Abstract
Objective
To determine risk factors for intestinal failure (IF) in infants undergoing surgery for necrotizing enterocolitis (NEC).
Study design
Infants were enrolled in a multicenter prospective cohort study. IF was defined as the requirement for parenteral nutrition for ≥ 90 days. Logistic regression was used to identify predictors of IF.
Results
Among 473 patients enrolled, 129 had surgery and had adequate follow-up data, and of these patients, 54 (42%) developed IF. Of the 265 patients who did not require surgery, 6 (2%) developed IF (OR 31.1, 95% CI, 12.9 –75.1, P < .001). Multivariate analysis identified the following risk factors for IF: use of parenteral antibiotics on the day of NEC diagnosis (OR = 16.61, P = .022); birth weight < 750 grams, (OR = 9.09, P < .001); requirement for mechanical ventilation on the day of NEC diagnosis (OR = 6.16, P = .009); exposure to enteral feeding before NEC diagnosis (OR=4.05, P = .048); and percentage of small bowel resected (OR = 1.85 per 10 percentage point greater resection, P = .031).
Conclusion
The incidence of IF among infants undergoing surgical treatment for NEC is high. Variables characteristic of severe NEC (low birth weight, antibiotic use, ventilator use, and greater extent of bowel resection) were associated with the development of IF.
Intestinal failure (IF) is a chronic condition characterized by a reduction in the functional intestinal mass necessary for adequate digestion and absorption for nutrient, fluid, and growth requirements.1 The leading cause of IF in infancy is short bowel syndrome (SBS), in which there is reduced mucosal surface area as a result of surgical or congenital conditions. The consequences of IF in infants are severe and include an increased mortality rate, recurrent central venous catheter infections, parenteral nutrition–associated liver disease, chronic diarrhea, multiple micronutrient deficiencies, osteopenia, and poor growth.2–4 The incidence of and risk factors for SBS have previously been determined in small, retrospective, single-center case series.2,5–9 Variables that have been identified as predictors of SBS include low birth weight,9 extent of bowel resection (>45 cm),2 resection of the ileocecal valve and reduced colonic remnant.8 Limited data also support an association between IF in infants and postoperative nutritional management, including early enteral feeding, and the use of breast milk and elemental formulas.6
Necrotizing enterocolitis is the leading cause of SBS in infancy, with a reported prevalence ranging from 13% to 45% in several cases series of premature infants.8,10–12 As survival for infants with premature birth is increasing with better medical and surgical management,13,14 the potential exists for more children to survive with compromised gastrointestinal function and IF, especially because of necrotizing enterocolitis (NEC). The aim of our study was to determine the risk factors for IF in infants undergoing surgery for NEC in a prospective multicenter fashion.
Methods
This observational, prospective cohort study was conducted at six academic medical centers in the United States: Children’s Hospital Boston, Boston, Massachusetts; Texas Children’s Hospital, Houston, Texas; Lucille S. Packard Children’s Hospital, Palo Alto, California; Mattel Children’s Hospital, Los Angeles, California; University of California at San Francisco Children’s Hospital, San Francisco, California and Yale–New Haven Children’s Hospital, New Haven, Connecticut. The protocol was approved by the Institutional Review Board at each site and informed consent was obtained when required; consent waivers were granted at some sites. Entry criteria for this study were those commonly used to identify infants with suspected or confirmed NEC.15 Patients were enrolled on the day they met these criteria, which included having at least 1 finding from each of a prespecified set of historical factors, physical examination findings, and radiographic findings. Historical factors included feeding intolerance, apneic/bradycardic episodes, oxygen desaturation, or grossly blood stools. Physical examination findings included abdominal distention, capillary refill time > 2 seconds, abdominal wall discoloration, or abdominal tenderness. Radiographic findings included pneumatosis intestinalis, dilated bowel, portal venous gas, ileus, pneumoperitoneum, air/fluid levels, thickened bowel walls, ascites, peritoneal fluid, or abnormal bowel gas pattern. Subjects were excluded if they had any major gastrointestinal anomalies or had undergone abdominal surgery before enrollment. The main results of the cohort study have been published.16
Surgical treatment for NEC was defined as either placement of a peritoneal drain, or exploratory laparotomy with or without intestinal resection. The decision for drain placement versus laparotomy, as well as the extent and location of bowel resection, was made by the treating surgeon. We included all operations within 14 days of the first procedure when constructing surgical predictors of IF. We defined IF as dependence on parenteral nutrition for ≥90 days17 after meeting the study entry criteria. Full enteral feeding was defined as receiving at least 100 kcal/kg/day from enteral nutrition and demonstrating a mean weight gain of >15 g/d over 7 days or being discharged home after discontinuing parenteral nutrition. Subjects who died or were lost to followup before reaching 90 days after entry (without first meeting the criteria for full enteral feeding) could not be classified as developing or not developing IF and therefore were excluded from the analysis.
