Abstract
BACKGROUND
Serial transverse enteroplasty (STEP) is a novel surgical therapy for short bowel syndrome and is being used with increasing frequency worldwide. Because no single center is likely to obtain sufficient experience for meaningful analysis, we created the International STEP Data Registry to allow for larger, multicenter patient accrual and followup. This report describes patient characteristics, operative parameters, and early results of STEP in the first 38 patients enrolled in the International STEP Data Registry.
STUDY DESIGN
After IRB approval, data were entered online through password-protected enrollment and followup forms. Patient and procedural characteristics were analyzed. Pre- and postoperative small bowel length and enteral feeding tolerance were compared with the paired t-test.
RESULTS
Between September 1, 2004, and April 30, 2006, 19 centers from 3 countries enrolled 38 patients. Median followup from STEP procedure to analysis was 12.6 months (range 0 to 66.9 months). Indications for STEP were short bowel syndrome (SBS, n = 29), bacterial overgrowth (n = 6), and neonatal atresia (n = 3). Mean small intestine length was substantially increased in all groups (68 ± 44 cm versus 115 ± 87 cm, p < 0.0001, n = 27). Notable complications included intraoperative staple line leak (n = 2), bowel obstruction (n = 2), and fluid collection or abscess (n = 3). Late outcomes included progression to transplantation (n = 3) and mortality (n = 3). For the short bowel syndrome cohort, enteral tolerance was notably increased from 31% ± 31% to 67% ± 37% of calories (p < 0.01, n = 21).
CONCLUSIONS
STEP has been performed at multiple centers with minimal complications and encouraging outcomes. Indications for the procedure have broadened beyond short bowel syndrome to include bacterial overgrowth and neonatal intestinal obstruction with dilated proximal intestine. Continued accrual and followup of patients in the International STEP Data Registry will elucidate the longterm safety and efficacy of the procedure, with the goal of improved patient selection and operative timing.
Serial transverse enteroplasty (STEP) was introduced in 2003 as a novel surgical technique for the treatment of patients with intestinal failure secondary to short bowel syndrome (SBS).1,2 Patient and outcomes measures must be analyzed to aid in appropriate patient selection and counseling as this procedure gains acceptance. This report describes results of a multiinstitutional international registry designed to aid in this process.
SBS is increasingly being recognized as a major pediatric illness. Estimates of its frequency from population studies suggest an incidence of 24.5 per 100,000 live births, increasing in premature neonates to 353.7 per 100,000 live births.3 In addition, mortality from SBS is staggering. A recent cohort study demonstrated a mortality of 37.5% in neonates with SBS, with the major causes of death being liver failure and sepsis.4 Reported longterm survival rates historically range from 70% to 80%,5–7 although some recent reports note survival rates as high as 85% to 90%.8,9
Medical treatment of SBS includes parenteral nutrition, deliberate and patient introduction and acceleration of enteral feeding, acid blockade, elemental formulas, and potentially, the use of hormonal factors aimed at augmenting the process of intestinal adaptation.10
Surgical management of SBS includes not only the initial operative treatment of neonatal pathology, but also decisions about additional therapies such as bowel lengthening procedures or transplantation.11–13 Factors involved in this decision include residual bowel length, degree of bowel dilation, presence of liver injury, degree of enteral tolerance, and the patient’s growth curve. Many studies have correlated the ability to wean completely from parenteral nutrition with survival, highlighting the need to maximize intestinal function in these patients.5
The STEP procedure involves serial transverse application of a surgical stapler to incompletely staple and divide the intestine, taking advantage of the intestine’s natural adaptive dilation in SBS. Applying the stapler from alternating sides of the intestine creates a new intestinal channel that directs the enteric contents through a longer and narrower lumen. Like previously described surgical therapies for SBS, the STEP procedure simultaneously accomplishes the tasks of eliminating the adaptive, but dysfunctional, intestinal dilation, elongating the foreshortened bowel, and preserving mucosal surface area.2,14,15 Unlike previously described operations, however, the STEP procedure does not require uniform dilation of the bowel, preserves the natural vascular supply to the intestine, and avoids the need for enterotomies and the associated risk of contamination of the peritoneal cavity with enteric contents. In light of these advantages, the STEP procedure has enjoyed rapid acceptance among pediatric surgeons and is being performed at an increasingly frequent rate.
