Summary
The T-cell immunoglobulin domain and mucin domain (TIM) family, including TIM-1, TIM-2, TIM-3 and TIM-4, is a relatively newly described group of molecules with a conserved structure and important immunological functions, including T cell activation, induction of T-cell apoptosis and T-cell tolerance, and the clearance of apoptotic cells. TIM-1 costimulates T-cell activation and enhances cytokine production. In humans, TIM-1 also serves as a susceptibility gene for allergy and asthma. TIM-3, expressed on T cells and dendritic cells, regulates T-cell apoptosis and immune tolerance. In contrast, TIM-4, which is expressed primarily on antigen-presenting cells and which is a receptor for phosphatidylserine, regulates T-cell activation and tolerance, in part by mediating the uptake and engulfment of apoptotic cells. The TIM molecules thus have surprisingly broad activities affecting multiple aspects of immunology.
Keywords: TIM, costimulation, T cell, allergy, tolerance, autoimmunity
Introduction
The T-cell immunoglobulin domain and mucin domain (TIM) family of genes was positionally cloned in 2001 from within the Tapr (T cell and airway phenotype regulator) locus as a novel allergy and asthma susceptibility gene (1). The TIM family consists of Type I cell surface molecules with a unique structure and important broad immune functions that include the regulation of allergy and asthma, and also the regulation of autoimmunity and transplantation immunity. The TIM gene family consists of eight members (TIM-1–8) on mouse chromosome 11B1.1, and three members (TIM-1, TIM-3 and TIM-4) on human chromosome 5q33.2, a chromosomal region that has been repeatedly linked with asthma, allergy and autoimmunity (2).
TIM-1, the first family member, was initially identified in 1996 as the receptor for the hepatitis A virus (HAVCR1) in monkeys (3) and then in humans in 1998 (4). TIM-1 was subsequently identified as a kidney injury molecule (KIM-1) in 1998, using representational difference analysis of ischemic kidney cells (5). Concurrently with the positional cloning of the TIM gene family in 2001, TIM-3 was expression cloned independently using an antibody specific to T helper type 1 (Th1) cells (6). TIM-1/HAVCR1/KIM-1 is expressed on kidney epithelial cells, and along with TIM-2 and TIM-3, is also expressed on T cells. Other hematopoietic and non-hematopoietic cells can express TIM-1, TIM-2 and TIM-3, such as mast cells and hepatocytes. In contrast, TIM-4 is expressed primarily by antigen-presenting cells (APCs).
All of the TIMs possess a similar structure as Type 1 membrane proteins, consisting of an N-terminal Cys-rich immunoglobulin variable (IgV)-like domain, a mucin-like domain, a transmembrane domain, and an intracellular tail. The intracellular tails of TIM-1, TIM-2 and TIM-3, but not TIM-4, contain tyrosine phosphorylation motifs that are involved in transmembrane signaling. Within the mucin domain, TIM-1 contains 60 predicted O-linked glycosylation sites, whereas TIM-3 has only three predicted glycosylation sites. The N-terminal Cys-rich regions of the TIM homologs have 40% sequence identity, whereas sequence identity between the mouse and human orthologues is approximately 60% (7). The structural similarities between all of the TIMs suggest that they arose from an ancestral gene by successive gene duplication events.
The TIM gene family: linkage and association studies
The TIM gene family was identified using linkage analysis in a mouse model of allergy and asthma. In this analysis, asthma and allergy, which are very complex genetic traits, were reduced to a single gene trait using congenic mice. The congenic strains were generated by genetically moving discrete chromosomal segments from one mouse strain, DBA/2 (asthma resistant), into another strain, BALB/c (asthma susceptible), by repeated backcrossing (8). One congenic strain, called C.D2/Es-3/Hba, exhibited the DBA/2 phenotype (asthma resistance) and contained a chromosome 11 segment that was syntenic to human chromosome 5q23–35, a region that had been repeatedly linked to asthma and allergy in humans (9). Offspring from this strain and from crosses with BALB/c mice were used to identify the novel atopy (i.e., asthma, allergy, and eczema/atopic dermatitis) susceptibility gene locus called Tapr and within it, the TIM gene family (1).
Human TIM-1, hepatitis A virus (HAV) infection and asthma
Of the TIM genes, TIM-1 is the most polymorphic in both mice and humans, and differences in the presumed function of the distinct versions of TIM-1 drove the cloning of mouse TIM-1. In humans, TIM-1 is also extremely polymorphic, with single nucleotide polymorphisms (SNPs) as well as insertion/deletion variants in the mucin domain. Association studies were performed in sight of the significant polymorphic variants, and these studies demonstrated that certain polymorphic variants of TIM-1 were associated with protection against atopic diseases. This relationship was strongest in individuals who had prior infection with HAV (10). Identification of the association between TIM-1 and atopy, which indicated that TIM-1 was a potent asthma and allergy susceptibility gene, was remarkable for several reasons. First, the human studies suggesting the importance of TIM-1 in atopy reflected previous studies in mice that had shown the importance of TIM-1 in airway disease and Th2 responses (1). This further supported that genes identified as important in mice could guide the identification of important human genes.
Second, the relationship between TIM-1 and atopy was important, since prior epidemiological studies performed first in 1997, but repeated in different populations in subsequent years showed that infection with HAV, a ligand of TIM-1, was associated with a reduced incidence of allergy and asthma. In these studies, the prevalence of allergy and asthma, as well as peanut food allergy, was significantly lower in HAV seropositive individuals compared to that in HAV seronegative individuals (11–13). However, because HAV is not a respiratory virus, and because HAV is transmitted through fecal-oral routes, HAV infection was assumed to be merely a marker of poor hygiene and that poor hygiene was responsible for the protective effects against atopy associated with HAV infection, the ‘Hygiene Hypothesis’. Nevertheless, the observation that TIM-1 was associated with protection against atopy (10) suggested that HAV might directly affect the development of atopy by altering the function of TIM-1-expressing cells. The association between HAV, TIM-1 and protection against atopy is also remarkable, because antibody titers against very few other specific microorganisms have been associated with protection against atopy, even though the Hygiene Hypothesis predicts that infection indeed protects against atopy (14). For example, antibody titers against a few other gastrointestinal infectious organisms, such as Salmonella, Helicobacter pylori, and Toxoplasma gondii, and herpes simplex virus 1, have been inversely associated with atopy (11, 12, 15, 16), but titers to other infectious agents, e.g., microorganisms affecting the respiratory tract, have not been associated with protection against atopy. Infection with mycobacteria or Bacillus Calmette-Guérin (BCG) vaccination has been reported to protect against asthma and allergy (17), but this has been controversial.
The studies demonstrating an association between TIM-1 and atopy and that TIM-1 is an atopy susceptibility gene have been reproduced in a number of other populations, including in African-American asthmatics (18), children with atopic dermatitis in Arizona (19), and Australia (20), Koreans with asthma and atopic dermatitis (21), but not in Japanese children with asthma (22). The lack of association between TIM-1 and asthma in Japanese children may be due to a reduced incidence of HAV infection in Japan, which is now close to zero in young Japanese children. However, the precise immunological mechanisms by which HAV infection and TIM-1 alter the immune system to protect against atopy are not yet clear. The immunology of TIM-1 is only beginning to be understood, and the results so far indicate that TIM-1 potently regulates immune responses through novel mechanisms. These results support the possibility that infection with HAV, by stimulating the immune system through TIM-1, can prevent the development of atopy, at least in children with a particular variant of TIM-1. Polymorphisms in the promoter region of TIM-3 have also been associated with allergic rhinitis in a Korean population (23), although this has not been confirmed by others (18).