Demographic, maternal, prenatal, and intrapartum data, medication history (for both mother and child), and newborn history before study entry were abstracted from medical records. Data collected daily during the week before diagnosis included mode of feeding (parenteral or enteral), method (bolus or continuous), type of enteral nutrition (breast milk, formula, or a combination), volume of formula, and the weight of the infant. After study entry, detailed clinical data were collected prospectively on a daily basis until subjects underwent surgery, reached full feedings, received another diagnosis, or died. If an operation was performed, surgical findings were recorded and data on postoperative course were collected weekly. Data collected included medications, laboratory and imaging evaluations, ventilation status, fluid/hemodynamic status, and blood culture results. Study endpoints for this analysis were the development of IF or the achievement of full enteral feedings as defined above.
Statistics
Factors were selected in a multistage analysis from a large set of demographic, clinical, and laboratory variables that would be available in clinical practice at the time of NEC diagnosis, as well as several surgical variables. The first stage of the modeling process produced 10 to 15 variables deemed clinically or statistically promising on the basis of univariate analysis with the Fisher exact tests or t-tests. The rest of the modeling process used multivariable logistic regression, with associations expressed as odds ratios.18 Models were fit with the logistic procedure in SAS 9.1 (SAS Institute, Cary, North Carolina).19 Confidence intervals were based on profile likelihoods, and P values were based on Wald tests. All P values are 2-sided.
Several of the variables selected at the first stage of the modeling process were subsequently discarded after considering their impact in the context of small groups of clinically related variables. This left 9 candidate variables. The discarded variables were either clinically or statistically related to other variables (eg, gestation age was dropped in favor of birth weight). The final stage of the modeling process was based on step-down selection among the 9 variables, with a final check for the potential addition of previously discarded variables. Both statistical and clinical considerations were used during the model building process.
Factors with missing data for substantial proportions of the sample were transformed into pairs of binary variables, one indicating missingness and the other indicating the outcome of interest. This transformation allowed patients with missing data on some predictors to remain in the multivariable models, thus contributing information about their other characteristics. The pair of variables was always considered together in a model, but the decision on whether to retain or drop the pair was based only on the effect of the variable of interest, and when reporting results we report only the variable of interest.
Although some authors have expressed small bowel resection as a percent of small bowel length,20,21 this calculation requires a measurement of both total and resected small bowel lengths, which were available for only 14 of our 49 patients with resection. We therefore imputed the percent of small bowel resected in a uniform manner by assigning a value of 0% for the 55 patients with no resection, and calculating the percent for the 49 patients with resection on the basis of an imputed total small bowel length by use of previously published data (Appendix; available at www.jpeds.com).20,21
The percent of small bowel effectively resected was constructed as a composite of the percent of small bowel actually resected and whether a diverting jejunostomy was created. Both original variables (actual resection and the creation of a jejunostomy) were individually important predictors but were also highly related to each other. The composite variable was considered clinically meaningful and also served to avoid the unreliability of jointly estimated effects of highly correlated factors. This variable was derived by assuming that the jejunostomy bypassed the distal 50% of the small bowel. If the jejunostomy was taken down within 90 days, the final composite effective percent resected was calculated as a weighted average of 2 percents, one while the jejunostomy was functional, and the other while it was not.