Soon after introduction of the STEP procedure, the International STEP Data Registry was formed. The goal of the Registry is to accrue patients who have undergone the STEP procedure, collect their baseline characteristics, and follow them longitudinally. By incorporating patients from around the world, the Registry is able to overcome the relatively small number of patients treated at single institutions and provide a more complete picture of the STEP procedure. The mission of the Registry is to act as a tool that allows surgeons to make educated decisions about patient selection and timing of intervention and to more accurately counsel patients and their families about indications for and expected outcomes of the STEP procedure. This article is the first report of the International STEP Data Registry, and it aims to establish the indications, efficacy, and complications of the STEP procedure.
METHODS
The Registry was created after approval by the Institutional Review Board of Children’s Hospital, Boston. An online portal was established (http://www.stepoperation.org) and the Web site was advertised to pediatric surgeons around the world through international meetings and mail correspondence with all members of the American Pediatric Surgical Association.
Registration is voluntary and does not mandate enrollment of patients. Participating surgeons are encouraged to follow the requirements of their individual institutional review boards. All patient entry is performed on the Web site using a secure online form by surgeons themselves or with the assistance of Registry personnel. Followup is accomplished at regular intervals through the use of electronic mail and telephone communication with participating surgeons. All patient records are maintained in a password-protected format, and surgeons are encouraged to deidentify patient records on input of data. Individual patient records are available for access only by the referring surgeon and Registry personnel.
The online form requests patient demographics, including date of birth, gender, and gestational age. Additional clinical information collected includes primary diagnosis, coexisting medical conditions, and surgical history. Operative information collected includes date of STEP, preoperative enteral tolerance expressed as percentage of total caloric intake, pre- and postoperative intestinal length, pre- and postoperative intestinal width, and intraoperative complications. Followup information collected includes date of most recent followup, transplantation, or death. Also collected are data on postoperative complications, late complications and outcomes, results of postoperative upper gastrointestinal series, and most recent enteral tolerance. The variables collected are summarized in Table 1.
Table 1.
Select Patient Characteristics Collected Using the Online Data Entry Form at the International Serial Transverse Enteroplasty Data Registry Web Site
| Demographics | Clinical | Operative | Followup |
|---|---|---|---|
| Date of birth | Primary diagnosis | Date of STEP | Date of followup |
|
| |||
| Gender | Coexisting medical conditions | Pre-STEP enteral tolerance | Postoperative upper gastrointestinal series |
|
| |||
| Gestational age | Earlier surgical history | Pre-STEP length | Postoperative complications |
| Post-STEP length | Late complications | ||
| re-STEP width | Most recent enteral tolerance | ||
| Post-STEP width | |||
| Intraoperative complications | |||
STEP, serial transverse enteroplasty.
To allow for variation between different practice styles and methods while attempting to remain consistent with the data, the terms used in this report were defined. SBS was defined functionally, rather than anatomically, as prolonged dependence on parenteral nutrition for appropriate growth. All bowel length measurements used for calculations were intraoperative measurements performed along the antimesenteric border of the small intestine from the ligament of Treitz to the enterocolic junction or end ostomy. The diagnosis (and indication) of bacterial overgrowth was made by the enrolling surgeon in all cases, and varied from clinical diagnosis based on symptoms and radiographic examination to hydrogen breath test to quantitative cultures obtained at endoscopy. The Registry relies on individual surgeons to follow standards of care for these patients. So, “satisfactory growth” requires that the patient is following appropriate growth curves. Enteral tolerance consists of the percentage of total caloric intake, which is administered by the enteral route (oral or tube feeding). The patient, however, must be growing appropriately and be without overt symptoms of malabsorption (inappropriate growth, high stool or stoma output resulting in skin maceration or dehydration, or laboratory tests revealing high stool reducing substances or fecal fats suggesting malabsorption of enteral nutrients).
