Abstract
Introduction
About one quarter of people having an acute myocardial infarction (MI) in the USA will die of it, half of them within 1 hour of the onset of symptoms. Cardiogenic shock develops in over 5% of people surviving the first hour after an acute MI, with a mortality of 50% to 80% in the first 48 hours.
Methods and outcomes
We conducted a systematic review and aimed to answer the following clinical questions: Which treatments improve outcomes in acute MI? Which treatments improve outcomes for cardiogenic shock after MI? We searched: Medline, Embase, The Cochrane Library, and other important databases up to October 2009 (Clinical Evidence reviews are updated periodically, please check our website for the most up-to-date version of this review). We included harms alerts from relevant organisations such as the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA).
Results
We found 52 systematic reviews, RCTs, or observational studies that met our inclusion criteria. We performed a GRADE evaluation of the quality of evidence for interventions.
Conclusions
In this systematic review we present information relating to the effectiveness and safety of the following interventions: angiotensin-converting enzyme (ACE) inhibitors, aspirin, beta-blockers, calcium channel blockers, early cardiac surgery, early invasive cardiac revascularisation, glycoprotein IIb/IIIa inhibitors, intra-aortic balloon counterpulsation, nitrates (with or without thrombolysis), positive inotropes, primary percutaneous transluminal coronary angioplasty (PTCA), pulmonary artery catheterisation, thrombolysis (with or without low molecular weight heparin, with or without unfractionated heparin), vasodilators, and ventricular assistance devices and cardiac transplantation.
Key Points
About one quarter of people who have a myocardial infarction (MI) in the USA will die from it, half of them within 1 hour of the onset of symptoms.
Cardiogenic shock develops in over 5% of people who survive the first hour after an MI, with a mortality of 50% to 80% in the first 48 hours.
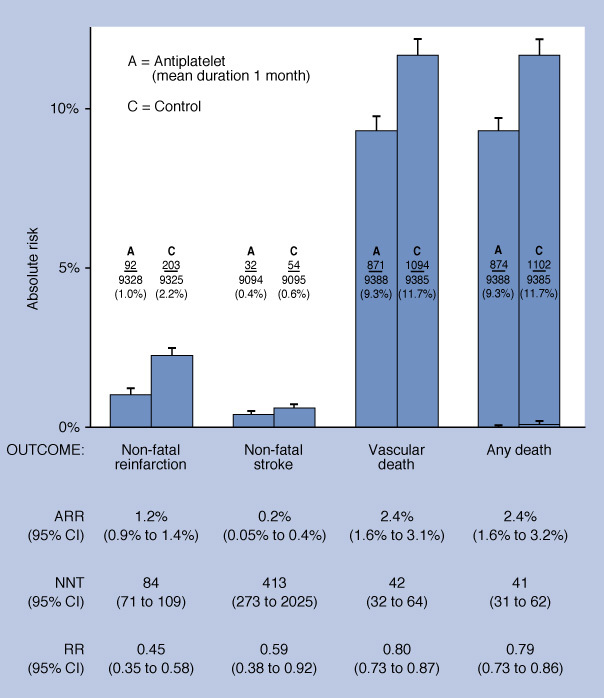
Aspirin reduces mortality, reinfarction, and stroke at 1 month compared with placebo in people with an acute MI.
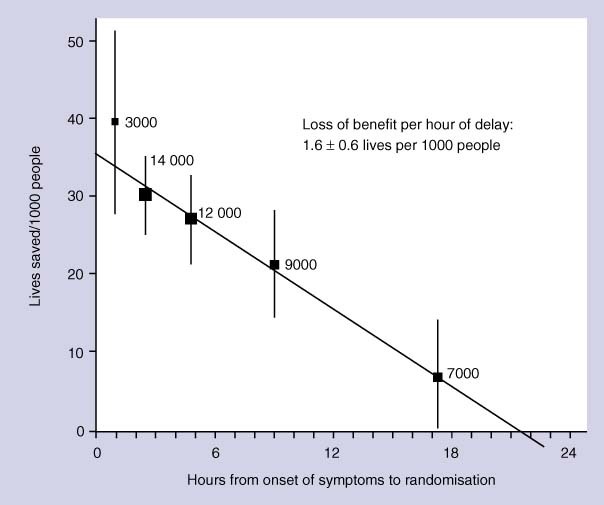
Thrombolysis within 6 hours reduces mortality but increases the risk of stroke or major bleeding in people with acute MI, with different agents seeming to have similar efficacy.
Adding low molecular weight heparin to thrombolytics may reduce the risk of further cardiovascular events, but the combination has not been shown to improve survival.
Beta-blockers reduce reinfarction in people with acute MI, but have no effect on mortality in the short term, and increase cardiogenic shock.
ACE inhibitors reduce mortality in people with acute MI compared with placebo.