Results
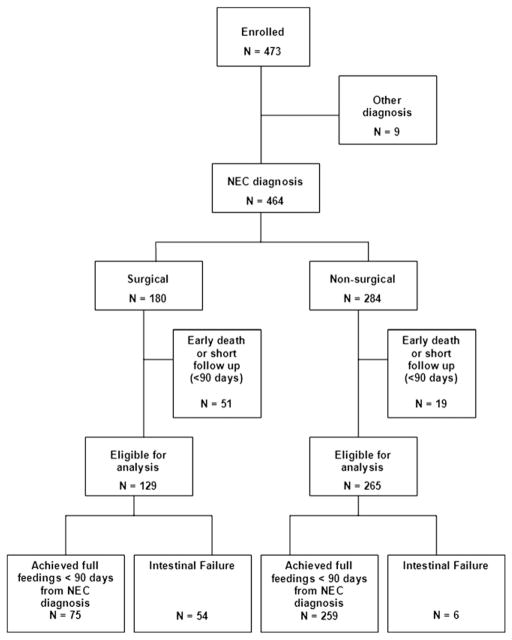
We enrolled 473 patients from February 2004 through February 2007 (Figure). Most subjects excluded either had diagnoses other than NEC or died early; only 9 were lost to follow-up. Of the 129 surgical NEC infants eligible for analysis, 54 (42%) had development of IF and 75 (58%) achieved full feedings before 90 days. The incidence of IF ranged from 20% to 60% across the 6 sites (P = .64). Among 265 infants who required only medical therapy for NEC and who had adequate follow-up, only 6 (2%) had development of IF. The incidence of IF in patients who required surgical therapy versus those who did not was significantly different (42% vs 2%, OR 31.1, 95% CI 12.9 to 75.1, P < .001). The small number of nonsurgical patients who had development of IF precluded meaningful analysis of risk factors in this subgroup, so the remainder of this analysis focuses on the surgical cohort.
Figure.
Study cohort enrolled from February 2004 through February 2007.
Baseline demographic and clinical characteristics of the surgical cohort are presented in Table I. As expected in a group of patients with NEC, they were predominantly premature (mean ± SD gestational age 28.6 ± 4.2 weeks), of low birth weight (1183 ± 680 g) and 69% were male; 65% of the infants were born by Cesarean section, and 61% were intubated at delivery. Seventy-one percent received surfactant, and 13% received steroids before NEC diagnosis. Most patients received enteral nutrition before the diagnosis of NEC, and more than one third of those enterally fed received breast milk (36%). The most common findings at diagnosis of NEC were the presence of abdominal distention in 90% of the patients, followed by acidosis (76%), and the need for respiratory support (72%), characteristics reflecting the severity of NEC.
Table I.
Baseline characteristics of 129 infants undergoing surgical treatment for necrotizing enterocolitis
| Characteristics (n = 129 unless otherwise indicated) | |
|---|---|
| Demographic factors | |
| Gestational age, weeks (Mean ± SD) | 28.6 ± 4.2 |
| Birth weight, g (n = 126) (Mean ± SD) | 1183 ± 680 |
| Birth length, cm (n = 113) (Mean ± SD) | 36.5 ± 5.8 |
| Head circumference, cm (n = 111) (Mean ± SD) | 25.2 ± 3.6 |
| Sex, male | 89 (69%) |
| Maternal factors | |
| Age of mother, years (n = 126) (Mean ± SD) | 27.2 ± 6.7 |
| History of gestational hypertension (n = 118) | 26 (22%) |
| History of gestational diabetes (n = 116) | 8 (7%) |
| Steroid use during pregnancy (n = 120) | 91 (76%) |
| Infant factors before diagnosis of NEC | |
| Vaginal delivery | 45 (35%) |
| Intubated at delivery | 79 (61%) |
| Resuscitation drugs used at delivery (n = 128) | 18 (14%) |
| Surfactant use before diagnosis | 89 (71%) |
| Steroid use before diagnosis | 16 (13%) |
| Positive blood culture within 5 days before diagnosis (n = 102) | 37 (36%) |
| Feeding history | |
| Received enteral nutrition before diagnosis of NEC (n = 122) | 100 (82%) |
| Received breast milk before diagnosis of NEC* (n = 100) | 36 (36%) |
| Received parenteral nutrition before diagnosis of NEC (n = 128) | 115 (90%) |
| Characteristics at diagnosis of NEC | |
| Abdominal distention | 116 (90%) |
| Antibiotic use on day of diagnosis of NEC (n = 128) | 114 (89%) |
| Acidosis† (n = 106) | 81 (76%) |
| Ventilator use at diagnosis (n = 129) | 93 (72%) |
| Feeding intolerance (n = 128) | 77 (60%) |
| Patent ductus arteriosus‡ (n = 90) | 49 (54%) |
| Pneumatosis intestinalis | 59 (46%) |
| Presence of gross blood in stool (n = 128) | 34 (27%) |
| Portal venous gas | 19 (15%) |
Among those fed enterally before diagnosis.
Defined as pH < 7.3, or HCO3 < 16 mEq/L.