Patient demographics, preoperative characteristics, and postoperative outcomes were evaluated and analyzed for indications, efficacy, and complications of the STEP procedure. All patients enrolled in the International STEP Data Registry from its inception on September 1, 2004, to April 30, 2006, were included in the analysis, with followup through August 30, 2006. Statistical analysis was performed using the Statistical Package for Social Sciences for Windows, version 14.0 (SPSS). Unless otherwise stated, comparison of means was performed using the paired t-test with significance set at p < 0.05. All data are presented as median (range) or mean ± standard deviation.
RESULTS
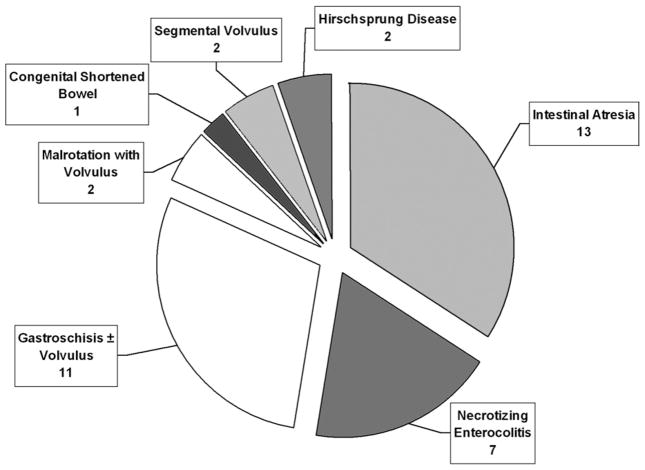
Nineteen centers, including 2 from outside North America, submitted 1 to 10 patients each, for a total of 38 patients. The baseline patient characteristics are summarized in Table 2. Median age at STEP was 1.3 years (range 0 to 19.9 years), and median followup period from STEP procedure to most recent update, death, or transplantation was 12.6 months (range 0 to 66.9 months). Primary diagnoses in these patients are shown in Figure 1 and most frequently included intestinal atresia (n = 13), gastroschisis with or without volvulus (n = 11), and necrotizing enterocolitis (n = 7). Three patients undergoing the STEP procedure had previously undergone intestinal lengthening and tapering using the method described by Bianchi.14 Interestingly, one patient underwent a successful STEP procedure after previously having an attempted Bianchi procedure that was aborted because the bowel was noted to be nonuniformly dilated at laparotomy.
Table 2.
Select Characteristics of 38 Patients Undergoing Serial Transverse Enteroplasty
| Characteristics | Value |
|---|---|
| Median age at STEP, y | 1.3 |
| Male:female | 20:18 |
| Indication, n | |
| Short-bowel syndrome | 29 |
| Bacterial overgrowth | 6 |
| Neonatal obstruction with marginal bowel length | 3 |
STEP, serial transverse enteroplasty.
Figure 1.
Distribution of primary diagnoses in 38 patients undergoing the serial transverse enteroplasty procedure.
Indications for the STEP procedure included SBS with dependence on parenteral nutrition (n = 29), bacterial overgrowth in the setting of SBS (n = 6), and neonatal atresia with marginal residual bowel length (n = 3). The median age at STEP for each of these indications was 1.2 years (range 1 month to 19.9 years), 13.9 years (range 2.0 to 19.6 years), and 3 days of life (range 0 to 4 days of life), respectively (p < 0.05, Kruskal-Wallis test). Primary diagnoses in the 29 patients with SBS included intestinal atresia in 11, gastroschisis with or without volvulus in 9, necrotizing enterocolitis in 4, Hirschsprung disease in 2, segmental volvulus in 2, and malrotation with volvulus in 1. Of the six patients with bacterial overgrowth, three had necrotizing enterocolitis and one each had intestinal atresia, malrotation with volvulus, and congenital shortened bowel. Finally, of the three neonates with obstruction, two had gastroschisis with volvulus and atresia and one had pure intestinal atresia.