Nitrates reduce mortality and improve symptoms in people not receiving thrombolysis, but may not be beneficial in people after thrombolysis.
Calcium channel blockers have not been shown to reduce mortality after an acute MI, and early treatment with nifedipine may increase mortality.
Primary PTCA within 12 hours of onset of chest pain reduces the risk of death, reinfarction, and stroke compared with thrombolysis.
In people with cardiogenic shock, invasive cardiac revascularisation within 48 hours of acute MI reduces mortality at 12 months compared with medical treatment alone, but people aged over 75 years may not benefit.
We don't know whether thrombolysis, vasodilators, intra-aortic balloon counterpulsation, ventricular assistance devices and cardiac transplantation, or early cardiac surgery improve survival in people with cardiogenic shock.
There is a consensus that positive inotropes and pulmonary artery catheterisation are beneficial, but we found no trials that confirmed this.
About this condition
Definition
Acute MI: Acute MI is myocardial cell death caused by prolonged ischaemia due to sudden occlusion of a coronary artery. There are two types of acute MI: ST-segment elevation MI (STEMI; clinically appropriate symptoms with ST-segment elevation on ECG) and non-ST-segment elevation MI (NSTEMI; clinically appropriate symptoms with ST-segment depression or T-wave abnormalities on ECG). Cardiogenic shock: Defined clinically as a poor cardiac output plus evidence of tissue hypoxia that is not improved by correcting reduced intravascular volume. When a pulmonary artery catheter is used, cardiogenic shock may be defined as a cardiac index below 2.2 L/minute/m2 despite an elevated pulmonary capillary wedge pressure (at least 15 mm Hg).
Incidence/ Prevalence
Acute MI: Acute MI is one of the most common causes of mortality worldwide. In 1990, ischaemic heart disease was the world's leading cause of death, accounting for about 6.3 million deaths. The age-standardised incidence varies among and within countries. Each year, about 900,000 people in the US experience acute MI, about 225,000 of whom die. About half of these people die within 1 hour of the onset of symptoms and before reaching a hospital. Event rates increase with age for both sexes and are higher in men than in women and in poorer than richer people at all ages. The incidence of death from acute MI has fallen in many Western countries over the past 20 years. Cardiogenic shock: Cardiogenic shock occurs in about 7% of people admitted to hospital with acute MI. Of these, about half have established cardiogenic shock at the time of admission to hospital, and most of the others develop it during the first 24 to 48 hours after admission.
Aetiology/ Risk factors
Acute MI: Identified major risk factors for CVD include increasing age, male sex, raised low-density lipoprotein cholesterol, reduced high-density lipoprotein cholesterol, raised blood pressure, smoking, diabetes, family history of CVD, obesity, and sedentary lifestyle. For many of these risk factors, observational studies show a continuous gradient of increasing risk of CVD with increasing levels of the risk factor, with no obvious threshold level. The immediate mechanism of acute MI is rupture or erosion of an atheromatous plaque causing thrombosis and occlusion of coronary arteries and myocardial cell death. Factors that may convert a stable plaque into an unstable plaque (the "active plaque") have yet to be fully elucidated. Shear stresses, inflammation, and autoimmunity have been proposed. The changing rates of CHD in different populations are only partly explained by changes in the standard risk factors for ischaemic heart disease (particularly a fall in blood pressure and smoking). Cardiogenic shock: Cardiogenic shock after acute MI usually follows a reduction in functional ventricular myocardium, and is caused by left ventricular infarction (79% of people) more often than by right ventricular infarction (3% of people). Cardiogenic shock after acute MI may also be caused by cardiac structural defects, such as mitral valve regurgitation due to papillary muscle dysfunction (7% of people), ventricular septal rupture (4% of people), or cardiac tamponade after free cardiac wall rupture (1% of people). Major risk factors for cardiogenic shock after acute MI are previous MI, diabetes mellitus, advanced age, hypotension, tachycardia or bradycardia, congestive heart failure with Killip class II–III, and low left ventricular ejection fraction (ejection fraction under 35%).
Prognosis
Acute MI: May lead to a host of mechanical and cardiac electrical complications, including death, ventricular dysfunction, congestive heart failure, fatal and non-fatal arrhythmias, valvular dysfunction, myocardial rupture, and cardiogenic shock. Cardiogenic shock: Mortality for people in hospital with cardiogenic shock after acute MI vary between 50% to 80%. Most deaths occur within 48 hours of the onset of shock (see figure 1 ). People surviving until discharge from hospital have a reasonable long-term prognosis (88% survival at 1 year).
Figure 1.