Diagnosis confirmed by echocardiogram
A comparison of characteristics between patients who had development of IF and patients who achieved full feedings within 90 days is shown in Table II. Infants who had development of IF had shorter gestation by an average of almost 3 weeks (P ≤ .001) and lower birth weights by more than 400 g (P = .001). Among maternal factors, age, history of gestational hypertension, and use of steroids during pregnancy were not significantly different between the 2 groups. The need for intubation at delivery was significantly greater in the group who had development of SBS (72% vs 53%, P = .043). Exposure to enteral feedings before the diagnosis of NEC was not significantly different (P = .231) between the 2 groups, nor was the type of enteral feeds. Presence of portal venous gas (P = .048), use of parenteral antibiotics (P = .008) and ventilator use (P < .001) (both on the day of NEC diagnosis) were significantly different, suggesting a greater severity of NEC at diagnosis in those with development of IF.
Table II.
Characteristics of patients with development of IF and patients who achieved full feedings in less than 90 days
| IF (n = 54)* | Full feedings (n = 75)* | P value | |
|---|---|---|---|
| Demographic factors | |||
| Gestational age, weeks | 27.0 ± 2.9 | 29.7 ± 4.6 | <.001 |
| Birth weight, g | 948 ± 422 (n = 53) | 1355 ± 777 | .001 |
| Birth length, cm | 34.8 ± 4.8 (n = 49) | 37.8 ± 6.1 (n = 64) | .004 |
| Sex, male | 37 (68%) | 52 (69%) | 1.000 |
| Maternal factors | |||
| Age of mother, years | 27.9 ± 7.5 (n = 53) | 26.7 ± 6.1 (n = 73) | .343 |
| History of gestational hypertension | 13 (26%) (n = 50) | 13 (19%) (n = 68) | .380 |
| Steroid use during pregnancy | 44 (83%) (n = 53) | 47 (70%) (n = 67) | .133 |
| History at Birth | |||
| Intubated at delivery | 39 (72%) | 40 (53%) | .043 |
| Resuscitation drugs used at delivery | 10 (18%) | 8 (11%) | .353 |
| Feeding history | |||
| Received enteral nutrition before diagnosis of NEC | 44 (88%) (n = 50) | 56 (78%) (n = 72) | .231 |
| Received breast milk before diagnosis of NEC† | 20 (45%) (n = 44) | 16 (29%) (n = 56) | .096 |
| Received parenteral nutrition before diagnosis of NEC | 50 (93%) | 65 (88%) | .555 |
| Characteristics at diagnosis of NEC | |||
| Feeding intolerance | 29 (55%) (n = 53) | 48 (64%) | .360 |
| Pneumatosis intestinalis | 26 (48%) | 33 (44%) | .721 |
| Portal venous gas | 12 (22%) | 7 (9%) | .048 |
| Abdominal distention | 50 (93%) | 66 (88%) | .556 |
| Antibiotic use on day of diagnosis of NEC | 52 (98%) (n = 53) | 62 (83%) | .008 |
| Ventilator use on day of diagnosis with NEC | 48 (89%) | 45 (60%) | <.001 |
| Surgical factors | |||
| Small bowel resected, cm | 12.3 ± 18.1 (n = 43) | 3.8 ± 7.2 (n = 61) | .001 |
| Percent of small bowel resected | 13.6 ± 19.6 (n = 43) | 4.0 ± 7.8 (n = 61) | <.001 |
| Percent of small bowel effectively resected‡ | 21.6 ± 22.6 (n = 47) | 4.6 ± 9.7 (n = 61) | <.001 |
| Large bowel resected, cm | 1.2 ± 6.0 (n = 43) | 1.9 ± 4.2 (n = 55) | .495 |
| Creation of jejunostomy | 17 (31%) | 1 (1.3%) | <.001 |
| Creation of ileostomy | 27 (50%) | 44 (59%) | .372 |
| Resection of ileocecal valve | 9 (17%) | 20 (27%) | .205 |
Data are summarized by mean ± SD or n (%).
Unless otherwise indicated.
Among those fed enterally before diagnosis
Composite variable including percent resected or distal to jejunostomy. See Methods.