Human TIMs as susceptibility genes in autoimmune disease
TIM-1 has been associated not only with atopic diseases, but also with several autoimmune diseases, suggesting that TIM-1 regulates the immune system globally. For example, in rheumatoid arthritis, polymorphisms in the promoter region (24) and exon four of TIM-1 (25) have been associated with susceptibility to disease. How TIM-1 regulates autoimmune disease, or whether HAV infection is associated with protection from autoimmunity is not yet known. However, TIM-1 mRNA is expressed in cerebrospinal fluid mononuclear cells of patients with multiple sclerosis (MS), primarily in patients in remission, suggesting that TIM-1 regulates the development of MS. This regulation could perhaps occur through the development of tolerance to autoantigens (26). In one study, TIM-3 polymorphisms have been associated with susceptibility to rheumatoid arthritis (27). Furthermore, T-cell clones isolated from the cerebrospinal fluid of MS patients expressed lower levels of TIM-3 and were found to secrete higher levels of interferon-γ (IFN-γ) (26), suggesting that TIM-3 could play a role in regulating autoimmunity in humans.
Crystal structure of the TIM molecules
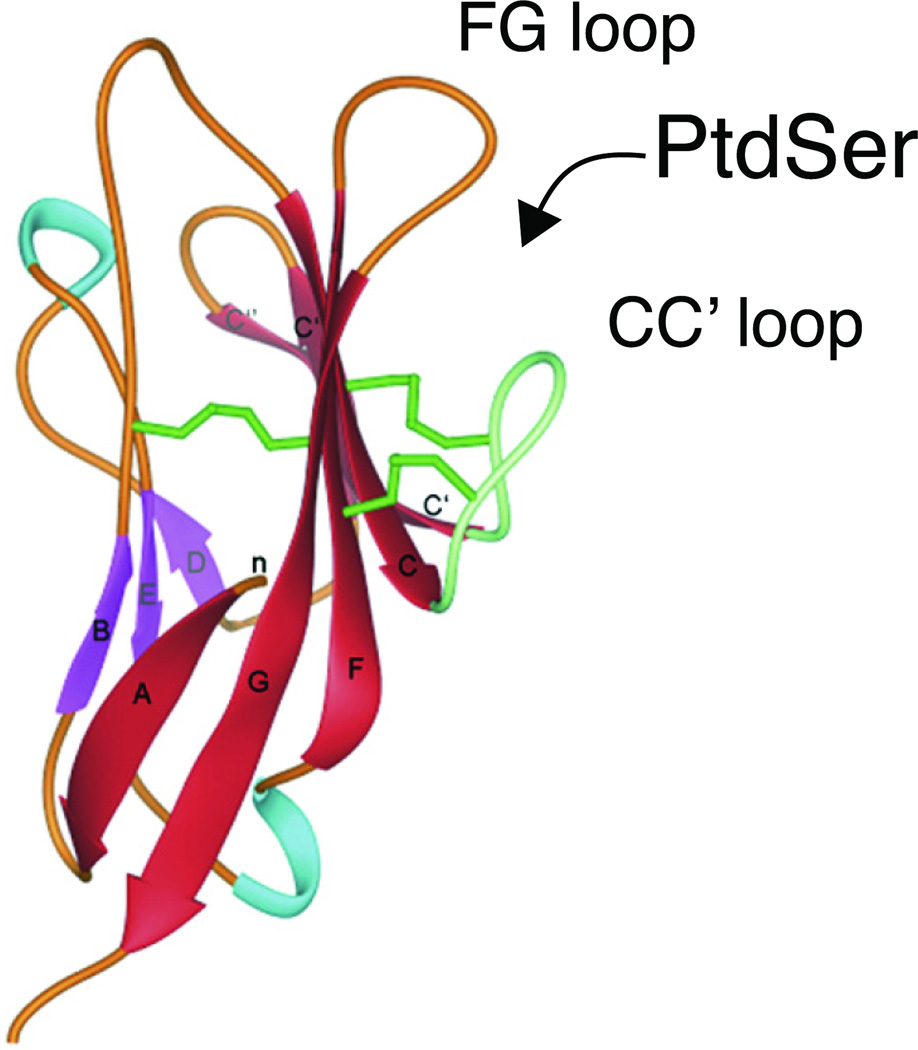
The function of the TIM molecules is being intensively investigated, but has been greatly aided by recent crystallographic studies demonstrating a conserved structural motif in the TIMs. This structural motif in the TIMs involves an IgV domain with two anti-parallel ®-sheets (Fig. 1), as in other Ig superfamily members, bridged by the first and last of six conserved Cys residues in the IgV domain. Four additional conserved Cys residues in the IgV domain link two loops, the FG loop and the CC′ loop, forming a cleft (called MILIBS, for metal-ion ligand binding site). The six conserved Cys residues in the IgV domains of all of the TIM molecules appear to provide a distinctive conserved structural feature, resulting in a MILIBS cleft in TIM-1, TIM-4 and TIM-3, but not TIM-2. The conserved structure of the IgV domain of the TIMs is important for the function of TIM-1, TIM-2, TIM-3 and TIM-4, which will be discussed below.
Fig. 1. Ribbon diagram of the mTIM-1 Ig domain.
β-sheets are in red and purple. The green sticks represent the three Cys-disulphide bonds. The FG and CC′ loops form a conserved cleft into which PtdSer fits snugly. Adapted from Santiago et al. 2007. Immunity 26:299.
TIM-1 biology and function
TIM-1 expression
In mouse, TIM-1 is expressed on activated but not naive CD4+ T cells (1, 28). Following differentiation, Th2 cells continue to express TIM-1 while Th1 and Th17 cells express little or no TIM-1 (28–30). TIM-1 is also expressed by mast cells (31) and at low levels on a subpopulation of B cells (32). TIM-1 was originally described as a renal epithelial cell protein that is upregulated and shed following injury to the kidney (5). In this venue, it plays an important role in tissue homeostasis by facilitating clearance of apoptotic and necrotic cells of the injured tubule (33).
TIM-1 as a costimulatory molecule
The in vivo function and effects of TIM-1 engagement are determined by its expression. Thus, cross-linking of TIM-1 on CD4+ T cells with an agonist mAb provided a potent costimulatory signal for T-cell activation that increased naive T-cell proliferation, and increased interleukin-4 (IL-4) production by differentiated Th2 cells (28). In vivo administration of agonist anti-TIM-1 mAb along with antigen also greatly increased antigen-specific T-cell proliferation and production of IL-4 and IFN-γ (28). TIM-1 mAb administered as adjuvant during vaccination with influenza enhanced the ensuing immune response (34), indicating that an agonistic anti-TIM-1 mAb can provide a potent adjuvant effect. Moreover, agonistic TIM-1 mAb blocked the development of respiratory tolerance (28), consistent with the idea that TIM-1 costimulation activates T cells. A recent study showed that TIM-1 costimulation prevents allogeneic transplant tolerance by reducing forkhead box p3 (Foxp3) expression and thereby preventing regulatory T-cell (Treg) development (35). Since development of respiratory tolerance to antigen is also associated with the generation of antigen-specific Tregs expressing Foxp3 (36), TIM-1 costimulation may prevent tolerance induction both by enhancing Th-cell development and hindering Treg-cell development.
Interestingly, TIM-1 mAbs recognizing distinct epitopes of TIM-1 have profoundly different effects on immunity, and the affinity with which an antibody binds to TIM-1 could affect the type of response that is induced. For example, TIM-1 mAbs recognizing exon four of the mucin domain greatly exacerbated airway inflammation and Th2-cytokine production, but another mAb reactive with the TIM-1 IgV domain blocked inflammation in a mouse model of asthma (32). Additionally, the strength of the signal provided by TIM-1 engagement, for example by distinct mAbs, may induce different outcomes. Thus, administration of the agonistic high affinity TIM-1 mAb 3B3 during the induction of autoimmunity enhanced pathogenic Th1 and Th17 responses and increased the severity of experimental autoimmune encephalomyelitis (EAE), whereas a lower affinity mAb, RMT1–10, increased Th2 responses and inhibited the development of EAE (37). Mechanistic studies revealed that while both antibodies bound to the TIM-1 IgV domain and induced CD3 capping, mAb 3B3 had a greater affinity and induced cytoskeletal reorganization (37). However, the precise events that regulate the various outcomes of TIM-1 signaling by distinct mAbs are not yet known.