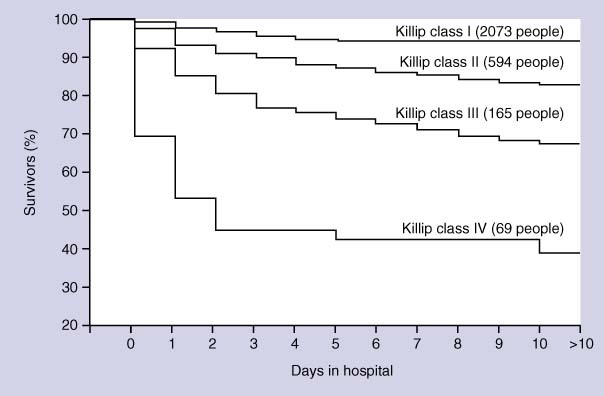
The AMIS registry Kaplan–Meier survival curves as a function of Killip class at hospital admission for 3138 people (2901 evaluable) admitted in 50 Swiss hospitals between 1977 and 1998. Published with permission: Urban P, Bernstein MS, Costanza MC, et al, for the AMIS investigators. An internet-based registry of acute MI in Switzerland. Kardiovasc Med 2000;3:430–441 (see text).
Aims of intervention
To decrease mortality, to prevent recurrent infarction and ischaemia, to reduce complications (such as congestive heart failure, myocardial rupture, valvular dysfunction, and fatal and non-fatal arrhythmia), to restore blood supply to heart muscle, and to relieve pain.
Outcomes
Mortality: some studies also reported composite outcomes of mortality or cardiovascular events. Cardiovascular events: major cardiovascular events including recurrent acute MI, refractory ischaemia, and stroke at up to 6 months. Bleeding: rates of major bleeding, intracranial haemorrhage, stroke possibly associated with treatment, at up to 6 months. Other adverse events: including cardiogenic shock at up to 6 months.
Methods
Clinical Evidence search and appraisal October 2009. The following databases were used to identify studies for this systematic review: Medline 1966 to October 2009, Embase 1980 to October 2009, and The Cochrane Database of Systematic Reviews 2009, Issue 4 (1966 to date of issue). An additional search within The Cochrane Library was carried out for the Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment (HTA). We also searched for retractions of studies included in the review. Abstracts of the studies retrieved from the initial search were assessed by an information specialist. Selected studies were then sent to the contributor for additional assessment, using predetermined criteria to identify relevant studies. Study design criteria for inclusion in this review were: published systematic reviews of RCTs and RCTs in any language, at least single blinded, and containing more than 20 individuals of whom more than 80% were followed up. There was no minimum length of follow-up required to include studies. We excluded all studies described as "open", "open label", or not blinded unless blinding was impossible. We included systematic reviews of RCTs and RCTs where harms of an included intervention were studied applying the same study design criteria for inclusion as we did for benefits. In addition we use a regular surveillance protocol to capture harms alerts from organisations such as the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA), which are added to the reviews as required. To aid readability of the numerical data in our reviews, we round many percentages to the nearest whole number. Readers should be aware of this when relating percentages to summary statistics such as relative risks (RRs) and odds ratios (ORs). We report outcomes of treatment from onset of symptoms up to 6 months in this review. We also include meta-analyses that combine outcomes from both before and after 6 months. We have performed a GRADE evaluation of the quality of evidence for interventions included in this review (see table ). The categorisation of the quality of the evidence (into high, moderate, low, or very low) reflects the quality of evidence available for our chosen outcomes in our defined populations of interest. These categorisations are not necessarily a reflection of the overall methodological quality of any individual study, because the Clinical Evidence population and outcome of choice may represent only a small subset of the total outcomes reported, and population included, in any individual trial. For further details of how we perform the GRADE evaluation and the scoring system we use, please see our website (www.clinicalevidence.com). Systematic reviews and RCTs that cover secondary prevention in mixed manifestations of atherosclerotic coronary artery disease are reported in the review on Secondary prevention of ischaemic cardiac events.
Table 1.
