Abstract
Background
Resting energy expenditure (REE) measurements are optimal for accurate assessment of energy requirements and precise provision of parenteral nutrients. We previously observed significant reduction in REE during a 4-week period in children undergoing hematopoietic stem cell transplantation (HSCT). The goal of this study was to determine if weekly REE measurements could accurately represent changes in REE in the peritransplant period compared with a more frequent standard of daily measurements.
Methods
Data are presented from a previously described cohort of 37 children undergoing HSCT. We performed weekly indirect calorimetry on 25 patients; of those 25, we performed daily measurements on a convenience sample of 5 children. The time course of REE was analyzed in each sample by repeated measures regression.
Results
The REE trend of the 20 weekly participants was similar to that of the 5 daily participants, reaching about 80% of predicted REE at 4 weeks posttransplant, with an average decline of 3.4% per week during 4 weeks.
Conclusion
The results suggest that weekly REE measurements accurately characterize REE changes 4 weeks after HSCT compared with daily measurements. Characterization of these trends using weekly measurements may help guide clinical and nutrition care of these patients.
Keywords: Energy expenditure, parenteral nutrition, hematopoietic stem cell transplantation, pediatrics
Following hematopoietic stem cell transplantation (HSCT), pediatric patients are at high risk of malnutrition due to their underlying disease and treatment. High-dose chemotherapy and total-body irradiation (TBI) used for preparative conditioning often cause painful oral mucositis, abdominal pain, nausea, diarrhea, and poor oral intake. Parenteral nutrition is therefore frequently used for nutrition support in these patients. Accurate estimation of energy expenditure during HSCT is a crucial factor for this population. Correct provision of parenteral nutrients could reduce risks associated with either overfeeding or underfeeding. Our previously published data1 suggest that standard approaches to the nutrition management of HSCT patients, which commonly provide 130%–150% of estimated basal energy needs,2 may provide excess energy intake. Long-term nutrition problems, including obesity,3 short stature,4 and insulin resistance, are common in these patients.5 Although it is commonly stated that children undergoing HSCT have high energy requirements,2 limited data exist to evaluate energy needs during this time, and provision of energy in excess of requirements could contribute to short- and long-term nutrition problems.
We previously observed that children undergoing HSCT had a significant decline in resting energy expenditure (REE).1 Among 25 subjects, weekly measurements of REE demonstrated normal energy expenditure prior to transplant with a decline over time, reaching a minimum at 4 weeks posttransplant of approximately 80% of predicted REE. Other studies have similarly found discrepancies between measured and estimated REE,6,7 suggesting the need to employ indirect calorimetry to improve the accuracy of energy assessment. Monitoring REE is important but consumes time and resources and also represents some burden to the patients. Convenience of REE measurement could be improved dramatically by the use of weekly instead of daily indirect calorimetry (IC). The goal of this study was to determine whether weekly measurements accurately characterize changes in REE after HSCT compared with more frequent daily measurements. The population for this study was selected from our previously described cohort of 37 children undergoing HSCT.1
Subjects and Methods
Study Population
Thirty-seven patients were enrolled in an open-label, multimodal intervention, including (1) reduction in the conventional practice of providing parenteral energy intake from 130% to 150% of estimated basal energy needs2 to 100% of estimated or measured REE; (2) routine oral supplementation with vitamin E (8 IU/kg/day for patients who weighed ≤25 kg and 400 IU/day for patients who weighed >25 kg) during the week before and the month after transplantation; (3) oral ursodeoxycholic acid (7.5 mg/kg/day for <40 kg, 300 mg/day for >40 kg, given twice a day) for the month after transplantation; and (4) the prescribed use of leucovorin (folinic acid) rescue after the methotrexate portion of graft versus host disease (GVHD) prophylaxis. Patients undergoing allogeneic SCT at Dana-Farber Cancer Institute/Children’s Hospital Boston were eligible for enrollment provided that “short course” methotrexate (given on days 1, 3, 6, and 11) and cyclosporine were used for GVHD prophylaxis. Patients undergoing unrelated donor SCT (n = 24) also received 1 mg methylprednisolone/kg/day intravenously from day 7 to day 21, tapered as appropriate for the grade of GVHD. Patients who were on experimental protocols that conflicted with this study and patients receiving alternative approaches to prophylaxis for GVHD were not eligible. Patients with known allergy or hypersensitivity to any of the study reagents were also ineligible. Study enrollment occurred between July 1998 and June 2000. Written informed consent was obtained from the parents or guardians of all subjects. Assent was obtained from subjects in an age-appropriate manner. The protocol was approved by the Dana-Farber Cancer Institute Scientific Review Committee and Institutional Review Board.