TIM-1, transplant tolerance, and Tregs
The role of TIM-1 as a costimulatory molecule was further elucidated in two transplant model studies, which also noted the opposing effects of different TIM-1 mAbs on Treg generation and survival. Treatment with TIM-1 mAb RMT1–10 prolonged survival of major histocompatibility complex (MHC)-mismatched mouse cardiac allografts. This prolongation was associated with inhibition of alloreactive Th1 responses, a Th1- to Th2- type cytokine switch, and preservation of CD4+CD25+ Tregs (38). In contrast, in vitro treatment of alloreactive T cells with the agonistic TIM-1 mAb 3B3 enhanced the expansion, commitment, and survival of IL-17- and IFN-γ-producing cells. Interestingly, treatment with TIM-1 mAb 3B3 also appeared to deprogram and inhibit Tregs (35). Thus, TIM-1 modulates T-cell responses, but the avidity of TIM-1 crosslinking can determine the profile of T-cell proliferation and cytokine production.
TIM-1 in signal transduction
Recent studies have begun to identify the signaling pathways triggered downstream of TIM-1 cross-linking. Reporter assays showed that TIM-1 overexpression resulted in increased transcription from the IL-4 promoter and NFAT/AP-1 transcriptional activation (39), and this was dependent on the presence of Y276 in the TIM-1 cytoplasmic tail. Studies utilizing human Jurkat T cells that expressed TIM-1 revealed that TIM-1 colocalizes on the T-cell surface with CD3, and is recruited to the T-cell receptor (TCR)-signaling complex (40). This study also showed that engagement of TIM-1 with agonistic TIM-1 mAbs led to rapid tyrosine phosphorylation of TIM-1, phosphorylation of zeta-chain-associated protein kinase 70 (Zap-70) and IL-2-inducible T-cell kinase (ITK), and recruitment of an ITK and phosphoinositide-3 kinase (PI3K) complex to the TCR-signaling complex (40). Additional insight was provided by a study that showed that the p85 subunit of PI3K is recruited directly to tyrosine 276 of TIM-1 after lymphocyte-specific protein tyrosine kinase (Lck)-dependent phosphorylation of the cytoplasmic tail (41). Another study using a TIM-4-Ig fusion protein showed that activation of T cells with TIM-4-Ig, anti-CD3, and anti-CD28 leads to phosphorylation of TIM-1, phosphorylation of thymoma viral proto-oncogene 1 (Akt1) and mitogen-activated protein kinase 1/2 (Erk1/2), and induction of B-cell chronic lymphocytic leukemia/lymphoma 2 (Bcl-2), suggesting that TIM-4 treatment can promote T-cell survival (42).
TIM-1 ligands
Several ligands for TIM-1 have been identified including TIM-4 (29), TIM-1 itself (7), IgA⌊ (43) and phosphatidylserine (PtdSer) (44–46). The structure of TIM-1 includes a glycosylated mucin domain, which imparts a degree of promiscuity to the TIM-1 molecule. This may explain the identification of multiple ligands, and has made it difficult to determine which is the primary functional ligand of TIM-1. TIM-4 was identified as a ligand of TIM-1 since TIM-1-Ig and TIM-1 tetramer bound to Chinese-hamster ovary (CHO) cells transfected with TIM-4 (29), and this interaction could be specifically inhibited by TIM-1 mAb. Support for the importance of the TIM-1-TIM-4 interaction came in the discovery that administration of TIM-4-Ig in vivo resulted in hyperproliferation of T cells and enhancement of T-cell cytokine production (29), similar to that observed with administration of the agonistic TIM-1 mAb 3B3 (28). The interaction between TIM-1 and TIM-4 was confirmed in a study that showed that TIM-1 tetramer bound to cells expressing full-length TIM-4. In addition, this study noted that homotypic TIM-1-TIM-1 binding could occur since TIM-1-Ig and TIM-1 tetramers bound to cells expressing TIM-1. These intrafamilial interactions of TIM family Ig domains with surface-expressed TIM-1 or TIM-4 was dependent on the presence of an intact TIM-1 or TIM-4 glycosylated mucin stalk, although the mucin stalk alone was not sufficient for TIM binding (47). There is also evidence that some TIM-1-TIM-1 or TIM-1-TIM-4 interactions could occur via a ‘bridge’ with two TIM proteins binding to a membrane fragment or exosome expressing exposed PtdSer. Observation by electron microscopy of TIM-1 and TIM-4 transfected cell lines indicated the presence of exosome-like vesicles bound to the cells (45).
TIM-1 crystal structure and phosphatidylserine
Elucidation of the crystal structure of TIM-1 in 2007 provided further insight into these observations. It was shown that TIM-1 binds to PtdSer, a membrane phospholipid expressed on the surface of apoptotic cells, as demonstrated by both crystallographic and biochemical studies (44–46). TIM-1 specifically recognizes PtdSer and not other phospholipids. The crystal structure analysis of TIM family members showed that the IgV domain of TIM-1 and TIM-4 display a distinctive cleft, termed the MILIBS, formed by the CC′ and FG loops of the GFC β sheet. Mouse TIM-1 and TIM-4 share high sequence similarity in the FG and CC′ loops that build the MILIBS, and they bound similarly to PtdSer (46). Importantly, PtdSer fits snugly in this cleft and coordinates with a metal ion bound to conserved residues in the TIM proteins. HAV also binds to human TIM-1 at this cleft region, with Ser37 in the CC′ loop possibly the critical virus-binding residue (7). Studies demonstrated a potentially important role of the MILIBS and its occupancy by PtdSer in the trafficking of TIM-1 to the cell surface, and in the conformation of the TIM-1 molecule displayed on the cell surface (46).
The crystal structure of TIM-1 also identified a homophilic TIM-1-TIM-1 interaction. The mouse TIM-1 IgV domain interactions observed suggested an intermolecular interaction occurring between two cells expressing TIM-1 on the cell surface as the TIM-1 domains were related by a rotation angle of about 180° and had their C-terminal ends extending in opposite directions. The crystal structure of the homophilic TIM-1 receptor interaction showed that the TIM-1 IgV domains contact through their BED faces, opposite from the FG-CC′ loop epitope that binds PtdSer (7).
TIM-1 mediates uptake of apoptotic cells through recognition of PtdSer
The lipid PtdSer is normally localized to the inner leaflet of the plasma membrane, but it is redistributed or exposed on the outer membrane when the cell undergoes apoptosis. Recognition of PtdSer provides a key signal to the phagocyte that triggers engulfment of the apoptotic cell (48, 49). Apoptotic cell death is a critical and evolutionally conserved process for elimination of unnecessary cells (50, 51). This process is essential for maintenance of tissue homeostasis and self-tolerance (52) since clearance of apoptotic cells by phagocytosis can result in powerful anti-inflammatory and immunosuppressive effects (53). Transfection of human TIM-1 into NIH 3T3 cells greatly augmented their capacity to engulf apoptotic cells, and this uptake was blocked by specific anti-TIM-1 mAb (44). The human kidney cell line 769P, which shows natural cell surface expression of TIM-1, was shown to phagocytose apoptotic cells through TIM-1 (44). Two other studies demonstrated that TIM-1 binds PtdSer and mediates uptake of apoptotic cells (33, 45). In one of these reports, TIM-1 was shown to transform kidney epithelial cells into semiprofessional phagocytes that engulfed apoptotic cells of the injured kidney tubule. This process was mediated through recognition by TIM-1 of PtdSer on the apoptotic cell (33).