GRADE evaluation of interventions for acute MI
| Important outcomes | Cardiovascular events, bleeding, mortality, adverse effects | ||||||||
| Number of studies (participants) | Outcome | Comparison | Type of evidence | Quality | Consistency | Directness | Effect size | GRADE | Comment |
| Which treatments improve outcomes in people with acute MI? | |||||||||
| 9 (18,773) | Mortality | Aspirin v placebo | 4 | 0 | 0 | 0 | 0 | High | |
| 9 (18,773) | Cardiovascular events | Aspirin v placebo | 4 | 0 | 0 | 0 | 0 | High | |
| 9 (58,600) | Mortality | Thrombolysis v placebo | 4 | 0 | −1 | 0 | 0 | Moderate | Consistency point deducted for different results for different subgroups |
| 3 (94,701) | Mortality | tPA v streptokinase | 4 | −2 | −1 | 0 | 0 | Very low | Quality points deducted for no blinding and incomplete reporting of results. Consistency point deducted for conflicting results across studies |
| 3 (47,086) | Mortality | tPA v other thrombolytics | 4 | −1 | 0 | −1 | 0 | Low | Quality point deducted for incomplete reporting of results. Directness point deducted for different selection criteria and protocols for different studies |
| 9 (58,600) | Bleeding | Thrombolysis v placebo | 4 | 0 | 0 | 0 | 0 | High | |
| 3 (at least 39,907) | Bleeding | Streptokinase v tPA | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for no blinding and incomplete reporting of results |
| 3 (47,086) | Bleeding | tPA v other thrombolytics | 4 | −1 | 0 | −1 | 0 | Low | Quality point deducted for incomplete reporting of results. Directness point deducted for different selection criteria and protocols for different studies |
| 1 (496) | Mortality | Thrombolytic plus low molecular weight heparin v thrombolytic plus placebo | 4 | 0 | 0 | 0 | 0 | High | |
| 1 (496) | Cardiovascular events | Thrombolytic plus low molecular weight heparin v thrombolytic plus placebo | 4 | 0 | 0 | 0 | 0 | High | |
| 8 (27,758) | Mortality | Thrombolytic plus low molecular weight heparin v thrombolytic plus unfractionated heparin | 4 | −1 | 0 | 0 | 0 | Moderate | Quality point deducted for weak methods (lack of blinding in some RCTs, incomplete reporting of results in 1 large RCT) |
| 8 (27,758) | Cardiovascular events | Thrombolytic plus low molecular weight heparin v thrombolytic plus unfractionated heparin | 4 | −1 | 0 | 0 | 0 | Moderate | Quality point deducted for weak methods (lack of blinding in some RCTs, incomplete reporting of results in 1 large RCT) |
| 8 (27,758) | Bleeding | Thrombolytic plus low molecular weight heparin v thrombolytic plus unfractionated heparin or placebo | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for weak methods (lack of blinding in some RCTs, incomplete reporting of background interventions which might affect bleeding) and incomplete reporting of results in 1 review |
| 2 (53,789) | Mortality | Thrombolytics plus unfractionated heparin v thrombolytics alone | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for no blinding and incomplete reporting of results |
| 2 (53,789) | Cardiovascular events | Thrombolytics plus unfractionated heparin v thrombolytics alone | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for no blinding and incomplete reporting of results |
| 2 (53,789) | Bleeding | Thrombolytics plus unfractionated heparin v thrombolytics alone | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for no blinding and incomplete reporting of results |
| 1 (16,588) | Mortality | Glycoprotein IIb/IIIa inhibitors plus thrombolysis v thrombolysis alone | 4 | −1 | 0 | −1 | 0 | Low | Quality point deducted for no blinding. Directness point deducted for different doses of thrombolytics in 2 groups |
| 1 (16,588) | Cardiovascular events | Glycoprotein IIb/IIIa inhibitors plus thrombolysis v thrombolysis alone | 4 | −1 | 0 | −1 | 0 | Low | Quality point deducted for no blinding. Directness point deducted for different doses of thrombolytics in 2 groups |
| 7 (at least 1101) | Mortality | Glycoprotein IIb/IIIa inhibitors plus PTCA v PTCA plus placebo | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for incomplete reporting of results and use of composite outcomes |
| 7 (at least 1101) | Cardiovascular events | Glycoprotein IIb/IIIa inhibitors plus PTCA v PTCA plus placebo | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for incomplete reporting of results and use of composite outcomes |
| 1 (unclear) | Bleeding | Glycoprotein IIb/IIIa inhibitors plus thrombolysis v thrombolysis alone | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for no blinding and incomplete reporting of results |
| 7 (at least 1101) | Bleeding | Glycoprotein IIb/IIIa inhibitors plus PTCA v PTCA plus placebo | 4 | −1 | −1 | 0 | 0 | Low | Quality point deducted for incomplete reporting of results. Consistency point deducted for inconsistent results depending on analysis |
| At least 52 (at least 60,772) | Mortality | Beta-blockers v placebo or no beta-blocker | 4 | −1 | 0 | −1 | 0 | Low | Quality point deducted for incomplete reporting of results. Directness point deducted for narrow range of inclusion criteria and lack of use of thrombolysis in many studies |
| 1 (45,852) | Cardiovascular events | Beta-blockers v placebo or no beta-blocker | 4 | 0 | 0 | 0 | 0 | High | |
| 15 (15,104) | Mortality | ACE inhibitors v placebo | 4 | 0 | 0 | 0 | 0 | High | |
| 2 (75,867) | Mortality | Nitrates plus thrombolysis v thrombolysis plus placebo | 4 | 0 | 0 | −1 | 0 | Moderate | Directness point deducted because of high numbers of people not receiving thrombolysis or receiving nitrates outside the study protocol |
| 1 (17,817) | Cardiovascular events | Nitrates plus thrombolysis v thrombolysis plus placebo | 4 | 0 | 0 | −1 | 0 | Moderate | Directness point deducted because of high numbers of people not receiving thrombolysis or receiving nitrates outside the study protocol |
| 10 (2041) | Mortality | Nitrates without thrombolysis v placebo | 4 | 0 | 0 | 0 | 0 | High | |
| 9 (12,024) | Mortality | Calcium channel blockers v placebo | 4 | 0 | 0 | 0 | 0 | High | |
| At least 22 (at least 6763) | Mortality | Primary PTCA v thrombolysis | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for lack of blinding and use of non-defined composite outcome in early studies |
| 23 (7739) | Bleeding | Primary PTCA v thrombolysis | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for lack of blinding and use of non-defined composite outcome in early studies |
| What treatments improve outcomes for cardiogenic shock after acute MI? | |||||||||
| 2 (357) | Mortality | Early invasive cardiac revascularisation v medical treatment alone | 4 | 0 | 0 | −2 | 0 | Low | Directness points deducted for uncertainty about comparators and uncertain generalisability of results |
| 1 (280) | Mortality | Thrombolysis v no thrombolysis | 4 | −2 | 0 | 0 | 0 | Low | Quality points deducted for retrospective subgroup analysis and blinding flaws |
Type of evidence: 4 = RCT. Consistency: similarity of results across studies. Directness: generalisability of population or outcomes. Effect size: based on relative risk or odds ratio.
