Abstract
Purpose
Children with intestinal failure (IF) often have gastrointestinal (GI) symptoms, including bleeding, increased stool output, and feeding intolerance. The use of endoscopic assessment of these symptoms has not been previously reported. This report evaluates the diagnostic yield of GI endoscopy in the setting of IF.
Methods
After institutional review board approval, we reviewed the medical records (including endoscopy, pathology and microbiology data) of patients with IF who underwent GI endoscopies between September 1999 and March 2007.
Results
Twenty-seven patients underwent 61 GI endoscopies: 34 esophagogastroduodenoscopies, 17 colonoscopies, 7 flexible sigmoidoscopies, and 3 ileoscopies. Indications for endoscopy, which were not mutually exclusive, included chronic diarrhea (39%, n = 24), GI bleeding (36%, n = 22), suspected bacterial overgrowth (36%, n = 22), and suspected peptic disease (15%, n = 9). Based on gross endoscopic appearance, histopathology, or microbiology, 43 (70%) procedures yielded abnormalities. These included infectious (20%, n = 12), anatomical (18%, n = 11), peptic (15%, n = 9), allergic (15%, n = 9), and other (2%, n = 1) findings. Eleven (73%) of 15 duodenal cultures grew a spectrum of 17 bacterial species. Overall, 24 (89%) of 27 patients had gross endoscopic, histopathologic, or microbiologic abnormalities.
Conclusions
In pediatric patients with IF, diagnostic upper and lower GI endoscopies yield high rates of abnormalities and can help guide clinical management.
Keywords: Short bowel syndrome, Intestinal failure, Endoscopy, Esophagogastroduodenoscopy, Colonoscopy, Bacterial overgrowth
Intestinal failure (IF) is a condition in which there is insufficient functional bowel to allow for nutrient and fluid absorption. The management of children with IF can be difficult because of a variety of gastrointestinal (GI) symptoms and signs, including poor weight gain, chronic diarrhea, GI bleeding, bacterial overgrowth of the small intestine, peptic disease, and GI allergies [1]. Some of these symptoms may be directly related to diagnoses resulting in IF, whereas others may be owing to complications of these conditions and/or their treatments. These symptoms can ultimately lead to enteral intolerance and the need for prolonged parenteral nutrition. Determining the etiology and treatment of these symptoms based on history and physical examination alone can be difficult, but treatment is often performed on an empirical basis because of the complex nature of these patients.
The diagnostic and therapeutic benefits of GI endoscopy in the management of patients with inflammatory bowel disease, gastroesophageal reflux, and other conditions are well known [2–5]. There are few published data, however, concerning the diagnostic yield of GI endoscopy in children with IF. Our goal was to determine the diagnostic yield of these procedures in a cohort of patients with IF followed at a single referral center.
1. Methods
After obtaining institutional review board approval, we reviewed the medical records (including endoscopy, pathology, and microbiology data) of all patients with IF who underwent upper and/or lower endoscopy between September 1999 and March 2007. All children were followed by the Boston Center for Advanced Intestinal Rehabilitation (CAIR), Children’s Hospital, Boston, Mass, a multidisciplinary program for pediatric patients with IF. The CAIR program consists of dedicated staff in general surgery, gastroenterology, transplant surgery, nutrition, pharmacy, nursing, and social work. Indications for endoscopy, which were not mutually exclusive, included (1) symptoms of bacterial overgrowth, (2) chronic diarrhea, (3) evaluation for GI bleeding, and (4) suspicion of peptic disease.
Abnormalities on endoscopy were categorized into infectious findings, anatomical abnormalities, evidence of peptic disease, evidence of allergic disease, and other findings. Infectious findings were defined as positive if there was growth greater than 105 organisms per milliliter from duodenal aspirates [6] and/or severe villous blunting of the small bowel mucosa on histopathology. Anatomical abnormalities included strictures, ulcerations, and esophageal or gastric varices, all grossly visualized on endoscopy. Peptic disease was defined as either gastritis or esophagitis and was diagnosed by standard histopathologic criteria [7]. Allergic disease was diagnosed by histopathologic evidence of greater than 15 eosinophils per high-powered field, presence of eosinophils in the intramuscular layers [8,9], or presence of clusters eosinophils in the esophageal mucosa [10].