Among operative factors reviewed, the amount of small intestine resected was significantly greater (12.3 ± 18.1 cm vs 3.8 ± 7.2 cm) (P = .001) among those infants with development of IF compared with those without. Similar results were observed for the percent of small bowel resected (13.3 ± 19.6 % vs 4.0 ± 7.8 %; P < .001). The extent of colon resection was not significantly different between the 2 groups (P = .495) nor was resection of the ileocecal valve (P = .205). Although creation of an ileostomy was not significantly different between the 2 groups (P = .372), the creation of a diverting jejunostomy was highly significant (P < .001). In fact, 17 of 18 patients who had a jejunostomy had development of IF. Among 88 subjects who underwent diverting ostomy placement, 34 of these (39%) underwent takedown within 90 days of study entry. The mean elapsed time for these 34 subjects was 60 days (range 42–84). The mean ± SD percentages of small bowel resected among subjects with and without a jejunostomy were 26.3 ± 25.7 and 5.1 ± 9.6, respectively. Our composite percent of small bowel effectively resected (percent of small bowel resected or distal to jejunostomy), also differed significantly between the groups (21.6 ± 22.6 vs 4.6 ± 9.7; P < .001).
Multivariate Analysis
In multivariate analysis, we identified five independent risk factors for the development of IF (Model 1, Table III). Antibiotic use at NEC diagnosis (OR = 16.61, P = .022); birth weight <750 g (OR = 9.09, P < .001), exposure to any enteral feedings before the diagnosis of NEC (OR = 4.05, P = .048), ventilator use at NEC diagnosis (OR = 6.16, P = .009), and percentage of small bowel resected (OR=1.85 per 10 percentage point resection, P = .031) were all independent and significant predictors for the development of IF. We chose to retain presence of portal venous gas in the model, although it was not significant at the 0.05 level (OR = 3.88, P = .086).
Table III.
Two multivariate models for the development of IF
| Variables | Model 1
|
Model 2
|
||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Antibiotic use at diagnosis of NEC | 16.61 | (2.08, 375.44) | .022 | 15.84 | (2.02, 353.36) | .024 |
| Birth weight < 750 g | 9.09 | (2.59, 38.12) | <.001 | 8.32 | (2.51, 32.83) | <.001 |
| Ventilator use at diagnosis of NEC | 6.16 | (1.76, 27.84) | .009 | 6.08 | (1.79, 26.27) | .007 |
| Received enteral nutrition before diagnosis of NEC | 4.05 | (1.10, 18.34) | .048 | 3.97 | (1.10, 17.42) | .047 |
| Portal venous gas | 3.88 | (0.85, 20.30) | .086 | 3.93 | (0.89, 20.09) | .078 |
| Creation of jejunostomy | 3.48 | (0.47, 71.85) | .284 | — | — | — |
| Percent of small bowel resected* | 1.85 | (1.09, 3.38) | .031 | — | — | — |
| Percent of small bowel effectively resected* † | — | — | — | 1.71 | (1.19, 2.69) | .008 |
Effect on odds per 10 percentage point larger resection
Composite variable including percent resected or distal to jejunostomy. See Methods.
Although jejunostomy creation was not statistically significant in this model at the 0.05 level, when small bowel resection was removed from the model, it was nearly significant (P = .057). When the percent of small bowel effectively resected (a composite variable based on intestinal resection and jejunostomy) was used in place of small bowel resection and jejunostomy, the effect was highly significant (OR= 1.71 per 10 percentage point increase in resection, P = .008). Other effect estimates were essentially unchanged in this model (Model 2, Table III).
Discussion
Surgical management of NEC often involves the competing priorities of resecting diseased bowel and controlling sepsis on one hand, and preserving bowel length on the other. Bowel-preserving strategies for NEC are designed to limit IF.11 Our results confirm that infants undergoing surgery for NEC have a considerably higher risk for the development of IF compared with those not requiring surgery (OR 31.1, 95% CI 12.9–75.1, P < .001). In addition, measures of disease severity at or before the day of NEC diagnosis are strong and important risk factors for the development of IF, as noted by the significant relationships between IF and antibiotic use, ventilator dependence, portal venous gas, and extent of bowel resection. The fact that ventilator dependence and antibiotic use on the day of NEC diagnosis were independent risk factors for IF also suggests that infants with more indolent courses of NEC may be at more risk of IF than those whose NEC presents abruptly.
Our finding of a high incidence of IF (42%) among infants undergoing surgical therapy for NEC is comparable with the findings of a multiinstitutional trial comparing laparotomy with peritoneal drainage for treatment for NEC.22 Among 76 infants surviving ≥ 90 days after surgery, 33 (43%) remained dependent on parenteral nutrition.22 In contrast, in a study comprising 175 neonates who underwent any gastrointestinal surgery, the incidence of IF was reported as 23%.9 However, the mean gestational age of patients in this study was higher than in our cohort (mean [SD] gestational age 34.7 [5.2] weeks vs 28.6 [4.3]) and only 23% of the cohort had NEC. These differences could explain the lower incidence of IF.