Glossary
- Cardiac index
A measure of cardiac output derived from the formula: cardiac output/unit time divided by body surface area (L/minute/m2).
- High-quality evidence
Further research is very unlikely to change our confidence in the estimate of effect.
- Intra-aortic balloon counterpulsation
A technique in which a balloon is placed in the aorta and inflated during diastole and deflated just before systole.
- Invasive cardiac revascularisation
A term used to describe either percutaneous transluminal coronary angioplasty or coronary artery bypass grafting.
- Killip class
A categorisation of the severity of heart failure based on easily obtained clinical signs. The main clinical features are Class I: no heart failure; Class II: crackles audible halfway up the chest; Class III: crackles heard in all the lung fields; Class IV: cardiogenic shock.
- Low-quality evidence
Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
- Moderate-quality evidence
Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
- Ventricular assistance device
A mechanical device placed in parallel to a failing cardiac ventricle that pumps blood in an attempt to maintain cardiac output. Because of the risk of mechanical failure, thrombosis, and haemolysis, ventricular assistance devices are normally used for short-term support while preparing for a heart transplant.
- Very low-quality evidence
Any estimate of effect is very uncertain.
Non ST-elevation acute coronary syndrome
Secondary prevention of ischaemic cardiac events
Prevention of cardiovascular events in diabetes
Disclaimer
The information contained in this publication is intended for medical professionals. Categories presented in Clinical Evidence indicate a judgement about the strength of the evidence available to our contributors prior to publication and the relevant importance of benefit and harms. We rely on our contributors to confirm the accuracy of the information presented and to adhere to describe accepted practices. Readers should be aware that professionals in the field may have different opinions. Because of this and regular advances in medical research we strongly recommend that readers' independently verify specified treatments and drugs including manufacturers' guidance. Also, the categories do not indicate whether a particular treatment is generally appropriate or whether it is suitable for a particular individual. Ultimately it is the readers' responsibility to make their own professional judgements, so to appropriately advise and treat their patients.To the fullest extent permitted by law, BMJ Publishing Group Limited and its editors are not responsible for any losses, injury or damage caused to any person or property (including under contract, by negligence, products liability or otherwise) whether they be direct or indirect, special, incidental or consequential, resulting from the application of the information in this publication.