2. Results
There were a total of 61 endoscopies performed on 27 patients (15 male) during the study period. Table 1 shows baseline characteristics and reasons for initial endoscopy in the cohort.
Table 1.
Characteristics of 27 children with IF and indications for GI endoscopy (n = 61)
| Median (interquartile range) age (y) |
1.8 (0.8, 4.5) | |
| N (%) male sex | 15 (55%) | |
| Conditions leading to IF (n = 27) | Intestinal atresias (n = 7) | 26% |
| Necrotizing enterocolitis (n = 6) | 22% | |
| Gastroschisis (n = 4) | 15% | |
| Other (n = 4) | 15% | |
| Pseudoobstruction | ||
| Twin conjoinment | ||
| Trauma | ||
| Chylous ascites | ||
| Hirschprung disease (n = 3) | 11% | |
| Segmental volvulus (n = 3) | 11% | |
| Indications for endoscopy (n = 61) a | Diarrhea (n = 36) | 39% |
| GI bleeding (n = 34) | 36% | |
| Suspected bacterial overgrowth (n = 34) | 36% | |
| Suspected peptic ulcer disease (n = 9) | 15% |
Not mutually exclusive.
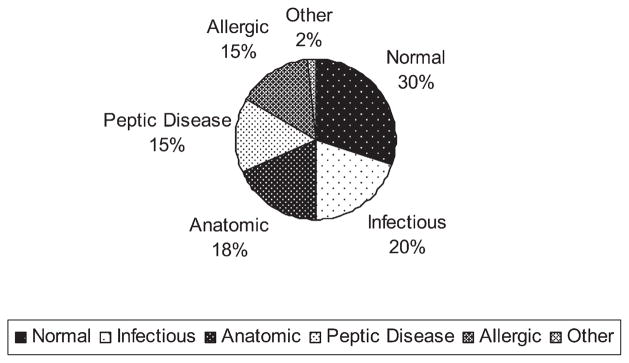
There were 61 total endoscopies: 34 esophagogastroduodenoscopies (EGD), 17 colonoscopies, 7 flexible sigmoidoscopies, and 3 ileoscopies. Seventy percent (n = 43) of the endoscopies revealed a gross, histopathologic, or microbiologic abnormality. These results, shown in Fig. 1, included those of normal (30%), infectious (20%), anatomical (18%), peptic (15%), allergic (15%), and other (2%) findings.
Fig. 1.
Distribution of findings on 61 GI endoscopies in 27 patients with IF.
Twelve patients were found to have infectious findings, and all but 2 of these patients had bacteria grown from duodenal aspirates, confirming a diagnosis of bacterial overgrowth [11,12]. For these 2 patients who did not have duodenal aspirates performed, histopathology revealed severe villous blunting consistent with bacterial overgrowth. In total, there were 15 duodenal aspirates obtained from 34 EGDs, and 11 (73%) grew a spectrum of 17 different bacterial species (Table 2).
Table 2.
Spectrum of bacterial species found on duodenal aspirates
| Findings on duodenal aspirates (n = 15) | |
|---|---|
| No Organisms | 4 |
| Gram-positive organisms (47%) | |
| Enterococcus faecium | 2 |
| Enterococcus not specified | 1 |
| Enterococcus faecalis | 1 |
| Staphylococcus aureus | 2 |
| Stomatococcus | 3 |
| S. viridans (alpha) | 4 |
| Corynebacterium | 1 |
| Lactobacillus not specified | 1 |
| Gram-negative organisms (53%) | |
| E. coli | 5 |
| Klebsiella oxytoca | 1 |
| Klebsiella pneumoniae | 6 |
| Haemophilus influenzae | 1 |
| Haemophilus not influenza | 2 |
| Proteus mirabilis | 2 |
| Serratia marcescens | 1 |
| Enterobacter cloacae | 1 |
| Pseudomonas aeruginosa | 1 |
Fifty-three percent of the bacteria were gram-negative organisms, and 47% were gram-positive organisms. The most common gram-negative organisms found were Klebsiella (35%) and Escherichia coli (20%), whereas the most common gram-positive organisms were Streptococcus viridans (27%) and Enterococcus (27%). Six duodenal cultures (55%) grew both gram-positive and gram-negative organisms, whereas 4 cultures (36%) grew strictly gram negatives, and 1 culture (9%) only grew gram-positive organisms. Nine of the 11 positive aspirates were associated with additional findings of erythema, villous atrophy, copious duodenal fluid, or gross mucosal ulcerations. One of the patients with severe bacterial overgrowth and lactic D-acidosis underwent a serial transverse enteroplasty after her endoscopic findings [13].