Nutrition Assessment and Management
Resting energy expenditure
In subjects able to cooperate (n = 25; mean age, 12.3 y; range, 1.3–19.1 y), REE was measured by IC with the Vmax 29n metabolic monitor (SensorMedics, Yorba Linda, CA). The Vmax 29n measures the inspiratory concentration of O2 (FiO2) and the difference between FiO2 and expiratory concentrations of O2 (FeO2) with a paramagnetic differential oxygen sensor. The expiratory carbon dioxide (FeCO2) is measured continuously with an infrared sensor. Both sensors were calibrated each morning with a gas mixture of known composition. The inspiratory concentration of room air (FiCO2) was measured every 2 minutes. Carbon dioxide output and oxygen consumption were calculated each minute and then converted to standard temperature and pressure using dry gas equations. The minute values of FiO2-FeO2, FeCO2-FiCO2, VO2, VCO2, and respiratory quotient (RQ = VCO2/VO2) were recorded for 20–30 minutes and averaged. REE was then calculated using the Weir equation and expressed as kcal/day. The percentage-predicted REE was calculated by using World Health Organization (WHO)8 and Schofield normative data.9
Patients were studied in a modified fasting state (6 hours without any oral/enteral intake; IV fluids/nutrition as needed). With the subject in the supine position, a transparent canopy was placed above the head. Twelve subjects (mean age, 2.4 y; range, 0.6–9.3 y) did not undergo REE measurements due to inability to comply with the procedure. Initial (baseline) REE was measured between 6 and 8 days before transplantation. Of the 25 patients who underwent weekly indirect calorimetry measurements, a convenience sample of 5 patients was also measured 5 times per week (daily participants). Studies were performed between the hours of 0700 and 0900 until hospital discharge.
Anthropometric assessment
Body weight was measured daily from the time of admission until hospital discharge with an electronic digital scale accurate to 0.1 kg. Standing height was measured on the day of admission with a stadiometer to the nearest 0.1 cm. Triceps skinfold thickness was measured to the nearest 0.5 mm with Lange skinfold calipers (Cambridge Scientific Industries Inc, Cambridge, MD) at the time of admission and at discharge. Midarm circumference was measured to the nearest 0.1 cm with a flexible nonstretchable plastic tape at the time of admission and at discharge. Arm measurements were made by a single observer (LB). Arm anthropometric data were compared with published norms.10 Weight, height, and body mass index z scores were calculated with EPI INFO 2002 (Centers for Disease Control and Prevention, Atlanta, GA).
Dietary intake assessment
All nutrient intake during hospitalization for HSCT was measured by calorie counts. The total intake of calories, protein, carbohydrate, and fat was calculated using nutrient analysis software (Nutritionist IV; First Data Bank, San Bruno, CA) and the pharmacy’s specifications for parenteral solutions.
Nutrition management
Parenteral nutrition was begun when oral dietary intake fell below REE for 5–7 days. Parenteral energy intake was titrated so that total energy intake (oral and IV) provided approximately 100% measured REE. Throughout the study, oral intake was allowed ad libitum. Parenteral nutrition was discontinued when oral intake met or exceeded REE needs for 2 consecutive days.
