Abstract
Objective
Meniscus lesions following trauma or associated with osteoarthritis (OA) have been described, yet meniscus aging has not been systematically analyzed. The objectives of this study were to (i) establish standardized protocols for representative macroscopic and microscopic analysis, (ii) improve existing scoring systems, and (iii) apply these techniques to a large number of human menisci.
Design
Medial and lateral menisci from 107 human knees were obtained and cut in two different planes (triangle/crossection and transverse/horizontal) in three separate locations (mid portion, anterior and posterior horns). All sections included vascular and avascular regions and were graded for i) surface integrity, ii) cellularity, iii) matrix/fiber organization and collagen alignment, and iv) Safranin-O staining intensity. The cartilage in all knee compartments was also scored.
Results
The new macroscopic and microscopic grading systems showed high inter-reader and intra-reader intraclass correlation coefficients. The major age-related changes in menisci in joints with no or minimal OA included increased Safranin-O staining intensity, decreased cell density, the appearance of acellular zones, and evidence of mucoid degeneration with some loss of collagen fiber organization. The earliest meniscus changes occurred predominantly along the inner rim. Menisci from OA joints showed severe fibrocartilaginous separation of the matrix, extensive fraying, tears and calcification. Abnormal cell arrangements included decreased cellularity, diffuse hypercellularity along with cellular hypertrophy and abnormal cell clusters. In general, the anterior horns of both medial and lateral menisci were less affected by age and OA.
Conclusions
New standardized protocols and new validated grading systems allowed us to conduct a more systematic evaluation of changes in aging and OA menisci at a macroscopic and microscopic level. Several meniscus abnormalities appear to be specific to aging in the absence of significant OA. With aging the meniscal surface can be intact but abnormal matrix organization and cellularity was observed within the meniscal substance. The increased Safranin-O staining appears to represent a shift from fibroblastic to chondrocytic phenotype during aging and early degeneration.
Keywords: meniscus, cartilage, aging, osteoarthritis and histopathology
Introduction
The menisci play an important role in the complex biomechanics of the knee joint, which is emphasized by the fact that meniscal tears, partial and total meniscectomy, and meniscal degeneration contribute to the development or progression of knee osteoarthritis (OA) [1–8].
Macroscopic and microscopic changes in human menisci and in menisci from animal models of OA have been reported [9–15]. While these prior studies described certain changes of aged or torn knee menisci, they focused on specific pathological features of interest and did not provide a global quantification of relevant changes throughout this tissue. The precise relationship between aging-associated changes in meniscus and articular cartilage in human knee joints also remains to be established.
To enable a better understanding of meniscus pathophysiology, a comprehensive system to evaluate macroscopic and microscopic changes is required. Over the past few decades, improved grading systems evaluating the histological characteristics of cartilaginous tissues have been developed including the Mankin [16], OARSI [17], and the ICRS scoring systems. Similar accepted systems for macroscopic and histologic assessment of meniscus are not available. Most studies of meniscus evaluation systems concentrated on narrow features of interest such as cellularity and matrix organization [11, 18–21], cellular phenotype [22, 23] or meniscal tears [24, 25]. However, no scoring approach integrates the status of the tissue surface, cells, tissue morphology and histochemical analyses to characterize normal aging and diseased tissue at both macroscopic and microscopic levels. In addition, collection of material from the different meniscal regions varies widely among studies and often does not provide a comprehensive representation of the different regions of the tissue.
The aims of the present study were to (1) develop and validate a macroscopic meniscus grading system to quantify zonal degeneration within the human knee by assigning individual scores for each region (anterior, mid and posterior) for an improved assessment of progressive changes with aging and disease, (2) establish standardized protocols for harvesting and processing meniscus samples to provide a consistent and reproducible overview of tissue structure, (3) integrate and enhance existing methods into a systematic evaluation of the human meniscus, and (4) document changes due to aging and contrast these changes with those due to OA.
Materials and methods
Human knee joints
Entire human knee joints were collected by resection of femur, tibia and fibula 15 cm above and below the joint line from tissue banks (approved by Scripps institutional review board). The knees were received within 72 hours postmortem. For macroscopic grading and histology menisci were harvested from 108 knee joints from 54 donors (26 males, 28 females; age range = 23 to 92, 76% Whites and 24% Hispanics). Subjects with a history of arthritis or knee trauma were excluded. Macroscopic and microscopic grading of the articular cartilage in all knee compartments was performed as described [16, 17, 26]. For macroscopic grading of articular cartilage nine scores per condyle, three scores per trochlear region, nine scores for each tibial plateau and nine scores for the patella were assessed according to the ICRS map by using a modified Outerbridge system [16, 17, 26].