Among the 11 anatomical findings of ulcerations, strictures, and varices, one patient underwent a small bowel resection and stricturoplasty. This patient had GI bleeding and was found to have gross evidence of perianastomotic ulcers that were initially managed with sulfasalazine. She had continued GI bleeding, and when repeat lower endoscopy revealed persistent anastomotic ulcers, she underwent surgical resection of these lesions.
Seven patients were found to have evidence of peptic disease, either gastritis or esophagitis. Six of these patients were subsequently started on a proton pump inhibitor (PPI) or had their PPI dose increased [14].
Previously undiagnosed allergic disease was identified in 5 patients, based on their initial endoscopic findings. All diagnoses were made by correlating gross endoscopic appearance with histopathologic findings [7,8,15]. As a result of these diagnoses, all 5 patients were transitioned to a hypoallergenic formula or diet [16], and 1 patient was started on sulfasalazine [16].
Overall, 24 (89%) of 27 patients were found to have at least one abnormality on endoscopy, and 8 patients (30%) were found to have multiple abnormalities. Changes in clinical management were defined as surgical intervention or a change in medical or nutritional therapy. We noted a change in clinical management in 20 patients (83% of the 24 patients with abnormal endoscopic findings and 74% of the 27 total patients) after their initial endoscopies (Table 3).
Table 3.
Results of initial endoscopy in 27 patients (44 total endoscopies)
| Patient | Age (y) a | Procedure(s) | Indication(s) | Finding(s) | Change in management |
|---|---|---|---|---|---|
| 1 | 5.0 | EGD | Suspected bacterial overgrowth | Infectious, peptic | Tailored antibiotics, started PPIs |
| 2 | 0.7 | EGD, colonoscopy | Suspected bacterial overgrowth | Infectious | Tailored antibiotics |
| 3 | 0.8 | Colonoscopy | GI bleeding | Anatomical | None |
| 4 | 0.8 | EGD, colonoscopy | Diarrhea | Normal | None |
| 5 | 25.9 | EGD, colonoscopy | Suspected bacterial overgrowth, diarrhea | Peptic | Started PPI |
| 6 | 1.8 | EGD, colonoscopy | Diarrhea | Infectious | Started antibiotics |
| 7 | 0.7 | EGD, colonoscopy | GI bleeding | Allergic | Started sulfasalazine |
| 8 | 1.3 | EGD | GI bleeding | Infectious | Tailored antibiotics |
| 9 | 3.2 | EGD | Suspected peptic disease | Allergic | Change in diet |
| 10 | 4.1 | EGD, colonoscopy | GI bleeding | Peptic | None |
| 11 | 4.5 | EGD, Flex Sig | Suspected bacterial overgrowth, suspected peptic disease, diarrhea | Normal | None |
| 12 | 7.5 | EGD, ileoscopy | Suspected bacterial overgrowth, suspected peptic disease | Anatomical, infectious | Small bowel resection, stricturoplasty, tailored antibiotics |
| 13 | 1.5 | EGD, ileoscopy | Diarrhea | Infectious | Started antibiotics |
| 14 | 1.8 | EGD, colonoscopy | Suspected bacterial overgrowth, diarrhea | Anatomical, infectious | Tailored antibiotics |
| 15 | 0.9 | EGD, colonoscopy | GI bleeding | Infectious | None |
| 16 | 6.9 | EGD, colonoscopy | Suspected bacterial overgrowth diarrhea, suspected peptic disease | Anatomical, infectious | Tailored antibiotics |
| 17 | 0.6 | EGD | Suspected peptic disease | Peptic | Started PPI |
| 18 | 1.5 | Flex Sig | GI bleeding, diarrhea | Allergic | Change in diet |
| 19 | 18.1 | EGD | Suspected bacterial overgrowth | Infectious, peptic | Tailored antibiotics, started PPI, serial transverse enteroplasty |
| 20 | 3.8 | EGD, colonoscopy | Suspected bacterial overgrowth | Normal | None |
| 21 | 0.5 | EGD | Suspected peptic | Peptic | Increased PPI dose |
| 22 | 4.1 | EGD, colonoscopy | GI bleeding | Anatomical | Started sulfasalazine |
| 23 | 13.4 | EGD, Flex Sig | Suspected bacterial overgrowth, suspected peptic disease | Allergic | Change in diet |
| 24 | 4.7 | EGD | Suspected peptic disease | Anatomical | None |
| 25 | 2.2 | EGD, colonoscopy | Suspected bacterial overgrowth diarrhea | Anatomical, infectious | Tailored antibiotics |
| 26 | 0.2 | Flex Sig | GI bleeding | Allergic | Change in diet |
| 27 | 0.6 | EGD, Flex Sig | GI bleeding | Allergic | Change in diet |
Flex Sig = flexible sigmoidoscopy.