References
- 1.Califf RM, Bengtson JR. Cardiogenic shock. N Engl J Med 1994;330:1724–1730. [DOI] [PubMed] [Google Scholar]
- 2.Hochman JS, Sleeper LA, Webb JG, et al, for the SHOCK investigators. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med 1999;341:625–634. [DOI] [PubMed] [Google Scholar]
- 3.Urban P, Stauffer JC, Khatchatrian N, et al. A randomized evaluation of early revascularization to treat shock complicating acute myocardial infarction. The (Swiss) Multicenter Trial of Angioplasty for Shock — (S)MASH. Eur Heart J 1999;20:1030–1038. [DOI] [PubMed] [Google Scholar]
- 4.Murray C, Lopez A. Mortality by cause for eight regions of the world: global burden of disease study. Lancet 1997;349:1269–1276. [DOI] [PubMed] [Google Scholar]
- 5.National Heart, Lung, and Blood Institute. Morbidity and mortality: chartbook on cardiovascular, lung, and blood diseases. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health; May 1992. [Google Scholar]
- 6.Goldberg RJ, Samad NA, Yarzebski J, et al. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med 1999;340:1162–1168. [DOI] [PubMed] [Google Scholar]
- 7.Hasdai D, Califf RM, Thompson TD, et al. Predictors of cardiogenic shock after thrombolytic therapy for acute myocardial infarction. J Am Coll Cardiol 2000;35:136–143. [DOI] [PubMed] [Google Scholar]
- 8.Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction — etiology, management and outcome: a report from the SHOCK trial registry. J Am Coll Cardiol 2000;36(3 suppl A):1063–1070. [DOI] [PubMed] [Google Scholar]
- 9.Urban P, Bernstein M, Costanza M, et al. An internet-based registry of acute myocardial infarction in Switzerland. Kardiovasc Med 2000;3:430–441. [Google Scholar]
- 10.Berger PB, Tuttle RH, Holmes DR, et al. One year survival among patients with acute myocardial infarction complicated by cardiogenic shock, and its relation to early revascularisation: results of the GUSTO-1 trial. Circulation 1999;99:873–878. [DOI] [PubMed] [Google Scholar]
- 11.Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of people. BMJ 1994;308:81–106. Search date 1990. [PMC free article] [PubMed] [Google Scholar]
- 12.Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest 1982;69:1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Second International Study of Infarct Survival (ISIS-2) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction. Lancet 1988;2:349–360. [PubMed] [Google Scholar]
- 14.Baigent BM, Collins R. ISIS-2: four year mortality of 17,187 patients after fibrinolytic and antiplatelet therapy in suspected acute myocardial infarction study. Circulation 1993;88(suppl I):I-291–I-292. [Google Scholar]
- 15.Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results of all randomized trials of more than 1000 patients. Lancet 1994;343:311–322. [PubMed] [Google Scholar]
- 16.French JK, Hyde TA, Patel H, et al. Survival 12 years after randomization to streptokinase: the influence of thrombolysis in myocardial infarction flow at three to four weeks. J Am Coll Cardiol 1999;34:62–69. [DOI] [PubMed] [Google Scholar]
- 17.Collins R, Peto R, Baigent BM, et al. Aspirin, heparin and fibrinolytic therapy in suspected acute myocardial infarction. N Engl J Med 1997;336:847–860. [DOI] [PubMed] [Google Scholar]
- 18.Gruppo Italiano per lo studio della streptochinasi nell'infarto miocardico (GISSI). GISSI-2: a factorial randomised trial of alteplase versus streptokinase and heparin versus no heparin among 12,490 patients with acute myocardial infarction. Lancet 1990;336:65–71. [PubMed] [Google Scholar]
- 19.Third International Study of Infarct Survival (ISIS-3) Collaborative Group. ISIS-3: a randomised comparison of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41,299 cases of suspected acute myocardial infarction. Lancet 1992;339:753–770. [PubMed] [Google Scholar]
- 20.The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–682. [DOI] [PubMed] [Google Scholar]
- 21.The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO III) investigators. A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med 1997;337:1118–1123. [DOI] [PubMed] [Google Scholar]
- 22.Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) investigators. Single bolus tenecteplase compared to front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet 1999;354:716–722. [DOI] [PubMed] [Google Scholar]
- 23.InTIME-II Investigators. Intravenous NPA for the treatment of infarcting myocardium early; InTIME-II, a double-blind comparison of single-bolus lanoteplase vs accelerated alteplase for the treatment of patients with acute myocardial infarction. Eur Heart J 2000;21:2005–2013. [DOI] [PubMed] [Google Scholar]
- 24.Simoons MI, Maggioni AP, Knatterud G, et al. Individual risk assessment for intracranial hemorrhage during thrombolytic therapy. Lancet 1993;342:1523–1528. [DOI] [PubMed] [Google Scholar]