Oncologic management
Of the cohort of 37 patients, 33 patients received TBI-containing regimens, and in each case TBI was given at 175 cGy twice a day for 4 days, for a total dose of 1400 cGy. Most of the patients (n = 32) also received cyclophosphamide (1,800 mg/M2 per day for 2 days), whereas 1 patient received etoposide. Six patients received antithymocyte globulin in addition to cyclophosphamide and TBI, while 1 patient also received VP-16. Two patients with aplastic anemia received cyclophosphamide (1,500 mg/M2 per day for 4 days) and antithymocyte globulin, whereas 2 patients received busulfan (4 mg × kg−1 × d−1 for 4 days) and cyclophosphamide (1,500 mg/M2 per day for 4 days). Transplants were carried out in laminar flow rooms. All patients received oral, nonabsorbable antibiotics for gut decontamination and prophylactic antipneumocystis, antiviral, and antifungal treatment per standard guidelines. IV immunoglobulin was administered at 400–500 mg/kg per dose to maintain immunoglobulin G concentrations at ≥500 mg/dL.
Data Analysis
Baseline characteristics of the 5 daily participants and 20 weekly participants were compared by Student t test for continuous measures and Fisher exact test for dichotomous variables.
From the 25 participants’ data, we constructed 5 overlapping samples:
Sample A: daily participants (5), all data (97 observations);
Sample B: daily participants (5), weekly data only (25 observations);
Sample C: weekly participants (20), weekly data (86 observations);
Sample D: all participants (25), combining weekly data (B and C, 111 observations);
Sample E: all participants (25), combining all data (A and C, 183 observations).
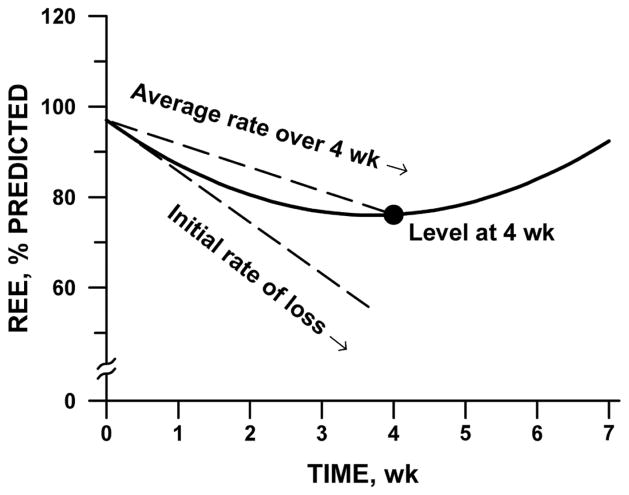
In each sample separately, we analyzed the time course of REE by repeated-measures regression. We modeled REE as a quadratic function of time, declining at a decelerating rate from week 0 to week 4. To account for within-subject correlation, we employed a spatial-power covariance structure and allowed the constant, linear, and quadratic coefficients to vary randomly among subjects. We constructed 3 summary parameters from the mean coefficients of the fitted model: the initial rate of decrease of REE; the level of REE at week 4; and the average rate of change in REE during the first 4 weeks. The geometric interpretation of these parameters is illustrated in Figure 1.
Figure 1.
Schematic diagram of quadratic curve for characterizing the time course of resting energy expenditure (REE) after stem cell transplantation. Three descriptive parameters are initial slope (tangent to curve at time 0); level at week 4 (●); and average rate of decline during the first 4 weeks (chord slope). For fit to data, see Figure 2.
To test whether the fitted curve describing the time course of REE was affected by more frequent sampling in the daily participants, we compared the results from Samples A and B. We compared the parameters from Sample C with those from A and B to test whether the curve differed between the 5 daily participants and the 20 weekly participants (C). To make that comparison formally, we analyzed the combined data with interaction terms added to the regression model (measurement frequency × coefficient). We performed the interaction test separately for the combined weekly data (Sample D) and the full data (Sample E), and compared the results to determine whether the frequency of sampling affected the comparison between daily and weekly participants. We took the parameters from the full data (Sample E) as a definitive summary of the time course of REE in these patients.