At time of initial endoscopy.
Four patients had follow-up endoscopies (15%) to follow progression of the initially diagnosed disease (eg, allergic colitis, varices). Three patients had additional endoscopies at a later date, but most had developed new indications. One patient has required multiple endoscopies because of persistent GI bleeding.
3. Discussion
Among 27 children with IF, because of a broad range of conditions who underwent GI endoscopy, we found a high rate of abnormalities. Seventy percent of all procedures demonstrated an abnormality on gross endoscopic appearance, on histopathology, or from microbiology data. Of these 27 patients, 89% were found to have some abnormality, and 30% were noted to have multiple findings. Our data are the first to our knowledge to document a high diagnostic yield of GI endoscopy in pediatric patients with IF.
The high incidence of peptic disease was not surprising because increased gastric secretions [17] and hypergastrinemia [18] are well-known features of short bowel syndrome. The findings of friability, loss of vascular pattern, and patchy erythema, and prominent eosinophilia are consistent with prior reports of allergic disease [15]. Patients with persistent symptoms of bacterial overgrowth [11] despite antibiotics, or a complicated clinical picture that did not easily lend to this diagnosis, underwent diagnostic endoscopies. Because treatment of bacterial overgrowth is best based on speciation and sensitivities, we tailored patients’ antibiotic regimens accordingly [12].
As with any retrospective review, selection bias may have been one factor that contributed to our findings. Patients who were more ill and who had more complex medical issues may have been more likely to undergo diagnostic endoscopy. The strength of our report is the minimal variability in the interpretation of the gross appearances of the intestinal mucosa; nearly all (57 of 61 procedures) were performed by the same pediatric gastroenterologist.
In our retrospective study, we noted a change in clinical management in 74% of these patients based on initial endoscopic findings. Of the 27 patients, all closely followed at our CAIR, only 4 patients required repeat endoscopies for persistent symptoms or the development of new symptoms.
The diagnostic yield of GI endoscopy varies greatly depending on the patients who are undergoing evaluation [19]. Among children with recurrent abdominal pain, the yield of upper GI endoscopy is low [20], whereas among patients with proximal GI mucosal disease [21], there appears to be a higher yield. Our overall diagnostic yield of 70% exceeds reports of endoscopic yield in patients with Crohn’s disease [21] and other conditions.
Our patients with IF are seen in a multidisciplinary program, where their management is discussed and formulated. If initial laboratory work, radiologic studies, and clinical examination fail to provide a clear diagnosis for GI symptoms, GI endoscopies are typically used for diagnostic purposes. Our findings of a high yield for GI endoscopy, as well as therapeutic changes that generally follow these procedures, confirm that endoscopy is an important diagnostic tool in pediatric IF.
Footnotes
Presented at the 39th Annual Meeting of the Canadian Association of Pediatric Surgeons, August 23–26, 2007, St John's Newfoundland, Canada.