- 25.Gore JM, Granger CB, Simoons MI, et al. Stroke after thrombolysis: mortality and functional outcomes in the GUSTO-1 trial. Circulation 1995;92:2811–2818. [DOI] [PubMed] [Google Scholar]
- 26.Berkowitz SD, Granger CB, Pieper KS, et al. Incidence and predictors of bleeding after contemporary thrombolytic therapy for myocardial infarction. Circulation 1997;95:2508–2516. [DOI] [PubMed] [Google Scholar]
- 27.Simoons M, Krzeminska-Pakula M, Alonso A, et al. for the AMI-SK Investigators. Improved reperfusion and clinical outcome with enoxaparin as an adjunct to streptokinase thrombolysis in acute myocardial infarction. The AMI-SK study. Eur Heart J 2002;23:1282–1290. [DOI] [PubMed] [Google Scholar]
- 28.De Luca G, Marino P. Adjunctive benefits from low-molecular-weight heparins as compared to unfractionated heparin among patients with ST-segment elevation myocardial infarction treated with thrombolysis. A meta-analysis of the randomized trials. Am Heart J 2007;154:1085.e1−e6. [DOI] [PubMed] [Google Scholar]
- 29.Singh S, Bahekar A, Molnar J, et al. Adjunctive low molecular weight heparin during fibrinolytic therapy in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Clin Cardiol 2009;32:358−364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mega JL, Morrow DA, Ostor E, et al. Outcomes and optimal antithrombotic therapy in women undergoing fibrinolysis for ST-elevation myocardial infarction. Circulation 2007;115:2822–2828. [DOI] [PubMed] [Google Scholar]
- 31.The GUSTO V investigators. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet 2001;357:1905–1914. [DOI] [PubMed] [Google Scholar]
- 32.Kandzari DE, Hasselblad V, Tcheng JE, et al. Improved clinical outcomes with abciximab therapy in acute myocardial infarction: a systematic overview of randomized clinical trials. Am Heart J 2004;147:457–462. [DOI] [PubMed] [Google Scholar]
- 33.Montalescot G, Antoniucci D, Kastrati A, et al. Abciximab in primary coronary stenting of ST-elevation myocardial infarction: a European meta-analysis on individual patients' data with long-term follow-up. Eur Heart J 2007;28:443−449. [DOI] [PubMed] [Google Scholar]
- 34.Montalescot G, Borentain M, Payot L, et al. Early vs late administration of glycoprotein IIb/IIIa inhibitors in primary percutaneous coronary intervention of acute ST-segment elevation myocardial infarction: a meta-analysis. JAMA 2004;292:362–366. [DOI] [PubMed] [Google Scholar]
- 35.Lee DP, Herity NA, Hiatt BL, et al. Adjunctive platelet glycoprotein IIb/IIIa receptor inhibition with tirofiban before primary angioplasty improves angiographic outcomes: results of the TIrofiban Given in the Emergency Room before Primary Angioplasty (TIGER-PA) pilot trial. Circulation 2003;107:1497–1501. [DOI] [PubMed] [Google Scholar]
- 36.van 't Hof AW, Ernst N, de Boer MJ, et al. Facilitation of primary coronary angioplasty by early start of a glycoprotein 2b/3a inhibitor: results of the ongoing tirofiban in myocardial infarction evaluation (On-TIME) trial. Eur Heart J 2004;25:837–846. [DOI] [PubMed] [Google Scholar]
- 37.Cutlip DE, Ricciardi MJ, Ling FS, et al. Effect of tirofiban before primary angioplasty on initial coronary flow and early ST-segment resolution in patients with acute myocardial infarction. Am J Cardiol 2003;92:977–980. [DOI] [PubMed] [Google Scholar]
- 38.Zorman S, Zorman D, Noc M, et al. Effects of abciximab pretreatment in patients with acute myocardial infarction undergoing primary angioplasty. Am J Cardiol 2002;90:533–536. [DOI] [PubMed] [Google Scholar]
- 39.Yusuf S, Peto R, Lewis S, et al. Beta-blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335–371. Search date not reported. [DOI] [PubMed] [Google Scholar]
- 40.Freemantle N, Cleland J, Young P, et al. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730–1737. Search date 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1622–1632. [DOI] [PubMed] [Google Scholar]
- 42.Roberts R, Rogers WJ, Mueller HS, et al. Immediate versus deferred beta-blockade following thrombolytic therapy in patients with acute myocardial infarction: results of the thrombolysis in myocardial infarction (TIMI) II-B study. Circulation 1991;83:422–437. [DOI] [PubMed] [Google Scholar]
- 43.Domanski MJ, Exner DV, Borkowf CB, et al. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. A meta-analysis of randomized clinical trials. J Am Coll Cardiol 1999;33:598–604. Search date 1997. [DOI] [PubMed] [Google Scholar]
- 44.ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomised trials. Circulation 1998;97:2202–2212. Search date not reported. [DOI] [PubMed] [Google Scholar]
- 45.Latini R, Tognoni G, Maggioni AP, et al. Clinical effects of early angiotensin-converting enzyme inhibitor treatment for acute myocardial infarction are similar in the presence and absence of aspirin. Systematic overview of individual data from 96,712 randomized patients. J Am Coll Cardiol 2000;35:1801–1807. Search date not reported. [DOI] [PubMed] [Google Scholar]