Changing partners: TIM-1 consorting with multiple ligands
Further investigation is needed to determine the roles of the various ligands that have been described for TIM-1. The relative importance of each ligand may be determined by the cell type expressing TIM-1. Therefore, recognition of PtdSer and uptake of apoptotic cells by a TIM-1-expressing kidney epithelial cell could play a significant role in maintenance of tissue homeostasis in the injured kidney. The most ‘functional’ ligand for a T cell expressing TIM-1 is more difficult to predict. TIM-1-expressing T cells avidly bind apoptotic cells (unpublished data), and this suggests that crosslinking TIM-1 on T cells by apoptotic-cell binding may modulate the immune response downstream of TIM-1. Thus, TIM-1 on the T cell could function to sense death in its environment and stimulate T-cell expansion. Interaction with TIM-1 expressed on other T cells or TIM-4 on APC could enhance T-cell expansion and survival.
TIM-3: biology and function
TIM-3 expression
Attempts to distinguish Th1 and Th2 cells based on cell surface expression profiles led to the identification of TIM-3 as a surface molecule expressed on Th1 cells but not found on Th2 cells (6, 54, 55). Recent findings, however, demonstrate that TIM-3 may also be expressed on a newly described proinflammatory T cell subset called Th17 cells (30). Galectin-9 was identified as the ligand for TIM-3, and an interaction between TIM-3 and galectin-9 was shown to induce cell death in Th1 cells (56). These data together with in vivo blocking studies suggest that TIM-3 may be an inhibitory molecule that terminates Th1 immunity.
Later it was shown that TIM-3 expression is not limited to cells of the adaptive immune response. Subsets of cells in the innate immune system also express TIM-3, including human natural killer cells, monocytes (26), and dendritic cells (57). Mast cells, macrophages (58), and dendritic cells (59) in mice, were also shown to constitutively express TIM-3. In addition to full-length, cell surface-expressed TIM-3, an alternate splice form was found in mice that lacks the mucin and transmembrane domains and is therefore thought to be a soluble form of TIM-3 (54, 60). Little is known about how this alternate TIM-3 splice form influences immune responses, but the presence of another soluble TIM molecule, metalloproteinase-cleaved TIM-1 (61), suggests that these alternate spliced or cleaved forms of TIM proteins could have relevant biological roles.
Tim-3 ligand
Galectin-9, a member of the S-type lectins (62) was identified as a ligand for TIM-3 by immunoprecipitation of cell surface proteins that bound to TIM-3-Ig. Galectin-9 is upregulated by IFNγ (63), and upon interaction with TIM-3 induces cell death in Th1 cells (56). Galectin-9 can be potentially expressed on almost all cells, but in the unactivated immune system, Galectin-9 is predominantly expressed on naive CD4+ T effector and CD4+CD25+ Treg cells. Upon activation, galectin-9 is downregulated on the effector T-cell population, but is maintained on Tregs (54, 55). Sustained expression of TIM-3L on Tregs could suggest that the TIM-3/TIM-3L pathway is utilized by Tregs to mediate suppression by inhibiting effector T cells expressing TIM-3. In addition to attenuating Th1 responses, the TIM-3 ligand galectin-9 inhibits the differentiation of naive T cells to IL-17-producing Th17 cells in vitro, and when given in vivo, can ameliorate a mouse model of arthritis (64). These findings point to a broader role for the galectin-9/TIM-3 pathway in downmodulating inflammatory responses that could involve both the Th1 and Th17 branches of adaptive immunity.
The cell death induced by galectin-9 is a mixture of both apoptotic and necrotic events (56), and although it has been shown that galectin-9 can induce tyrosine phosphorylation of TIM-3 (65), little else is known about the signaling pathways that mediate this functional outcome. Galectin-9 binds to TIM-3 in a glycosylation dependent manner (56) by forming a β-glycosidase bond, and the observation that nonglycosylated TIM-3-Ig binds to various cell types suggests that TIM-3 may have additional ligands (66). The nature of this additional binding partner remains unknown, but it is thought to be highly conserved across species (66). More knowledge about TIM-3 ligands is required in order to fully understand the biological functions of this molecule in health and disease.
TIM-3 in regulatory immune responses
The pattern and kinetics of TIM-3 expression on terminally differentiated Th1 cells, and the ability of galectin-9 to specifically downregulate this population generated interest in the potential involvement of the TIM-3/galectin-9 pathway in autoimmune disease associated with Th1 cells. To this end, blocking TIM-3 by administering anti-TIM-3 antibody during the induction of EAE, a mouse model for human MS, worsened disease progression. In this study, blocking TIM-3 resulted in a severe form of demyelinating disease characterized by higher numbers of inflammatory foci in the central nervous system (CNS). Upon examination of the CNS of treated mice, it was found that anti-TIM-3 led to higher numbers of activated macrophages in demyelinating lesions, concurrent with the expansion of this population in the periphery (6). Conversely, in vivo treatment with galectin-9 could attenuate Th1 responses, whereas in vivo ablation of galectin-9 expression resulted in exacerbation of EAE (56).
TIM-3 in humans
In human autoimmune disease, analysis of TIM-3 expression and cytokine production by T cell clones isolated from cerebrospinal fluid showed higher levels of secreted IFN-γ with concomitant decrease in TIM-3 expression in T cell clones from MS patients compared to the healthy controls (67). TIM-3 expression on T cells was restored in MS patients upon in vivo therapy with glatiramer acetate, implicating TIM-3 in the pathogenesis of human autoimmune diseases involving dysregulated Th1 immune responses. Dysregulated TIM-3 expression might also be associated with systemic lupus erythematosus (SLE) (68), although a role for TIM-3 in the pathogenesis of SLE has not yet been defined. New data have also implicated TIM-3 in functionally impaired T-cell responses to human immunodeficiency virus type 1 (HIV-1) infection (69). In this study, it was found that PBMC from acute/early and chronic progressive HIV-1-infected patients had higher frequencies of TIM-3+CD8+ T cells. When compared to the TIM-3− fraction of CD8+ PBMC, TIM-3lo and TIM-3hi CD8 cells failed to make IFN-γ in response to viral antigen. Polyclonal stimulation of TIM-3+CD8+ PBMCs could not drive cell division in this population, whereas cell division in the TIM-3−/lo population was observed, thus suggesting that TIM-3 may be another molecule that is involved in T cell ‘exhaustion’ in chronic viral infections. The inability of TIM-3+ cells to either proliferate or produce IFN-γ was reversed upon treatment with either soluble TIM-3 or anti-TIM-3 mAb (69). Taken together, these observations support the inhibitory nature of galectin-9-mediated immunoregulation and strengthen the potential for therapeutic targeting of the TIM-3 pathway in infectious and autoimmune diseases.
TIM-3 in tolerance
Besides inducing cell death of Th1 cells, the TIM-3/TIM-3 ligand interaction has also been implicated in establishing tolerance. Tolerance to allogenic grafts can be achieved by costimulation blockade and donor-specific transfusion (DST) (55). By blocking the CD40/CD40L costimulatory pathway at the same time as administering DST, it is possible to induce indefinite tolerance to MHC mismatched transplanted islet allografts, an effect due to enhanced donor-specific Treg suppression. Blocking TIM-3-mediated immunoregulation by administering full-length TIM-3-Ig compromised the generation of functional, donor-specific Tregs, and prevented the induction of tolerance to transplanted islets. More striking however was the finding that TIM-3-deficient BALB/c mice could not be tolerized by DST and CD40/CD40L blockade, even though BALB/c mice are biased towards generating Th2 immune responses (55). Peripheral tolerance induced with high-dose aqueous antigen causes the hypoproliferation of antigen-specific T cells and abrogates IL-2 production. However, blocking the TIM-3 pathway using TIM-3-Ig during tolerance induction was sufficient to prevent tolerance and led to increased proliferation and cytokine production. Moreover, TIM-3-deficient mice could not be tolerized by treating with high-dose aqueous antigen (54), further implicating TIM-3 in regulating T-cell responses. These data strongly suggested that TIM-3 negatively regulates T-cell responses by directly inducing deletion of effector Th1 cells, but also by inducing tolerance. Whether these two phenomena are related has not been addressed.