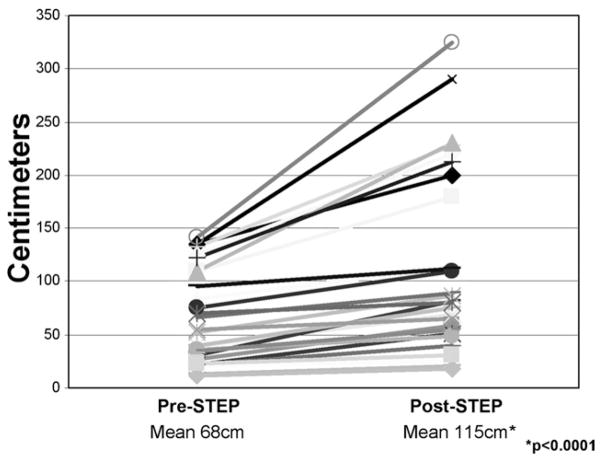
All patients experienced some degree of intestinal lengthening and tapering. In patients with operative measurements, pre-STEP intestinal length ranged from 12 cm to 190 cm; post-STEP length ranged from 18 cm to 325 cm. Overall, mean intestinal length increased considerably, from 68 ± 44 cm to 115 ± 87 cm (n = 27, p < 0.0001; Fig. 2). This represented a relative 69% increase in overall small intestinal length. Likewise, mean intestinal width was considerably tapered by the STEP procedure, from 6.3 ± 3.9 cm to 2.1 ± 0.9 cm (n = 30, p < 0.0001), representing a relative three-fold tapering of the dilated intestinal diameter. Pre-STEP intestinal width ranged from 2 cm to 24 cm; post-STEP width ranged from 1 cm to 5 cm.
Figure 2.
Graphic representation of the increase in intestinal length after the serial transverse enteroplasty (STEP) procedure in 27 of the 38 patients.
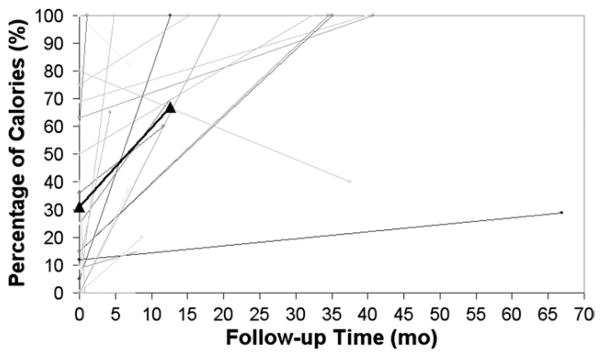
For patients in whom the STEP procedure was performed for SBS with dependence on parenteral nutrition, the percentage of total calories tolerated enterally increased from 31% ± 31% to 67% ± 37% of calories (n = 21, p < 0.01) at a median followup of 12.6 months (Fig. 3). This represented an overall post-STEP increase in enteral tolerance of 116% relative to pre-STEP baseline. This analysis excluded neonates and patients who progressed to transplantation or death. Ten of these 21 patients are now completely weaned from parenteral nutrition. Of three patients who had a decrease in enteral tolerance after the STEP procedure, two patients are in the early postoperative period and are expected to increase their enteral tolerance, and one has had a slight decrease and plateau of his enteral tolerance, now at 37 months followup (Fig. 3).
Figure 3.
Graphic representation of the change in tolerance of enteral calories for 21 short bowel syndrome patients undergoing the serial transverse enteroplasty procedure, based on length of followup. The increase in mean enteral tolerance from 31% to 67% of total calories at median followup of 12.6 months (p < 0.01) is depicted by the thick line with triangular end points.
Of the six patients who underwent the STEP procedure for bacterial overgrowth, five have experienced complete resolution of their symptoms. One patient who had neurogenic anorectal incontinence is still having diarrhea but is reported to have a better quality of life, with improved bowel control. Of the three neonates who underwent the STEP procedure, two are currently tolerating 100% and 80% of their calories enterally. Progressive liver failure developed in the third and was referred for liver and small intestine transplantation, but died after the family elected not to pursue transplantation.
Complications related to the STEP procedure include those that occurred intraoperatively, those that occurred within 30 days postoperatively, and late complications occurring more than 30 days postoperatively (Table 3). The majority of complications occurred in patients with SBS, most likely because this cohort represented the vast majority of the patients.
Table 3.
Complications Arising after the Serial Transverse Enteroplasty Procedure in 38 Patients
| Complications
| |||||
|---|---|---|---|---|---|
| Intraoperative | n | Postoperative | n | Late | n |
| Staple line leak | 2 | Bowel obstruction | 2 | Mortality | 3 |
|
| |||||
| Aspiration | 1 | Hypertension | 1 | Transplantation | 3 |
| Hematoma | 1 | ||||
| Abscess | 1 | ||||
| Pleural effusion | 1 | ||||
Complications are labeled as intraoperative, postoperative (within 30 d postoperatively), and late (greater than 30 d postoperatively).