Results
Twenty-five patients underwent weekly measurements of REE and of these, 5 were studied with daily REE measurements. The demographics, diagnoses, and nutrition characteristics of the 2 groups are described in Table 1. There were no significant differences in age, weight, height, and nutrition status between the 2 groups.
Table 1.
Demographic, Diagnoses, and Nutrition Characteristics at the Time of Hospital Admission in 25 Children Undergoing Allogeneic Stem Cell Transplantationa
| Daily Participants (n = 5) | Weekly Participants (n = 20) | |
|---|---|---|
| Age, y | 14.0 ± 3.7 | 12.3 ± 5.01 |
| Gender, male | 2 (40) | 8 (40) |
| Diagnosis | ||
| Acute lymphoblastic leukemia | 3 | 5 |
| Aplastic anemia/myelodysplastic syndrome | 0 | 6 |
| Chronic myelogenous leukemia | 2 | 4 |
| Acute myelogenous leukemia | 0 | 4 |
| Other | 0 | 1 |
| Type of stem cell transplantation | ||
| Matched, unrelated | 2 | 12 |
| Sibling, matched | 3 | 8 |
| Weight, kg | 56.8 ± 13.8 | 44.4 ± 18.9 |
| Weight, z score for age | 0.89 ± 0.39 | 0.31 ± 1.00 |
| Height, cm | 160 ± 24 | 144 ± 28 |
| Height, z score for age | 0.41 ± 1.99 | −0.03 ± 1.20 |
| BMI, kg/m2 | 22.1 ± 3.0 | 20.1 ± 3.2 |
| BMI, z score | 0.76 ± 0.90 | 0.45 ± 0.90 |
| Triceps skinfold thickness, % of median | 171 ± 79 | 163 ± 71 |
| Midarm circumference, % of median | 112 ± 9 | 107 ± 15 |
| Midarm muscle area, % of median | 110 ± 24 | 102 ± 22 |
| Prealbumin, mg/dL | 22.4 ± 5.0 | 22.7 ± 4.7 |
| Baseline REE, kcal/d | 1501 ± 418 | 1238 ± 329 |
| REE, % of predicted (WHO)8 | 96 ± 12 | 95 ± 16 |
| REE, % of predicted (Schofield)9 | 97 ± 10 | 98 ± 21 |
REE, resting energy expenditure; BMI, body mass index; WHO, World Health Organization.
Data are mean ± SD or n (%).
Figure 2 illustrates the time course of REE in the 5 daily participants, using daily measurements (Sample A, left panel) and weekly measurements (Sample B, right panel). Summary parameters are listed in Table 2. After an initial decline at the rate of about 10% per week, REE leveled off at approximately 80% at week 4. The average rate of decline during this entire period was 4%–5% per week. These estimates of rate and level prevailed regardless of the frequency of sampling, although Sample A (daily measurement) yielded somewhat smaller standard errors than Sample B (weekly measurement) because of the difference in sample size.
Figure 2.
Resting energy expenditure (REE) after stem cell transplant in 5 daily participants. Left, indirect calorimetry performed Monday through Friday. Right, weekly measurements selected. Solid lines, indicating fitted quadratic repeated-measures regression, are similar; see Table 2.
Table 2.