TIM-3 in innate immunity
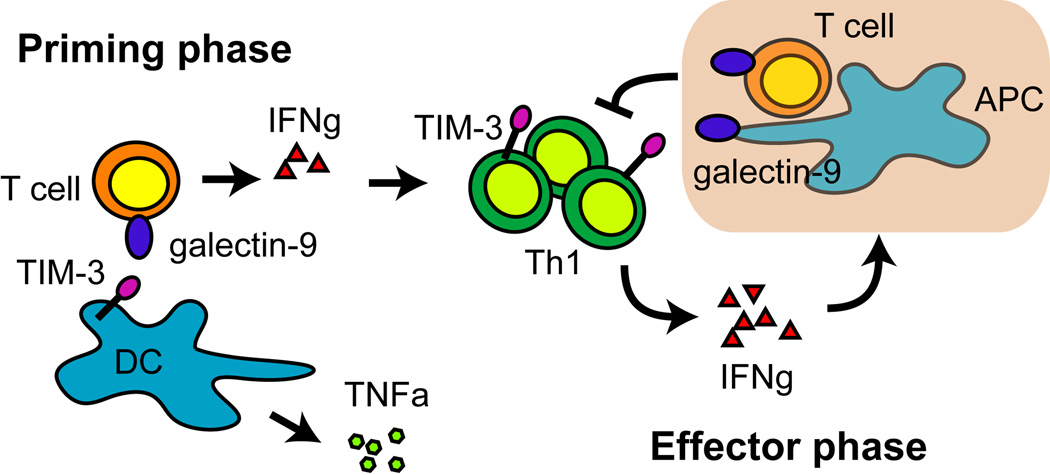
Whereas the functional consequences of TIM-3 expression in adaptive immunity appear to involve downregulation of T-cell responses, TIM-3 expression on innate immune cells appears to play a more complex role in innate immunity and inflammation. TIM-3 is rapidly expressed on mast cells and macrophages obtained from the peritoneum, spleen, and heart of virally infected mice, suggesting that TIM-3 could be involved in the earlier stages of an immune response (58). In a virally induced model of inflammatory heart disease, blocking TIM-3 at the time of viral infection was sufficient to increase inflammation in the heart. Although the mechanism responsible for this observation remains unclear, it involves downregulation of CD80 on mast cells and macrophages (58). Interestingly, male mice develop more severe inflammation, which is concurrent with fewer TIM-3+ cell infiltrates in the heart, a decrease in IL-4 production, and an increase in the levels of IFN-γ (70). These observations suggest that blocking TIM-3 could either directly affect cells of the innate immune system, or could indirectly alter the inflammatory response by shaping adaptive immunity. Furthermore, crosslinking TIM-3 on the surface of mast cells increases IL-4, IL-6, and IL-13 production (31), which then indirectly modulate T-cell responses. However, there is also evidence that supports a role for TIM-3 in directly activating innate immunity. TIM-3-deficient dendritic cells do not efficiently produce inflammatory cytokines after stimulation with LPS and the TIM-3 ligand galectin-9 (57). Similar to findings in mice, human dendritic cells and monocytes also express TIM-3, and blocking TIM-3 on the surface of monocytes inhibits tumor necrosis factor α (TNFα)-production following galectin-9 stimulation (57). These data suggest that TIM-3 plays a complex role in regulating both innate and adaptive immunity. It is possible that TIM-3 expressed on dendritic cells and macrophages synergizes with TLR signals that would lead to the activation of these APCs to produce proinflammatory cytokines. In turn, activated APCs could drive Th1-cell responses that, once established, would result in the upregulation of TIM-3 on Th1 cells. IFN-γ-produced by these Th1 cells would induce galectin-9 and lead to the eventual termination of the Th1 response. Expression and ligation of TIM-3 on mast cells could result in further attenuation of Th1 responses by the production of Th2 cytokines (Fig. 2).
Fig. 2. TIM-3 engaged on APC during the priming phase stimulates the innate immune system to produce inflammatory cytokines that drive T effector responses.
In the effector phase, Th1 cells upregulate TIM-3 and produce IFN-γ, which drives galection-9 expression. Galectin-9 induces the termination of Th1 immune responses through TIM-3.
TIM-4: biology and function
TIM-4 expression
TIM-4 is another TIM molecule conserved in both humans and mice and is similar to all TIM proteins in that it contains an extracellular IgV domain, a glycosylated mucin domain, and an intracellular tail. Unlike other members of the TIM family, however, TIM-4 is not expressed in T cells, but is instead exclusively expressed in APC. Although TIM-4 transcripts were detected in spleen and lymph node, and to a lesser degree in lung, liver, and thymus, further studies revealed that expression is restricted to dendritic cells and macrophages (29), and in particular to macrophages in the splenic marginal zone (71, 72). In addition to its unique expression pattern, TIM-4 differs from other TIM proteins in that it contains an Arg-Gly-Asp (RGD) motif in its IgV domain, which is a hallmark for adhesive proteins. This structural motif could potentially mediate adhesion and migration of TIM-4-expresing cells, although this possibility has not been investigated. Lastly, TIM-4 does not contain any predicted signaling motifs in its intracellular tail, unlike other TIM proteins, which have intracellular tyrosine phosphorylation motifs, hence raising the question of whether TIM-4 can directly mediate transmembrane signaling.
TIM-4 as a T-cell costimulatory molecule
Given that other members of the TIM family of proteins regulate T-cell immune responses, studies were undertaken to determine whether TIM-4 could similarly regulate immunity. Administration of TIM-4-Ig fusion protein during in vivo T-cell priming increased basal T-cell proliferation and enhanced production of T cell IL-2 and IFN-γ (29). These results could be due to blocking of an endogenous TIM-4/TIM-4L interaction, or by crosslinking a TIM-4 ligand. To identify potential binding partners, TIM-4-Ig fusion protein was used and found to bind to unstimulated splenic B cells, as well as activated B cells and T cells (29). The kinetics of TIM-4L expression on T cells were similar to those seen with TIM-1 expression on activated T cells. This observation, together with the capacity of heterotypic binding within the TIM family, led to the discovery that TIM-4 binds to TIM-1 (29). In order to further understand the role of a TIM-4/ TIM-1 interaction in T-cell immune responses, additional studies were undertaken in vitro using a system devoid of APCs, and therefore lacking any endogenous TIM-4/TIM-1 engagements. The data suggest that at low concentrations, TIM-4 inhibits T-cell responses, but at high concentrations, TIM-4 mediates positive costimulation (29). The ability of TIM-4 to costimulate T-cell responses was characterized biochemically in that TIM-4 crosslinking of its receptors on T cells induced cell division and increased cell survival, along with increased phosphorylation of key signaling molecules linker for activation of T cells (Lat), Akt1 and Erk1/2. It was also shown that TIM-4 could induce the phosphorylation of endogenously expressed TIM-1 (42).