Among risk factors identified in our analysis for the development of IF, the creation of a jejunostomy was highly associated with IF in an unadjusted analysis, but the association was not significant after adjusting for the amount of small bowel resected. Although this seems to suggest that, after controlling for the amount of bowel resected, creation of jejunostomy is not associated with IF, there are compelling statistical and clinical reasons not to dismiss jejunostomy as a potential predictor. First, patients with jejunostomy tended to have a greater length of bowel resected and there was only 1 patient with jejunostomy who did not have development of IF. This correlation between covariates, coupled with sparse counts in the outcome variable, produced imprecise estimates in the model which included both variables, leaving uncertainty about the results. Second, the creation of a jejunostomy may reflect more extensive or more proximal intestinal disease, which in turn is manifest by the need for a proximal diversion. The diversion temporarily renders the distal bowel effectively nonfunctional, just as resecting that portion would have done. It is for these reasons that we created a single composite variable which combined jejunostomy with the amount of small bowel resected (Model 2, Table III). This composite variable attempts to measure the percent of small bowel effectively resected, that is, rendered nonfunctional either by having been resected or by being distal to a jejunostomy. More proximal bowel disease or resection may also indicate greater need for parenteral nutrition, as the absorptive area of the jejunum and ileum is bypassed. Our study did not allow us to investigate whether jejunostomy and associated diversion of the fecal stream is inherently damaging or whether jejunostomy is simply a marker of severe disease.
In our study, resection of the colon and creation of ileostomy did not differ significantly between those infants who did and did not have development of IF. In contrast, Wales et al23 reported that the creation of ileostomy was an independent risk factor for the development of IF. This may reflect the different definitions for IF used in the 2 studies noted above. Dependence on parenteral nutrition for only 42 days was considered sufficient evidence of SBS. The creation of an ileostomy and resection of the colon may not be as prognostically important as a jejunostomy for a longer term parenteral nutrition (ie, more than 90 days) dependence.
A novel outcome in our study was the finding that exposure to enteral feeds before the diagnosis of NEC was associated with subsequent IF. This finding contrasts with analysis from this data set showing that infants with development of NEC and who have been fed are less likely to require surgery than infants who have not been previously fed.16 Enteral feeds are encouraged in premature infants, and evidence linking trophic feedings to the development of NEC is unclear.24 It is possible that NEC among infants who have been fed may be harmful, especially if peritoneal contamination by enteral feeds ensues.24 The mode of enteral feeding (bolus or continuous) was not associated with the development of IF in our study.
Previous retrospective data had suggested that the use of breast milk or elemental formulas was associated with less dependence on parenteral nutrition,6 but our current study did not confirm this association. Among infants with surgical NEC, it may well be that any presumed advantages of these types of enteral nutrition do not apply but that in infants with medical NEC or other causes of IF, they might still be advantageous. Randomized controlled clinical trials of different types of enteral feeding in SBS are needed to fully address this question.
Select maternal and infant clinical characteristics were unable to predict the occurrence of IF, although variables characteristic of more severe NEC were associated with the development of IF. These findings raise a question about whether more severe NEC at presentation predisposes infants to IF solely because of the need for more bowel resection, or because of more severe intestinal ischemia that would later interfere with gastrointestinal absorption of nutrients. The observation that both more extensive resection and characteristics indicative of more severe NEC remained significant predictors in multivariate analysis suggests that more severe intestinal ischemia at presentation contributes to the risk of IF beyond simply leading to more bowel being removed or rendered nonfunctional because of being distal to a jejunostomy.
One limitation of our study is related to referral bias. The hospitals involved in this study are all academic medical centers, and are referral centers for infants with complicated NEC. Therefore the population studied is likely to represent more severe patients than those with NEC in primary care centers and thus represent patients more likely to require surgical care, resulting in an increased incidence of IF. In addition explicit standardization of surgical therapy for NEC was not performed in this study.