Measurement of Changes in Resting Energy Expenditure (REE), as Affected by Sampling Schedule
| Sample | Measurement Schedule | Participants | Data Analyzed | Measurementsa | REE, % (Predicted, SE)b |
||
|---|---|---|---|---|---|---|---|
| Change/Wk, Initial | Level at 4 Wk | Change/Wk, Wk 1–4 | |||||
| A | Daily | 5 | Mon–Fri | 97 | −10.6 (2.6) | 79.4 (4.8) | −4.5 (1.2) |
| B | Daily | 5 | Weekly | 25 | −11.0 (4.0) | 80.1 (5.0) | −4.0 (1.6) |
| C | Weekly | 20 | Weekly | 86 | −5.3 (2.1) | 79.8 (3.0) | −3.4 (0.9) |
| D | All | 25 | Weekly | 111 | −6.0 (1.9) | 79.2 (2.5) | −3.7 (0.8) |
| E | All | 25 | All | 183 | −6.6 (1.6) | 79.6 (2.6) | −3.6 (0.7) |
SE, standard error.
Five participants were measured most weekdays, for a period varying between 24 and 40 d (Sample A). Measurements on days 0, 7, 14, etc, or following soonest after, were selected for Sample B. Twenty additional participants were measured on a weekly schedule for a period varying between 2 and 7 wk (Sample C). Sample D is the aggregate of weekly measurements (B and C). Sample E is the aggregate of all measurements (A and C).
Estimate (SE), from repeated-measures analysis using quadratic function of time.
Table 2 shows that the time course in the 20 weekly participants (Sample C) was similar to that in daily participants, reaching about 80% at 4 weeks with an average decline of 3.4% during that period. The initial rate of decline in Sample C appeared to be somewhat slower (5.3%/wk) than in daily participants, but the difference was not statistically significant, as indicated by analysis of the combined samples. Specifically, the coefficients of the quadratic time course did not differ significantly between daily and weekly participants according to the test of interaction, regardless of whether the analysis was limited to weekly data (Sample D, Fisher F ratio [F] = 0.62; degrees of freedom [df] = 2,23; P = .55) or included daily data as well (Sample E, F = 0.75; df = 2,23; P = .48).
Parameters for the combined data, summarized at the end of Table 2, show a mean initial decline of about 7%/wk, slowing to 4%/wk during the first 4 weeks and ending at an REE of 80% of predicted at week 4 after transplant.
Discussion
Currently, indirect calorimetry is considered to be the clinically feasible “gold standard” for REE measurement. This study demonstrates that daily IC measurements might not be necessary for HSCT patients and that weekly IC measurements were of equal merit and did not compromise accuracy. Even though daily measurements can be performed for complex cases, the majority of patients do not require such frequent measurements. Weekly measurements give an accurate portrayal of the REE throughout the treatment course and are much more convenient for both patients and medical staff.
We observed significant decreases in REE following HSCT, with some recovery toward baseline values after engraftment. The shape of the REE vs time curve followed a quadratic function trend. The declines were substantial, ranging from 5% to 11% per week compared with baseline measures. REE measurements showed a close correlation in initial slope, 4-week change, and 4-week level for all participants. There was no significant difference in REE trend during 4 weeks between weekly and daily measurements.
Several limitations to our study should be noted. First, only 5 patients were used for daily measurements. A larger patient population would have provided more data to better define the relationship with the 20 patients measured weekly. Second, by definition, daily participants had to be able to comply with daily IC measurements. Because occasionally some patients are unable to tolerate the procedure, limiting the analysis to those with a higher tolerance of IC may have introduced a bias into the study. These patients might have been healthier originally, or may not have experienced transplant-related toxicities to the same extent as other patients. Important baseline factors (Table 1), however, were not significantly different between the 2 groups.
Evaluation of the optimal frequency and duration of REE measurements has been the subject of only a limited number of studies. In a study of mechanically ventilated patients in the intensive care unit, IC of 30 minutes in duration was compared with the results of REE measured for 24 hours.11 The average REE measured during 24 hours was comparable with the average REE predicted by extrapolation from the 30-minute studies (1490 ± 486 kcal/d vs 1501 ± 503 kcal/d with a mean difference of 0 ± 209 kcal/d).
