheal system, they have a number of ancestral features, and are aquatic as nymphs and are semiaquatic as adults (17–19). Thus, it is likely that the presence of a Hc in stoneflies reflects an intermediate state in the transformation from a crustacean to an insect respiratory system.
Supplementary Material
Acknowledgments
We thank J. Markl for excellent working facilities, K. Enting for specimens, and K. Kusche, E. Jaenicke, and A. Schmitz for discussions. This work is supported by the Deutsche Forschungsgemeinschaft Grants Bu956/3 and 956/5 (to T.B.) and De414/8 (to H.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: Hc, hemocyanin.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AJ555403 and AJ555404).
References
- 1.Hwang, U. W., Friedrich, M., Tautz, D., Park, C. J. & Kim, W. (2001) Nature 413, 154–157. [DOI] [PubMed] [Google Scholar]
- 2.Giribet, G., Edgecombe, G. D. & Wheeler, W. C. (2001) Nature 413, 157–161. [DOI] [PubMed] [Google Scholar]
- 3.Mill, P. J. (1985) in Comprehensive Insect Physiology, Biochemistry, and Pharmacology: Integument, Respiration, and Circulation, eds. Kerkut, G. A. & Gilbert, L. I. (Pergamon, Oxford), Vol. 3, pp. 517–593. [Google Scholar]
- 4.Brusca, R. C. & Brusca, G. J. (1990) Invertebrates (Sinauer, Sunderland, MA).
- 5.Mangum, C. P. (1985) Am. J. Physiol. 248, 505–514. [Google Scholar]
- 6.Law, J. H. & Wells, M. A. (1989) J. Biol. Chem. 264, 16335–16338. [PubMed] [Google Scholar]
- 7.Willmer, P., Stone, G. & Johnston, I. (2000) Environmental Physiology of Animals (Blackwell, Oxford).
- 8.Weber, R. E. & Vinogradov, S. N. (2001) Physiol. Rev. 81, 569–628. [DOI] [PubMed] [Google Scholar]
- 9.Markl, J. & Decker, H. (1992) Adv. Comp. Environ. Physiol. 13, 325–376. [Google Scholar]
- 10.van Holde, K. E. & Miller, K. I. (1995) Adv. Protein Chem. 47, 1–81. [DOI] [PubMed] [Google Scholar]
- 11.van Holde, K. E., Miller, K. I. & Decker, H. (2001) J. Biol. Chem. 276, 15563–15566. [DOI] [PubMed] [Google Scholar]
- 12.Burmester, T. (2002) J. Comp. Physiol. B 172, 95–117. [DOI] [PubMed] [Google Scholar]
- 13.Kusche, K., Ruhberg, H. & Burmester, T. (2002) Proc. Natl. Acad. Sci. USA 99, 10545–10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telfer, W. H. & Kunkel, J. G. (1991) Annu. Rev. Entomol. 36, 205–228. [DOI] [PubMed] [Google Scholar]
- 15.Burmester, T. (1999) Eur. J. Entomol. 96, 213–225. [Google Scholar]
- 16.Sánchez, D., Ganfornina, M. D., Gutiérrez, G. & Bastiani, M. J. (1998) Mol. Biol. Evol. 15, 415–426. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda, R. (1970) Mem. Entomol. Soc. Can. 76, 1–334. [Google Scholar]
- 18.Wichard, W., Arens, W. & Eisenbeis, G. (1995) Atlas zur Biologie der Wasserinsekten (Fischer, Stuttgart).
- 19.Thomas, M. A., Walsh, K. A., Wolf, M. R., McPheron B. A. & Marden, J. H. (2000) Proc. Natl. Acad. Sci. USA 97, 3178–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burmester, T. (2001) Mol. Biol. Evol. 18, 184–195. [DOI] [PubMed] [Google Scholar]
- 21.Loewe, R. (1978) J. Comp. Physiol. 128, 161–168. [Google Scholar]
- 22.Huelsenbeck J. P. & Ronquist, F. (2001) Bioinformatics 17, 754–755. [DOI] [PubMed] [Google Scholar]
- 23.Whelan, S. & Goldman, N. (2001) Mol. Biol. Evol. 18, 691–699. [DOI] [PubMed] [Google Scholar]
- 24.Hazes, B., Magnus, K. A., Bonaventura, C., Bonaventura, J., Dauter, Z., Kalk., K. H. & Hol, W. G. J. (1993) Protein Sci. 2, 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangum, C. P. (1983) in The Biology of Crustacea, eds. Bliss, D. E. & Mantel, L. H. (Academic, New York), Vol. 5, pp. 373–429. [Google Scholar]
- 26.Adams, M. D., Celniker, S. E., Holt, R. A., Evans, C. A., Gocayne, J. D., Amanatides, P. G., Scherer, S. E., Li, P. W., Hoskins, R. A., Galle, R. F., et al. (2000) Science 287, 2185–2195. [DOI] [PubMed] [Google Scholar]
- 27.Holt, R. A., Subramanian, G. M., Halpern, A., Sutton, G. G., Charlab, R., Nusskern, D. R., Wincker, P., Clark, A. G., Ribeiro, J. M., Wides, R., et al. (2002) Science 298, 129–149.12364791 [Google Scholar]
- 28.Burmester, T. & Hankeln, T. (1999) Mol. Biol. Evol. 16, 1809–1811. [DOI] [PubMed] [Google Scholar]
- 29.Hankeln, T., Jaenicke, V., Kiger, L., Dewilde, S., Ungerechts, G., Schmidt, M., Urban, J., Marden, M., Moens, L. & Burmester, T. (2002) J. Biol. Chem. 277, 29012–29017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.