In summary, the incidence of prolonged dependence on parenteral nutrition was quite high in a cohort of infants with NEC undergoing surgical treatment (42%) compared with infants with NEC not undergoing surgery (2%) (OR 31.1, 95% CI, 12.9–75.1, P < .001). Risk factors for the development of IF included use of parenteral antibiotics on the day of NEC diagnosis (OR=16.61, P = .022); birth weight < 750 grams, (OR = 9.09, P < .001); requirement for mechanical ventilation on the day of NEC diagnosis (OR = 6.16, P = .009); exposure to enteral feeding before NEC diagnosis (OR = 4.05, P = .048); and percentage of small bowel resected (OR = 1.85 per 10 percentage point greater resection, P = .031).
Of note, most of these factors seem related to the severity of NEC and degree of prematurity, and none seem easily amenable to preoperative or postoperative modification. New risk factors to identify NEC patients at risk of developing IF are needed, and may include clinical, genetic and other biomarkers.25,26 Early identification of patients at risk of prolonged parenteral nutrition dependence may help in the design of clinical trials of new therapies to either prevent IF or optimize the treatment of NEC.
Acknowledgments
Supported by the Glaser Pediatric Research Network and the Gerber Foundation. D.D. was supported by the following grants: Institutional Grant from NIH T32-HD43034-05A1; Pilot Feasibility from the Clinical Nutrition Research Center, NIH P30 DK40561-13; and the Junior Faculty Career Development Award from Children’s Hospital, Boston.
The following persons and institutions (funded by the Glaser Pediatric Research Network) participated in this study: Joyce Simpson, RN, MPH, Yale-New Haven Hospital; Geneva Shores, RN and Pam Gordon, RN, Texas Children’s Hospital; Janet Mooney, RN, Department of Pediatrics, UCLA; Keniki McNeil, RN, Stanford University and Lucille Packard Children’s Hospital; Carol Sweeney, BSN, David Wypij, PhD, and Laura Boger, BA, Children’s Hospital, Boston; and Marcia Wertz, RN, University of California Children’s Hospital, San Francisco.
Glossary
- IF
Intestinal failure
- NEC
Necrotizing enterocolitis
- SBS
Short bowel syndrome
Appendix
Imputation of Total Small Bowel Length
Using published autopsy studies reporting small bowel lengths for different corrected gestational ages (CGA) at the time of death20,21 we constructed a piecewise linear model for bowel length as a function of CGA. We then compared these autopsy based predicted bowel lengths with in vivo lengths measured during surgery in 14 infants from the current study as well as an additional cohort of 15 infants who had intestinal surgery at Children’s Hospital Boston.6 We regressed in vivo length on the predicted autopsy based length using a generalized linear model with no intercept and an assumed proportionality between standard deviation and mean. Results were very similar for the two cohorts, yielding an overall “scaling factor” of 0.531, indicating the tendency for autopsy based lengths to be almost double in vivo lengths. (Post mortem measurements overestimate in vivo lengths due to relaxation and lengthening of the intestines.20,21) We then applied this scaling factor to the autopsy based predicted length of each patient, based on their CGA at the time of surgery, to obtain our final value of the total small bowel length, from which we calculated the percent of small bowel resected.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:S16–28. doi: 10.1053/j.gastro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Goulet O, Baglin-Gobet S, Talbotec C, et al. Outcome and long-term growth after extensive small bowel resection in the neonatal period: a survey of 87 children. Eur J Pediatr Surg. 2005;15:95–101. doi: 10.1055/s-2004-821214. [DOI] [PubMed] [Google Scholar]
- 3.Duro D, Jaksic T, Duggan C. Multiple micronutrient deficiencies in a child with short bowel syndrome and normal somatic growth. J Pediatr Gastroenterol Nutr. 2008;46:461–4. doi: 10.1097/MPG.0b013e3181373b91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;47(Suppl 1):S33–6. doi: 10.1097/MPG.0b013e3181819007. [DOI] [PubMed] [Google Scholar]
- 5.Carbonnel F, Cosnes J, Chevret S, et al. The role of anatomic factors in nutritional autonomy after extensive small bowel resection. JPEN J Parenter Enteral Nutr. 1996;20:275–80. doi: 10.1177/0148607196020004275. [DOI] [PubMed] [Google Scholar]