Two intraoperative complications were noted. One was a leak at the apex of a staple line, which was immediately identified and repaired in two patients with SBS. The second was aspiration of gastric contents on induction of anesthesia in one patient, also with SBS. This occurred despite venting of a preexisting gastrostomy tube before induction of anesthesia and resulted in a prolonged intensive care course for respiratory insufficiency. This patient subsequently experienced progressive liver failure and received a multivisceral transplant.
Five postoperative complications were noted. Two patients—one with SBS and one with bacterial overgrowth—experienced postoperative bowel obstructions. Both resolved successfully with expectant management and both patients had free passage of contrast on subsequent upper gastrointestinal series. The other four postoperative complications occurred once each in four different patients with SBS. Hypertension of unknown etiology developed in one patient after the procedure and is maintained on antihypertensive medications; the relationship of this condition to the operation is unclear. Finally, in one patient an intraabdominal abscess developed, in one patient an intraabdominal hematoma developed, and in one patient a serous pleural effusion developed. All three of these patients were managed using temporary, radiologically placed drainage catheters with full resolution and no additional sequelae.
The only late complications were the need for intestinal or multivisceral transplantation and mortality. Transplantation was required in three patients (7.9%), all with SBS, who experienced progressive liver failure despite the STEP procedure. Two other patients have been referred for transplantation because of ongoing feeding intolerance and progressive liver disease. One patient underwent a multivisceral transplantation, and the remaining four underwent or have been listed for a combined liver and intestine transplantation. Three other patients, who had previously been listed for liver and intestine transplantation, have been removed from the transplant list because of steady improvement in their enteral tolerance post-STEP.
Mortality occurred in three patients (7.9%): two with SBS and the neonate described previously. In all three patients, the cause of death was progressive liver failure and sepsis. All three were referred for transplantation before death. Two died while awaiting transplantation. The neonate, as discussed, died after being removed from the transplant list at the request of the family.
DISCUSSION
The STEP procedure has been performed successfully at many centers around the world for a variety of indications. Bowel lengthening and tapering for patients with SBS remains the standard indication for the procedure. Patients receive a substantial and effective increase in bowel length, and the end result is a 116% increase in tolerance of enteral calories. Various mechanisms have been proposed for this salutary effect, including improved absorption of enteral nutrients, decreased bacterial load, and improved motility, but definitive studies are ongoing. Preliminary studies in an animal model of SBS demonstrated improvement in a D-xylose assay of carbohydrate absorption and in surrogate markers of longterm nutrient absorption in STEP animals compared with paired controls.16 The same studies demonstrated a decrease in bacterial load in the STEP animals, and treatment of D-lactic acidosis from bacterial overload in SBS has been reported.16,17 No attempts at assessing post-STEP intestinal motility have been reported in the literature, but work in our laboratory is ongoing to elucidate this potential mechanism.
Use of the STEP procedure in bacterial overgrowth and neonatal atresia was proposed in the original description of the procedure and has been reported in several case reports recently.2,17–19 In this report, all patients with bacterial overgrowth experienced a subjective resolution of or improvement in their symptoms, suggesting a beneficial role for the STEP procedure in this patient population.
Two of the three neonates are doing well, with one at full enteral nutrition and the other advancing slowly toward enteral independence; one neonate died of progressive liver failure after the parents chose to forego transplantation. We do not endorse the use of the STEP procedure or establishment of enteric continuity at the time of primary gastroschisis closure. The experience with the three neonates described in this report, however, does suggest that subsequent use of these measures during the neonatal period is safe and feasible. Overall, these outcomes suggest that the increasing use of the STEP procedure itself in neonates with marginal residual bowel length is warranted and should be practiced with appropriate patient selection.