Another study in healthy infants using a metabolic chamber compared 24-hour REE, resting metabolic rate, and sleeping metabolic rate with short-term 4-hour measurements.12 The extrapolated 24-hour REE, resting metabolic rate, and sleeping metabolic rate were similar, respectively (78.2 ± 17.6, 74.7 ± 3.8, 65.1 ± 3.5 kcal/kg/d), to those measurements obtained during only 4 hours (72.2 ± 6.6, 65.9 ± 8.7, and 64.9 ± 6.4 kcal/kg/d). These results suggest that extrapolating measurements obtained during a short-term period can accurately estimate a 24-hour measurement.
In conclusion, children undergoing HSCT exhibit significant time-related decreases in REE in the first month after transplant. Our results suggest that weekly measurements of REE accurately characterize these trends, compared with more frequent daily measurements. Further studies in other metabolically labile patients should be undertaken.
Acknowledgments
We acknowledge and thank Colleen Holmes, RN, Kate Donovan, BS, and the research and nursing staff for their assistance in completing these studies.
Footnotes
Financial disclosure: Debora Duro was supported by a Institutional Grant from NIH T32-HD43034-05A1.
References
- 1.Duggan C, Bechard L, Donovan K, et al. Changes in resting energy expenditure among children undergoing allogeneic stem cell transplantation. Am J Clin Nutr. 2003;78:104–109. doi: 10.1093/ajcn/78.1.104. [DOI] [PubMed] [Google Scholar]
- 2.Muscaritoli M, Grieco G, Capria S, Iori AP, Fanelli FR. Nutrition and metabolic support in patients undergoing bone marrow transplantation. Am J Clin Nutr. 2002;75:183–190. doi: 10.1093/ajcn/75.2.183. [DOI] [PubMed] [Google Scholar]
- 3.Didi M, Didcock E, Davies HA, Ogilvy-Stuart AL, Wales JK, Shalet SM. High incidence of obesity in young adults after treatment of acute lymphoblastic leukemia in childhood. J Pediatr. 1995;127:63–67. doi: 10.1016/s0022-3476(95)70258-x. [DOI] [PubMed] [Google Scholar]
- 4.Birkebaek NH, Clausen N. Height and weight pattern up to 20 years after treatment for acute lymphoblastic leukemia. Arch Dis Child. 1998;79:161–164. doi: 10.1136/adc.79.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356:993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 6.Framson CM, LeLeiko NS, Dallal GE, Roubenoff R, Snelling LK, Dwyer JT. Energy expenditure in critically ill children. Pediatr Crit Care Med. 2007;8:264–267. doi: 10.1097/01.PCC.0000262802.81164.03. [DOI] [PubMed] [Google Scholar]
- 7.Ringwald-Smith KA, Heslop HE, Krance RA, et al. Energy expenditure in children undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;30:125–130. doi: 10.1038/sj.bmt.1703608. [DOI] [PubMed] [Google Scholar]
- 8.FAO/WHO. Energy and protein requirements. Report of a joint FAO/WHO ad hoc expert committee. Rome, March 22–April 2 1971. FAO Nutr Meet Rep Ser. 1973;(52):1–118. [PubMed] [Google Scholar]
- 9.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 10.Frisancho A. New norms of upper limb fat and muscle areas for assessment of nutrition status. Am J Clin Nutr. 1981;34:2540–2545. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 11.Smyrnios NA, Curley FJ, Shaker KG. Accuracy of 30-minute indirect calorimetry studies in predicting 24-hour energy expenditure in mechanically ventilated, critically ill patients. JPEN J Parenter Enteral Nutr. 1997;21:168–174. doi: 10.1177/0148607197021003168. [DOI] [PubMed] [Google Scholar]
- 12.Rising R, Duro D, Cedillo M, Valois S, Lifshitz F. Daily metabolic rate in healthy infants. J Pediatr. 2003;143:180–185. doi: 10.1067/S0022-3476(03)00362-7. [DOI] [PubMed] [Google Scholar]