- 46.Fourth International Study of Infarct Survival (ISIS-4) Collaborative Group. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58 050 patients with suspected acute myocardial infarction. Lancet 1995;345:669–685. [PubMed] [Google Scholar]
- 47.Gruppo Italiano per lo studio della streptochinasi nell'infarto miocardico (GISSI). GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet 1994;343:1115–1122. [PubMed] [Google Scholar]
- 48.Yusuf S, Collins R, MacMahon S, et al. Effect of intravenous nitrates on mortality in acute myocardial infarction: an overview of the randomised trials. Lancet 1988;1:1088–1092. [DOI] [PubMed] [Google Scholar]
- 49.Wilcox RG, Hampton JR, Banks DC, et al. Early nifedipine in acute myocardial infarction: the TRENT study. BMJ 1986;293:1204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldbourt U, Behar S, Reicher-Reiss H, et al. Early administration of nifedipine in suspected acute myocardial infarction: the Secondary Prevention Reinfarction Israel Nifedipine Trial 2 Study. Arch Intern Med 1993;153:345–353. [PubMed] [Google Scholar]
- 51.Pepine CJ, Faich G, Makuch R. Verapamil use in patients with cardiovascular disease: an overview of randomized trials. Clin Cardiol 1998;21:633–641. Search date 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yusuf S, Furberg CD. Effects of calcium channel blockers on survival after myocardial infarction. Cardiovasc Drugs Ther 1987;1:343–344. Search date not reported. [DOI] [PubMed] [Google Scholar]
- 53.Teo KK, Yusuf S, Furberg CD. Effects of prophylactic antiarrhythmic drug therapy in acute myocardial infarction: an overview of results from randomized controlled trials. JAMA 1993;270:1589–1595. [PubMed] [Google Scholar]
- 54.The Multicenter Diltiazem Post Infarction Trial Research Group. The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med 1988;319:385–392. [DOI] [PubMed] [Google Scholar]
- 55.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 56.Boersma E. The Primary Coronary Angioplasty vs.Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 2006;27:779–788. [DOI] [PubMed] [Google Scholar]
- 57.Yusuf S, Pogue J. Primary angioplasty compared to thrombolytic therapy for acute myocardial infarction [editorial]. JAMA 1997;278:2110–2111. [PubMed] [Google Scholar]
- 58.The GUSTO IIb Angioplasty Substudy Investigators. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. N Engl J Med 1997;336:1621–1628. [DOI] [PubMed] [Google Scholar]
- 59.Van de Werf F, Topol EJ, Lee KL, et al. Variations in patient management and outcomes for acute myocardial infarction in the United States and other countries: results from the GUSTO trial. JAMA 1995;273:1586–1591. [DOI] [PubMed] [Google Scholar]
- 60.Hochman JS, Sleeper LA, White HD, et al. One year survival following early revascularization for cardiogenic shock. JAMA 2001;285:190–192. [DOI] [PubMed] [Google Scholar]
- 61.GISSI-1. Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet 1986;1:397–401. [PubMed] [Google Scholar]
- 62.French, JK, Feldman, HA, Assmann SF, et al. Influence of thrombolytic therapy, with or without intra-aortic balloon counterpulsation, on 12-month survival in the SHOCK trial. Am Heart J 2003; 146: 804–810. [DOI] [PubMed] [Google Scholar]
- 63.Herbert P, Tinker J. Inotropic drugs in acute circulatory failure. Intensive Care Med 1980;6:101–111. [DOI] [PubMed] [Google Scholar]
- 64.Hollenberg SM, Kavinsky CJ, Parrillo JE. Cardiogenic shock. Ann Int Med 1999;131:47–59. [DOI] [PubMed] [Google Scholar]
- 65.Bernard GR, Sopko G, Cerra F, et al. Pulmonary artery catheterization and clinical outcomes. JAMA 2000;283:2568–2572. [DOI] [PubMed] [Google Scholar]
- 66.Hollenberg SM, Hoyt J. Pulmonary artery catheters in cardiovascular disease. New Horiz 1977;5:207–213. Search date 1996. [PubMed] [Google Scholar]
- 67.Participants. Pulmonary artery catheter consensus conference: consensus statement. Crit Care Med 1997;25:910–925. [DOI] [PubMed] [Google Scholar]
- 68.O'Rourke MF, Norris RM, Campbell TJ, et al. Randomized controlled trial of intraaortic balloon counterpulsation in early myocardial infarction with acute heart failure. Am J Cardiol 1981;47:815–820. [DOI] [PubMed] [Google Scholar]
- 69.Flaherty JT, Becker LC, Weiss JL, et al. Results of a randomized prospective trial of intraaortic balloon counterpulsation and intravenous nitroglycerin in patients with acute myocardial infarction. J Am Coll Cardiol 1985;6:434–446. [DOI] [PubMed] [Google Scholar]
- 70.Frazier OH. Future directions of cardiac assistance. Semin Thorac Cardiovasc Surg 2000;12:251–258. [DOI] [PubMed] [Google Scholar]
- 71.Pagani FD, Lynch W, Swaniker F, et al. Extracorporeal life support to left ventricular assist device bridge to cardiac transplantation. Circulation 1999;100(suppl 19):II-206–210. [DOI] [PubMed] [Google Scholar]
- 72.Mavroidis D, Sun BC, Pae WE. Bridge to transplantation: the Penn State experience. Ann Thorac Surg 1999;68:684–687. [DOI] [PubMed] [Google Scholar]