The complication rate associated with the STEP procedure is quite favorable when compared with those in previously described intestinal lengthening and tapering procedures. When compared with single-surgeon or single-institution outcomes after longitudinal intestinal lengthening and tapering (Bianchi procedure), our cohort demonstrated less overall mortality (7.9% compared with 55% or 21%) and less overall progression to transplantation (7.9% compared with 47% in the series from Pittsburgh).20,21 Differences obviously exist between the two groups of patients because of improvements in overall health care and the multicenter nature of the STEP Registry, but the improved outcomes are striking. In addition to these comparative outcomes, these STEP Registry data note another important outcome: the ability of three patients who were previously listed for transplantation to be fully weaned from total parenteral nutrition, obviating the need for transplantation.
This report does have limitations, including its retrospective nature and potential selection bias. Because of the limited and variable SBS patient population, it is unlikely that a prospective, randomized, controlled trial will ever be performed. It is therefore not possible to comment on the possibility that the SBS cohort could have achieved improved enteral tolerance through native adaptive mechanisms. In most cases, however, surgery for intestinal lengthening and tapering is recommended only after the patient has clearly reached a plateau in the ability of the bowel to adapt.
In an effort to reduce the potential selection bias, we have had direct communication with all centers that have submitted patients to this series, and we believe that we have achieved full reporting from these centers. This does not, of course, eliminate the potential for bias in the form of nonreporting from other centers. This potential for reporting or selection bias cannot be completely eliminated, as with any voluntary data registry, and the data must be viewed in this light.
In summary, the STEP procedure is being increasingly used throughout the world as a surgical option for intestinal lengthening and tapering. Although SBS remains the primary indication for the STEP procedure, these data support its potential use in patients with bacterial overgrowth or neonatal atresia with marginal bowel length. In addition, the overall complication rate appears acceptable despite a large number of participating centers and surgeons. Finally, the overall need for transplantation may be decreased when STEP is used as part of a multidisciplinary approach to intestinal rehabilitation, as seen in the three patients who were removed from the transplant list. It is our hope that continued followup and accrual of patients in this registry will improve our understanding of the indications, outcomes, and complications of serial transverse enteroplasty.
Acknowledgments
This work was supported by the Children’s Hospital Surgical Foundation and the Rappaport Surgical Fellowship (BPM).
We would especially like to thank all the surgeons and medical centers who have participated in this project. The surgical groups that enrolled these first 38 patients are: Austin Pediatric Surgery Association, Austin, TX; Baylor College of Medicine, Houston TX; Children’s Hospital Boston, Boston, MA; Children’s Hospital of Michigan, Detroit, MI; Children’s Hospital of New Jersey, Newark, NJ; Children’s Hospital of New York, New York, NY; Columbus Children’s Hospital, Columbus, OH; CS Mott Children’s Hospital at the University of Michigan, Ann Arbor, MI; Hospital S Joao, Porto, Portugal; Hospital Universitario Valle de Hebron, Barcelona, Spain; Kapiolani Pediatric Surgery, Honolulu, HI; Northwest Permanente, Portland, OR; Pediatric Surgery of Idaho, Boise, ID; Pediatric Surgical Associates of Minneapolis, Minneapolis, MN; Penn State University, State College, PA; Schneider Children’s Hospital, New Hyde Park, NY; Seattle Children’s Hospital, Seattle, WA; University of Utah, Salt Lake City, UT; and Vanderbilt Children’s Hospital, Nashville, TN.
Footnotes
Competing Interests Declared: None.