However, there is also evidence that TIM-4 can bind to naive T cells, which do not express TIM-1, further supporting the existence of another ligand for TIM-4 (71). These studies went on to show that when added to naive T cell and dendritic cell cocultures, anti-TIM-4 mAb could costimulate antigen-specific T-cell proliferation. Though, when the anti-TIM-4 was given in vivo during the induction phase of an immune response, both stimulation and inhibition of the ensuing response was observed (71). Specifically, in a model for contact hypersensitivity, anti-TIM-4 mAb administered at the time of sensitization resulted in increased T-cell reactivity seen after challenge of these cells in vitro. However, if given after elicitation of the delayed-type hypersensitivity (DTH) response in vivo, the antibody resulted in decreased DTH response and inflammation. In the same study, when administered during the induction of EAE, anti-TIM-4 caused attenuated disease progression, even though antigen-specific T-cell priming was enhanced in vitro (71). Therefore, it seems that TIM-4 can inhibit naive T cells, but once established, TIM-4 might play a role in sustaining the ongoing immune response. These data substantiate the idea that TIM-4 regulates T-cell responses in a bimodal fashion, possibly through multiple receptors on the surface of naive and activated T cells. It is possible that naive T cells that do not express TIM-1, engage TIM-4 on APCs using an as of yet unidentified receptor, which then mediates inhibitory signals downregulating T-cells responses. In the case of activated T cells that have upregulated TIM-1, TIM-4 could engage TIM-1 and costimulate T-cell expansion. Further studies using TIM-deficient animals are needed in order to better understand the role TIM-4 plays in vivo during the initiation and expansion of T-cell responses.
TIM-4 as a phosphatidylserine receptor
In searching for TIM-4 ligands, TIM-4-Ig fusion proteins were also found to bind to various long-term cultured cell lines, and in particular to cells with high scatter on flow cytometry, which usually include dying cells. The high scatter, TIM-4-Ig staining cells also stained with Annexin V and propidium iodide, indicating that the cells that bound TIM-4-Ig were indeed apoptotic cells (44). TIM-4-Ig was also found to bind to plates coated with PtdSer in a solid-phase enzyme-linked immunosorbent assay (ELISA) (44). TIM-1-Ig fusion proteins also bound PtdSer, and the binding of TIM-4-Ig and TIM-1-Ig fusion proteins was specific for PtdSer, since neither fusion protein bound to phosphatidylinositol (PI), phosphatidylcholine (PC), or phosphatidylethanolamine (PE). TIM-2-Ig fusion protein did not bind PtdSer (44).
These results strongly suggested that TIM-4 and TIM-1, but not TIM-2, specifically recognized PtdSer, a phospholipid that is expressed by dying cells. PtdSer is a canonical ‘eat me’ signal that can trigger the engulfment of apoptotic cells, suggesting that TIM-4 and TIM-1 might mediate the engulfment of apoptotic cells. Indeed, NIH 3T3 cells transfected with TIM-1 or TIM-4 quickly engulfed apoptotic U937 or PKH67 apoptotic cells, but not live cells, as assessed by flow cytometry, confocal microscopy or electron microscopy of fluorescently labeled cells (44). Phagocytosis of apoptotic cells by TIM-1 or TIM-4 transfected cells was inhibited by anti-TIM-1 or anti-TIM-4 mAbs, respectively, or by liposomes containing PtdSer but not liposomes containing PC alone (44). Moreover, as discussed above, TIM-4 is expressed exclusively by APCs, such as macrophages and DCs, including human tingible-body macrophages located in germinal centers of tonsil or white pulp of spleen (44). Tingible-body macrophages are cells in lymphoid tissue that engulf apoptotic cells. Namely, the tingible bodies within these cells are remnants of phagocytosed cells. These observations suggest that TIM-4-expressing macrophages recognize apoptotic cells by recognizing PtdSer, resulting in engulfment and clearance of the apoptotic cell.
The observation that TIM-4 binds PtdSer and mediates engulfment of apoptotic cells was independently discovered by another group of investigators, using an antibody that blocked PtdSer-dependent engulfment of apoptotic cells. These investigators, using expression cloning, identified the target of the antibody as TIM-4 (45). Administration of the anti-TIM-4 mAb to mice blocked engulfment of apoptotic cells by thymic macrophages and resulted in the development of autoantibodies. This observation was consistent with the idea that TIM-4 controlled the development of tolerance by mediating the clearance of apoptotic cells. Furthermore, these investigators found that intercellular bridging could occur among TIM-4 or TIM-1-expressing cells via exosomes expressing PtdSer. Exosomes are 50–90 nm vesicles usually created within endosomes by the invagination of membrane into the endosomal lumen. The endosomes that contain the intraluminal vesicles (precursors to exosomes) can fuse with the plasma membrane leading to release of the vesicles into the extracellular space. The vesicles, which are now termed exosomes, contain ligands from the cell membrane, including PtdSer. Electron microscopy studies demonstrated that exosomes can be present at the interface between TIM-1 expressing cells, and TIM-1- or TIM-4-expressing cells, suggesting that the PtdSer expressing exosomes can mediate interactions between TIM-1 and TIM-4. These observations suggest that TIM-1 and TIM-4 may also be involved in intercellular signaling via exosomes.
Crystal structure of TIM-4 and TIM-1: cocrystallization with PtdSer
The binding of PtdSer to TIM-4 and TIM-1 was confirmed by cocrystallization studies of TIM-4 and TIM-1 (46). In these studies, the crystal structure of TIM-4 identified a MILIBS in the Ig domain, created by the FG and CC′ loops (Fig. 1). PtdSer co-crystallized with TIM-4, with the hydrophilic head of PtdSer penetrating into the MILIBS of TIM-4 and coordinating with the metal ion. The PtdSer fatty acid chains were found to interact with aromatic residues of the TIM-4 FG loop. The binding of PtdSer to TIM-1 and TIM-4 and of apoptotic cells to cells expressing TIM-1 and TIM-4 depended on the localization of PtdSer into the cleft, since mutations in TIM-1 and TIM-4 in or near the MILIBS cavity prevented the engulfment of apoptotic cells by cells expressing the mutant TIM-4 and TIM-1 (44).
The precise role of TIM-4 in immune responses clearly appears to be complex. There may be several roles depending on what TIM-4 binds, and the effect may depend on the level of expression of TIM-4, and in which tissues and on what cell types TIM-4 is being expressed. It is clear, however, that TIM-4 is an important molecule that can both influence immunity directly by regulating T-cell responses, and also indirectly maintain tolerance to self by clearing dying cells.
TIM-2: biology and function
TIM-2 expression
TIM-2, unlike the other TIM molecules discussed in this review, does not have a structural orthologue in humans. TIM-2 is most homologous with mouse TIM-1, and similar to TIM-1, expression of TIM-2 is upregulated on activated T cells (73), but is more exclusively maintained in differentiated Th2 cells (74). Expression is also seen on B cells and on epithelial cells of the liver and kidney (75), although possible functions for TIM-2 in these tissues has not been investigated.
TIM-2 ligands
TIM-2 was first identified as binding to the class IV semaphorin, Sema4A, a transmembrane protein expressed in dendritic cells and B cells (73). In a separate study, using TIM-2-Ig to detect TIM-2 ligands, activated APCs were found to express a binding partner for TIM-2, while no expression was seen on T cells (74). This binding pattern was concordant with the expression of Sema4A. Further studies revealed that H-ferritin also binds to TIM-2 (75). H-ferritin is a component of the spherical protein complex ferritin involved in regulating iron mineralization and sequestration, and is secreted by macrophage cell lines (75).
TIM-2 as a Th2 regulator
The expression kinetics of TIM-2 on immune cell subsets suggested that this molecule could have a role in regulating immune responses. When administered in vivo, TIM-2-Ig fusion protein induced high basal proliferation of splenocytes, concomitant with elevated levels of IL-2, IL-4, and IL-10. This apparent induction of a Th2 immune response could explain why TIM-2-administration, either during or right before disease onset in EAE, was sufficient to ameliorate disease progression (74). When Sema4A-Ig fusion protein was administered during the induction of EAE, disease was also decreased (73). These observations, along with the finding that Sema4A-deficient mice have impaired Th1 responses (76), further implied that TIM-2 could serve to downregulate established Th2 responses. To this effect, no differences were observed when TIM-2-deficient T cells were activated with polyclonal activators (77), but upon immunization, draining lymph node CD4+ T cells from TIM-2-deficient mice proliferated more than wildtpe cells in response to antigen (77). Additional data support a role for TIM-2 in the expansion or survival of an established Th2 immune response, since late administration of TIM-2-Ig was sufficient to exacerbate lung inflammation. Furthermore, TIM-2-deficient mice were found to have worse lung inflammation (77). These findings are strengthened by biochemical analyses that show ectopic expression of TIM-2 sufficiently impairs the induction of NFAT/AP-1 transcriptional reporters (78) suggesting that TIM-2 induces inhibitory signals.