References
- 1.Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:S16–S28. doi: 10.1053/j.gastro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Lemberg DA, Clarkson CM, Bohane TD, et al. Role of esophagogastroduodenoscopy in the initial assessment of children with inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1696–700. doi: 10.1111/j.1440-1746.2005.03954.x. [DOI] [PubMed] [Google Scholar]
- 3.Abreu MT, Harpaz N. Diagnosis of colitis: making the initial diagnosis. Clin Gastroenterol Hepatol. 2007;5:295–301. doi: 10.1016/j.cgh.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Tutuian R. Update in the diagnosis of gastroesophageal reflux disease. J Gastrointestin Liver Dis. 2006;15:243–7. [PubMed] [Google Scholar]
- 5.Angtuaco TL, Reddy SK, Drapkin S, et al. The utility of urgent colonoscopy in the evaluation of acute lower gastrointestinal tract bleeding: a 2-year experience from a single center. Am J Gastroenterol. 2001;96:1782–5. doi: 10.1111/j.1572-0241.2001.03871.x. [DOI] [PubMed] [Google Scholar]
- 6.Dukowicz AC, Lacey BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol. 2007;3:112–22. [PMC free article] [PubMed] [Google Scholar]
- 7.Dahms BB. Reflux esophagitis: sequelae and differential diagnosis in infants and children including eosinophilic esophagitis. Pediatr Dev Pathol. 2004;7:5–16. doi: 10.1007/s10024-003-0203-5. [DOI] [PubMed] [Google Scholar]
- 8.Odze RD, Bines J, Leichtner AM, et al. Allergic proctocolitis in infants: a prospective clinicopathologic biopsy study. Hum Pathol. 1993;24:668–74. doi: 10.1016/0046-8177(93)90248-f. [DOI] [PubMed] [Google Scholar]
- 9.Odze RD, Wershil BK, Leichtner AM, et al. Allergic colitis in infants. J Pediatr. 1995;126:163–70. doi: 10.1016/s0022-3476(95)70540-6. [DOI] [PubMed] [Google Scholar]
- 10.Antonioli DA, Furuta GT. Allergic eosinophilic esophagitis: a primer for pathologists. Semin Diagn Pathol. 2005;22:266–72. doi: 10.1053/j.semdp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman SS, Loseke CA, Lupo JV, et al. Influence of bacterial overgrowth and intestinal inflammation on duration of parenteral nutrition in children with short bowel syndrome. J Pediatr. 1997;131:356–61. doi: 10.1016/s0022-3476(97)80058-3. [DOI] [PubMed] [Google Scholar]
- 12.Vanderhoof JA, Young RJ, Murray N, et al. Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome. J Pediatr Gastroenterol Nutr. 1998;27:155–60. doi: 10.1097/00005176-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Modi BP, Langer M, Duggan C, et al. Serial transverse enteroplasty for management of refractory D-lactic acidosis in short-bowel syndrome. J Pediatr Gastroenterol Nutr. 2006;43:395–7. doi: 10.1097/01.mpg.0000228116.52229.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel AS, Pohl JF, Easley DJ. What’s new: proton pump inhibitors and pediatrics. Pediatr Rev. 2003;24:12–5. doi: 10.1542/pir.24-1-12. [DOI] [PubMed] [Google Scholar]
- 15.Taylor SF, Sondheimer JM, Sokol RJ, et al. Noninfectious colitis associated with short gut syndrome in infants. J Pediatr. 1991;119:24–8. doi: 10.1016/s0022-3476(05)81033-9. [DOI] [PubMed] [Google Scholar]
- 16.Sicherer SH. Clinical aspects of gastrointestinal food allergy in childhood. Pediatrics. 2003;111:1609–16. [PubMed] [Google Scholar]
- 17.Frederick PL, Sizer JS, Osborne MP. Relation of massive bowel resection to gastric secretion. N Engl J Med. 1965;272:509–14. doi: 10.1056/NEJM196503112721004. [DOI] [PubMed] [Google Scholar]
- 18.Straus E, Gerson CD, Yalow RS. Hypersecretion of gastrin associated with the short bowel syndrome. Gastroenterology. 1974;66:175–80. [PubMed] [Google Scholar]
- 19.Thakkar K, Gilger MA, Shulman RJ, et al. EGD in children with abdominal pain: a systematic review. Am J Gastroenterol. 2007;102:654–61. doi: 10.1111/j.1572-0241.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 20.Ashorn M, Maki M, Ruuska T, et al. Upper gastrointestinal endoscopy in recurrent abdominal pain of childhood. J Pediatr Gastroenterol Nutr. 1993;16:273–7. doi: 10.1097/00005176-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Castellaneta SP, Afzal NA, Greenberg M, et al. Diagnostic role of upper gastrointestinal endoscopy in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2004;39:257–61. doi: 10.1097/00005176-200409000-00006. [DOI] [PubMed] [Google Scholar]