Author Contributions
Study conception and design: Modi, Javid, Jaksic, Kim
Acquisition of data: Modi, Javid, Piper, Langer
Analysis and interpretation of data: Modi, Jaksic, Duggan, Kamin, Kim
Drafting of manuscript: Modi, Jaksic, Langer, Kim
Critical revision: Modi, Javid, Jaksic, Piper, Langer, Duggan, Kamin, Kim
References
- 1.Kim HB, Lee PW, Garza J, et al. Serial transverse enteroplasty for short bowel syndrome: a case report. J Pediatr Surg. 2003;38:881–885. doi: 10.1016/s0022-3468(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 2.Kim HB, Fauza D, Garza J, et al. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg. 2003;38:425–429. doi: 10.1053/jpsu.2003.50073. [DOI] [PubMed] [Google Scholar]
- 3.Wales PW, de Silva N, Kim J, et al. Neonatal short bowel syndrome: population-based estimates of incidence and mortality rates. J Pediatr Surg. 2004;39:690–695. doi: 10.1016/j.jpedsurg.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 4.Wales PW, de Silva N, Kim JH, et al. Neonatal short bowel syndrome: a cohort study. J Pediatr Surg. 2005;40:755–762. doi: 10.1016/j.jpedsurg.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Andorsky DJ, Lund DP, Lillehei CW, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001;139:27–33. doi: 10.1067/mpd.2001.114481. [DOI] [PubMed] [Google Scholar]
- 6.Quiros-Tejeira RE, Ament ME, Reyen L, et al. Long-term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: a 25-year experience. J Pediatr. 2004;145:157–163. doi: 10.1016/j.jpeds.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403–409. doi: 10.1097/01.sla.0000179647.24046.03. discussion 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goulet O, Baglin-Gobet S, Talbotec C, et al. Outcome and long-term growth after extensive small bowel resection in the neonatal period: a survey of 87 children. Eur J Pediatr Surg. 2005;15:95–101. doi: 10.1055/s-2004-821214. [DOI] [PubMed] [Google Scholar]
- 9.Sudan D, DiBaise J, Torres C, et al. A multidisciplinary approach to the treatment of intestinal failure. J Gastrointest Surg. 2005;9:165–176. doi: 10.1016/j.gassur.2004.10.014. discussion 176–177. [DOI] [PubMed] [Google Scholar]
- 10.Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:S16–28. doi: 10.1053/j.gastro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Georgeson K, Halpin D, Figueroa R, et al. Sequential intestinal lengthening procedures for refractory short bowel syndrome. J Pediatr Surg. 1994;29:316–320. doi: 10.1016/0022-3468(94)90339-5. discussion 320–321. [DOI] [PubMed] [Google Scholar]
- 12.Thompson JS. Edgar J Poth Memorial Lecture. Surgical aspects of the short-bowel syndrome. Am J Surg. 1995;170:532–536. doi: 10.1016/s0002-9610(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JS, Langnas AN, Pinch LW, et al. Surgical approach to short-bowel syndrome. Experience in a population of 160 patients. Ann Surg. 1995;222:600–605. doi: 10.1097/00000658-199522240-00016. discussion 605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi A. Intestinal loop lengthening–a technique for increasing small intestinal length. J Pediatr Surg. 1980;15:145–151. doi: 10.1016/s0022-3468(80)80005-4. [DOI] [PubMed] [Google Scholar]
- 15.Kimura K, Soper RT. A new bowel elongation technique for the short-bowel syndrome using the isolated bowel segment Iowa models. J Pediatr Surg. 1993;28:792–794. doi: 10.1016/0022-3468(93)90328-i. [DOI] [PubMed] [Google Scholar]
- 16.Chang RW, Javid PJ, Oh JT, et al. Serial transverse enteroplasty enhances intestinal function in a model of short bowel syndrome. Ann Surg. 2006;243:223–228. doi: 10.1097/01.sla.0000197704.76166.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modi BP, Langer M, Duggan C, et al. Serial transverse enteroplasty for management of refractory D-lactic acidosis in short-bowel syndrome. J Pediatr Gastroenterol Nutr. 2006;43:395–397. doi: 10.1097/01.mpg.0000228116.52229.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wales PW, Dutta S. Serial transverse enteroplasty as primary therapy for neonates with proximal jejunal atresia. J Pediatr Surg. 2005;40:E31–4. doi: 10.1016/j.jpedsurg.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Ismail A, Alkadhi A, Alnagaar O, Khirate A. Serial transverse enteroplasty in intestinal atresia management. J Pediatr Surg. 2005;40:E5–6. doi: 10.1016/j.jpedsurg.2004.10.059. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi A. Longitudinal intestinal lengthening and tailoring: results in 20 children. J R Soc Med. 1997;90:429–432. doi: 10.1177/014107689709000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker SR, Nucci A, Yaworski JA, Barksdale EM., Jr The Bianchi procedure: a 20-year single institution experience. J Pediatr Surg. 2006;41:113–119. doi: 10.1016/j.jpedsurg.2005.10.015. discussion 113–119. [DOI] [PubMed] [Google Scholar]