Interestingly, structural analyses of TIM-2 suggest that TIM-2 exists as a dimer, possibly preventing binding to other TIM molecules, but potentially facilitating binding of multivalent ligands (7). Ferritin could represent such a ligand. Circulating ferritin has been shown to inhibit T-cell proliferation (75), but the effects H-ferritin might have on TIM-2-expressing cells has not been investigated. In summary, TIM-2 expressed on Th2 cells can function as a negative feedback loop that ensures downmodulation of ensuing Th2-immune responses.
Conclusions
The TIM family, discovered eight years ago using a genetic approach, represents a unique family of genes with surprising functions. All of the TIM family members have a conserved structure, and together the TIM molecules affect many aspects of immunology, including T-cell activation, cell survival, and death, as well as the capacity of APCs to clear apoptotic cells. TIM-1, TIM-2 and TIM-3 are expressed primarily on T cells, and as such, affect adaptive immunity by altering the activation and survival of CD4+ T cells. TIM-1 serves as a costimulatory molecule for T cells, particularly on Th2 cells, enhancing T cell proliferation and cytokine production. In contrast, TIM-2 provides an inhibitory signal primarily on Th2 cells, while TIM-3 costimulation of Th1 cells causes T-cell apoptosis. However, the realm of the TIMs goes beyond the regulation of Th1/Th2-cell differentiation, as initial studies suggested. Rather, the TIM molecules very broadly affect immunity, and other immune cell types. TIM-4, expressed by APCs, regulates immunity through several different pathways; including the clearance of PtdSer-expressing apoptotic cells or by activating TIM-1 expressing T cells. While TIM-1 is an atopy susceptibility gene that interacts with the environment, the activation/regulatory pathways of the TIMs can affect not only asthma and allergy, but also autoimmunity, transplantation, and the development of tolerance. As an increasing number of investigators from around the world focus on study of the TIM molecules, on the ligands of the TIMs, how these ligands, including HAV, activate T cells and how the TIMs clear apoptotic cells, it is likely that additional surprising functions of the TIM molecules will be uncovered in the future, which should provide us with a greater understanding of T-cell activation and costimulation.
Acknowledgements
This was supported by research funded by NIH grant awards.
References
- 1.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 2.McIntire J, Umetsu D, DeKruyff R. TIM-1, a novel allergy and asthma susceptibility gene. Springer Seminars in Immunopathology. 2004;25:335–348. doi: 10.1007/s00281-003-0141-3. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan G, Totsuka A, Thompson P, Akatsuka T, Moritsugu Y, Feinstone SM. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996;15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 4.Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan GG. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J Virol. 1998;72:6621–6628. doi: 10.1128/jvi.72.8.6621-6628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 6.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 7.Santiago C, Ballesteros A, Tami C, Martinez-Munoz L, Kaplan GG, Casasnovas JM. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruscetti S, Matthai R, Potter M. Susceptibility of BALB/c mice carrying various DBA/2 genes to development of Friend murine leukemia virus-induced erythroleukemia. J Exp Med. 1985;162:1579–1587. doi: 10.1084/jem.162.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh DG, Neely JD, Breazeale DR, Ghosh B, Freidhoff LR, Ehrlich-Kautzky E, Schou C, et al. Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science. 1994;264:1152–1156. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- 10.McIntire JJ, Umetsu SE, Macaubas C, Hoyte EG, Cinnioglu C, Cavalli-Sforza LL, Barsh GS, et al. Hepatitis A virus link to atopic disease. Nature. 2003;425:576–576. doi: 10.1038/425576a. [DOI] [PubMed] [Google Scholar]
- 11.Linneberg A, Ostergaard C, Tvede M, Andersen LP, Nielsen NH, Madsen F, Frolund L, et al. IgG antibodies against microorganisms and atopic disease in Danish adults: the Copenhagen Allergy Study. J Allergy Clin Immunol. 2003;111:847–853. doi: 10.1067/mai.2003.1335. [DOI] [PubMed] [Google Scholar]
- 12.Matricardi PM, Rosmini F, Riondino S, Fortini M, Ferrigno L, Rapicetta M, Bonini S. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ. 2000;320:412–417. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matricardi PM, Rosmini F, Ferrigno L, Nisini R, Rapicetta M, Chionne P, Stroffolini T, et al. Cross sectional retrospective study of prevalence of atopy among Italian military students with antibodies against hepatitis A virus. BMJ. 1997;314:999–1003. doi: 10.1136/bmj.314.7086.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 15.Illi S, von Mutius E, Lau S, Bergmann R, Niggemann B, Sommerfeld C, Wahn U. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ. 2001;322:390–395. doi: 10.1136/bmj.322.7283.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosunen TU, Hook-Nikanne J, Salomaa A, Sarna S, Aromaa A, Haahtela T. Increase of allergen-specific immunoglobulin E antibodies from 1973 to 1994 in a Finnish population and a possible relationship to Helicobacter pylori infections. Clin Exp Allergy. 2002;32:373–378. doi: 10.1046/j.1365-2222.2002.01330.x. [DOI] [PubMed] [Google Scholar]
- 17.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 18.Gao PS, Mathias RA, Plunkett B, Togias A, Barnes KC, Beaty TH, Huang SK. Genetic variants of the T-cell immunoglobulin mucin 1 but not the T-cell immunoglobulin mucin 3 gene are associated with asthma in an African American population. J Allergy Clin Immunol. 2005;115:982–988. doi: 10.1016/j.jaci.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Graves PE, Siroux V, Guerra S, Klimecki WT, Martinez FD. Association of atopy and eczema with polymorphisms in T-cell immunoglobulin domain and mucin domain-IL-2-inducible T-cell kinase gene cluster in chromosome 5 q 33. J Allergy Clin Immunol. 2005;116:650–656. doi: 10.1016/j.jaci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Page NS, Jones G, Stewart GJ. Genetic association studies between the T cell immunoglobulin mucin (TIM) gene locus and childhood atopic dermatitis. Int Arch Allergy Immunol. 2006;141:331–336. doi: 10.1159/000095459. [DOI] [PubMed] [Google Scholar]
- 21.Chae SC, Song JH, Lee YC, Kim JW, Chung HT. The association of the exon 4 variations of Tim-1 gene with allergic diseases in a Korean population. Biochem Biophys Res Commun. 2003;312:346–350. doi: 10.1016/j.bbrc.2003.10.125. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi E, Nakayama J, Kamioka M, Ichikawa K, Shibasaki M, Arinami T. Insertion/deletion coding polymorphisms in hHAVcr-1 are not associated with atopic asthma in the Japanese population. Genes Immun. 2003;4:170–173. doi: 10.1038/sj.gene.6363935. [DOI] [PubMed] [Google Scholar]
- 23.Chae SC, Park YR, Lee YC, Lee JH, Chung HT. The association of TIM-3 gene polymorphism with atopic disease in Korean population. Hum Immunol. 2004;65:1427–1431. doi: 10.1016/j.humimm.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Chae SC, Park YR, Song JH, Shim SC, Yoon KS, Chung HT. The polymorphisms of Tim-1 promoter region are associated with rheumatoid arthritis in a Korean population. Immunogenetics. 2005;56:696–701. doi: 10.1007/s00251-004-0743-5. [DOI] [PubMed] [Google Scholar]
- 25.Chae SC, Song JH, Shim SC, Yoon KS, Chung HT. The exon 4 variations of Tim-1 gene are associated with rheumatoid arthritis in a Korean population. Biochem Biophys Res Commun. 2004;315:971–975. doi: 10.1016/j.bbrc.2004.01.154. [DOI] [PubMed] [Google Scholar]