- 6.Andorsky DJ, Lund DP, Lillehei CW, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001;139:27–33. doi: 10.1067/mpd.2001.114481. [DOI] [PubMed] [Google Scholar]
- 7.Quiros-Tejeira RE, Ament ME, Reyen L, et al. Long-term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: a 25-year experience. J Pediatr. 2004;145:157–63. doi: 10.1016/j.jpeds.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: re-defining predictors of success. Ann Surg. 2005;242:403–9. doi: 10.1097/01.sla.0000179647.24046.03. discussion 409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wales PW, de Silva N, Kim J, Lecce L, To T, Moore A. Neonatal short bowel syndrome: population-based estimates of incidence and mortality rates. J Pediatr Surg. 2004;39:690–5. doi: 10.1016/j.jpedsurg.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Cikrit D, West KW, Schreiner R, Grosfeld JL. Long-term follow-up after surgical management of necrotizing enterocolitis: sixty-three cases. J Pediatr Surg. 1986;21:533–5. doi: 10.1016/s0022-3468(86)80227-5. [DOI] [PubMed] [Google Scholar]
- 11.Petty JK, Ziegler MM. Operative strategies for necrotizing enterocolitis: The prevention and treatment of short-bowel syndrome. Semin Pediatr Surg. 2005;14:191–8. doi: 10.1053/j.sempedsurg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Abbasi S, Pereira GR, Johnson L, Stahl GE, Duara S, Watkins JB. Long-term assessment of growth, nutritional status, and gastrointestinal function in survivors of necrotizing enterocolitis. J Pediatr. 1984;104:550–4. doi: 10.1016/s0022-3476(84)80545-4. [DOI] [PubMed] [Google Scholar]
- 13.Lundqvist P, Kallen K, Hallstrom I, Westas LH. Trends in outcomes for very preterm infants in the southern region of Sweden over a 10-year period. Acta Paediatr. 2008;98:648–53. doi: 10.1111/j.1651-2227.2008.01155.x. [DOI] [PubMed] [Google Scholar]
- 14.Tomashek KM, Shapiro-Mendoza CK, Davidoff MJ, Petrini JR. Differences in mortality between late-preterm and term singleton infants in the United States, 1995–2002. J Pediatr. 2007;151:450–6. 456, e1. doi: 10.1016/j.jpeds.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss RL, Kalish LA, Duggan C, et al. Clinical parameters do not adequately predict outcome in necrotizing enterocolitis: a multi-institutional study. J Perinatol. 2008;28:665–74. doi: 10.1038/jp.2008.119. [DOI] [PubMed] [Google Scholar]
- 17.Sondheimer JM, Cadnapaphornchai M, Sontag M, Zerbe GO. Predicting the duration of dependence on parenteral nutrition after neonatal intestinal resection. J Pediatr. 1998;132:80–4. doi: 10.1016/s0022-3476(98)70489-5. [DOI] [PubMed] [Google Scholar]
- 18.Agresti A. Analysis of Categorical Data. 2. New York: Wiley; 2002. [Google Scholar]
- 19.Cary NS. SAS 9.1.3 Help and Documentation, 2002–2004. SAS Institute Inc; [Google Scholar]
- 20.Touloukian RJ, Smith GJ. Normal intestinal length in preterm infants. J Pediatr Surg. 1983;18:720–3. doi: 10.1016/s0022-3468(83)80011-6. [DOI] [PubMed] [Google Scholar]
- 21.Weaver LT, Austin S, Cole TJ. Small intestinal length: a factor essential for gut adaptation. Gut. 1991;32:1321–3. doi: 10.1136/gut.32.11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss RL, Dimmitt RA, Barnhart DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006;354:2225–34. doi: 10.1056/NEJMoa054605. [DOI] [PubMed] [Google Scholar]
- 23.Wales PW, de Silva N, Kim JH, Lecce L, Sandhu A, Moore AM. Neonatal short bowel syndrome: a cohort study. J Pediatr Surg. 2005;40:755–62. doi: 10.1016/j.jpedsurg.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 24.Tyson JEKK. Trophic feedings for parenterally fed infants. Cochrane Database of Systematic Reviews. 2005 doi: 10.1002/14651858.CD000504.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Harris MC, D’Angio CT, Gallagher PR, Kaufman D, Evans J, Kilpatrick L. Cytokine elaboration in critically ill infants with bacterial sepsis, necrotizing enterocolitis, or sepsis syndrome: correlation with clinical parameters of inflammation and mortality. J Pediatr. 2005;147:462–8. doi: 10.1016/j.jpeds.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Upperman JS, Camerini V, Lugo B, et al. Mathematical modeling in necrotizing enterocolitis—a new look at an ongoing problem. J Pediatr Surg. 2007;42:445–53. doi: 10.1016/j.jpedsurg.2006.10.053. [DOI] [PubMed] [Google Scholar]