- 26.Khademi M, Illes Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, Brundin L, et al. T Cell Ig- and Mucin-Domain-Containing Molecule-3 (TIM-3) and TIM-1 Molecules Are Differentially Expressed on Human Th1 and Th2 Cells and in Cerebrospinal Fluid-Derived Mononuclear Cells in Multiple Sclerosis. J Immunol. 2004;172:7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- 27.Chae SC, Park YR, Shim SC, Yoon KS, Chung HT. The polymorphisms of Th1 cell surface gene Tim-3 are associated in a Korean population with rheumatoid arthritis. Immunol Lett. 2004;95:91–95. doi: 10.1016/j.imlet.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Umetsu SE, Lee W-L, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 29.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, et al. TIM-4 is the ligand for TIM, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 30.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 31.Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, et al. Tim-1 and Tim-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sizing ID, Bailly V, McCoon P, Chang W, Rao S, Pablo L, Rennard R, et al. Epitope-dependent effect of anti-murine Tim-1 monoclonal antibodies on T cell activity and lung immune responses. J Immunol. 2007;178:2249–2261. doi: 10.4049/jimmunol.178.4.2249. [DOI] [PubMed] [Google Scholar]
- 33.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soo Hoo W, Jensen ER, Saadat A, Nieto D, Moss RB, Carlo DJ, Moll T. Vaccination with cell immunoglobulin mucin-1 antibodies and inactivated influenza enhances vaccine-specific lymphocyte proliferation, interferon-gamma production and cross-strain reactivity. Clin Exp Immunol. 2006;145:123–129. doi: 10.1111/j.1365-2249.2006.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, Alexopoulos S, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 37.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, Imitola J, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueno T, et al. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118:742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Souza AJ, Oriss TB, O'Malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (Tim-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. PNAS. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binne LL, Scott ML, Rennert PD. Human Tim-1 associates with the TCR complex and up-regulates T cell activation signals. J Immunol. 2007;178:4342–4350. doi: 10.4049/jimmunol.178.7.4342. [DOI] [PubMed] [Google Scholar]
- 41.de Souza AJ, Oak JS, Jordanhazy R, Dekruyff RH, Fruman DA, Kane LP. T cell Ig and mucin domain-1-mediated T cell activation requires recruitment and activation of phosphoinositide 3-kinase. J Immunol. 2008;180:6518–6526. doi: 10.4049/jimmunol.180.10.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Manzanet R, Meyers JH, Balasubramanian S, Slavik J, Kassam N, Dardalhon V, Greenfield EA, et al. Tim-4 Expressed on APCs Induces T Cell Expansion and Survival. J Immunol. 2008;180:4706–4713. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tami C, Silberstein E, Manangeeswaran M, Freeman GJ, Umetsu SE, DeKruyff RH, Umetsu DT, et al. Immunoglobulin A (IgA) is a natural ligand of hepatitis A virus cellular receptor 1 (HAVCR1), and the association of IgA with HAVCR1 enhances virus-receptor interactions. J Virol. 2007;81:3437–3446. doi: 10.1128/JVI.01585-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, et al. Tim-1 and Tim-4 Glycoproteins Bind Phosphatidylserine and Mediate Uptake of Apoptotic Cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 46.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T Cell Immunoglobulin Mucin Protein 4 Show a Metal-Ion-Dependent Ligand Binding Site where Phosphatidylserine Binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilker PR, Sedy JR, Grigura V, Murphy TL, Murphy KM. Evidence for carbohydrate recognition and homotypic and heterotypic binding by the TIM family. Int Immunol. 2007;19:763–773. doi: 10.1093/intimm/dxm044. [DOI] [PubMed] [Google Scholar]
- 48.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 49.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 51.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 52.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 53.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 54.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 56.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 57.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 58.Frisancho-Kiss S, Nyland JF, Davis SE, Barrett MA, Gatewood SJ, Njoku DB, Cihakova D, et al. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J Immunol. 2006;176:6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 59.Oikawa T, Kamimura Y, Akiba H, Yagita H, Okumura K, Takahashi H, Zeniya M, et al. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol. 2006;177:4281–4287. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- 60.Geng H, Zhang GM, Li D, Zhang H, Yuan Y, Zhu HG, Xiao H, et al. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol. 2006;176:1411–1420. doi: 10.4049/jimmunol.176.3.1411. [DOI] [PubMed] [Google Scholar]
- 61.Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 62.Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J Biol Chem. 1997;272:6078–6086. doi: 10.1074/jbc.272.9.6078. [DOI] [PubMed] [Google Scholar]
- 63.Imaizumi T, Kumagai M, Sasaki N, Kurotaki H, Mori F, Seki M, Nishi N, et al. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J Leukoc Biol. 2002;72:486–491. [PubMed] [Google Scholar]
- 64.Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, Ito K, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 65.van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 66.Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, Zencheck WD, et al. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007;26:311–321. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Koguchi K, Anderson DE, Yang L, O'Connor KC, Kuchroo VK, Hafler DA. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006;203:1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Meng J, Wang X, Liu S, Shu Q, Gao L, Ju Y, et al. Expression of human Tim-1 and Tim-3 on lymphocytes from systemic lupus erythematosus patients. Scand J Immunol. 2008;67:63–70. doi: 10.1111/j.1365-3083.2007.02038.x. [DOI] [PubMed] [Google Scholar]
- 69.Jones RB, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, Rose NR, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 71.Mizui M, Shikina T, Arase H, Suzuki K, Yasui T, Rennert PD, Kumanogoh A, et al. Bimodal regulation of T cell-mediated immune responses by Tim-4. Int Immunol. 2008;20:695–708. doi: 10.1093/intimm/dxn029. [DOI] [PubMed] [Google Scholar]
- 72.Shakhov AN, Rybtsov S, Tumanov AV, Shulenin S, Dean M, Kuprash DV, Nedospasov SA. SMUCKLER/TIM4 is a distinct member of TIM family expressed by stromal cells of secondary lymphoid tissues and associated with lymphotoxin signaling. Eur J Immunol. 2004;34:494–503. doi: 10.1002/eji.200324590. [DOI] [PubMed] [Google Scholar]
- 73.Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419:629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- 74.Chakravarti S, Sabatos CA, Xiao S, Illes Z, Cha EK, Sobel RA, Zheng XX, et al. Tim-2 regulates T helper type 2 responses and autoimmunity. J Exp Med. 2005;202:437–444. doi: 10.1084/jem.20050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen TT, Li L, Chung DH, Allen CD, Torti SV, Torti FM, Cyster JG, et al. Tim-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J Exp Med. 2005;202:955–965. doi: 10.1084/jem.20042433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumanogoh A, Shikina T, Suzuki K, Uematsu S, Yukawa K, Kashiwamura S, Tsutsui H, et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity. 2005;22:305–316. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 77.Rennert PD, Ichimura T, Sizing ID, Bailly V, Li Z, Rennard R, McCoon P, et al. T Cell, Ig Domain, Mucin Domain-2 Gene-Deficient Mice Reveal a Novel Mechanism for the Regulation of Th2 Immune Responses and Airway Inflammation. J Immunol. 2006;177:4311–4321. doi: 10.4049/jimmunol.177.7.4311. [DOI] [PubMed] [Google Scholar]
- 78.Knickelbein JE, de Souza AJ, Tosti R, Narayan P, Kane LP. Cutting edge: inhibition of T cell activation by Tim-2. J Immunol. 2006;177:4966–4970. doi: 10.4049/jimmunol.177.8.4966. [DOI] [PubMed] [Google Scholar]