Meniscus harvesting and processing for histological assessment
High resolution digital photographs of the femoral and tibial sides of the menisci were captured before 1.5–2 cm long slices were resected from the anterior, middle and posterior region of each meniscus. The tissue samples were cut at 45°, 90° and 135° angles relative to the sagittal plane and fixed in Z-Fix (Anatech, Battle Creek, MI) for 2–3 days.
Each of the fixed tissue pieces was cut in two different planes to obtain a representative overview of the tissue structure (total six different tissue samples per meniscus). The vertical section (perpendicular to the longitudinally/circumferentially oriented collagen bundles) generated a triangular specimen, which provides an overview of the femoral and tibial surfaces of the meniscus as well as the inner rim and the vascular region. The second section extended horizontally from the inner rim to the vascular zone adjacent to the capsule at a 30° angle relative to the tibial plateau. The horizontal section revealed the parallel organization of the collagen bundles and the matrix morphology in greater detail. In addition, a representative overview of the cellular organization within the collagen bundles was obtained. We also recorded more detailed information concerning inner rim morphology, calcification, and cell cluster formation. Tissue sections were marked on the femoral sides with tissue marking dye (Cancer Diagnostics, Morrisville, NC) to maintain orientation during analysis.
Tissue processing and staining
After fixation, dehydration with alcohol, Pro-Par Clearant (Anatech) and infiltration with paraffin (Paraplast, McCormick Scientific, Richmond. IL) samples were embedded in paraffin and oriented according to the labeling with the marking dye. Four micrometer sections were cut and stained with hematoxylin and eosin (H&E) to evaluate cellularity, cellular morphology, collagen alignment and to obtain a general overview of tissue organization. Safranin O-Fast Green (Saf O) staining was performed for the semi-quantitative evaluation of proteoglycan content [27]. Alcian Blue stain was applied for assessment of mucoid degeneration. Picrosirius Red stain was used to qualitatively assess the collagen fiber organization and Alizarin Red stain was used to detect meniscus calcification.
Development of a macroscopic grading system
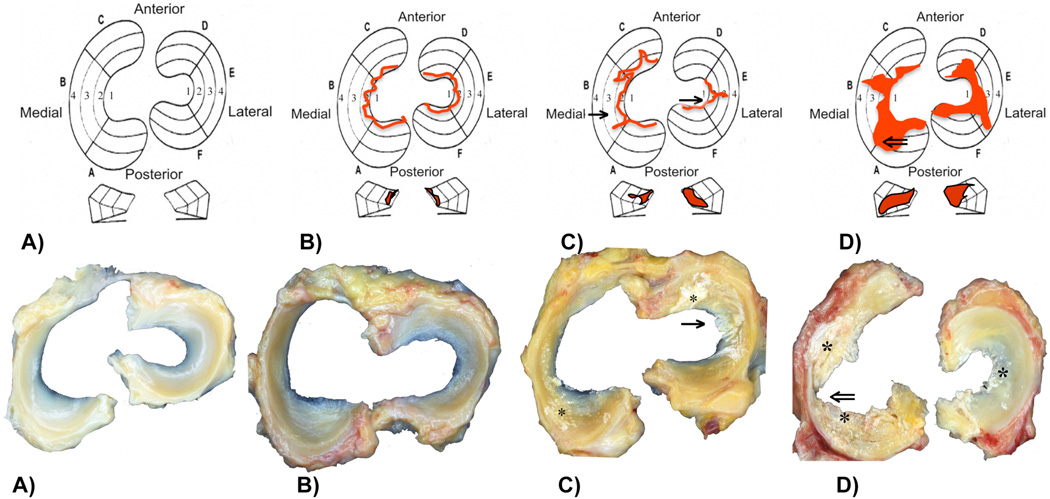
Both menisci of each knee were divided into 6 zones (zones A–F). Each zone was given a grade between 1 and 4. Grade 1: Normal intact menisci, attached at both ends with sharp inner borders, no tibial or femoral surface changes. Grade 2: Fraying at inner borders, tibial or femoral surface fibrillation, no tears. Grade 3: Partial substance tears, fraying, tibial or femoral side fibrillations. Grade 4: Full/complete substance tears, loss of tissue, tissue maceration. Calcium deposition was marked in addition to the grade (Fig. 1).
Fig. 1.
A) Macroscopic assessment: Grade 1: Normal intact menisci, attached at both ends with sharp inner borders, no tibial or femoral surface changes. B) Grade 2: Fraying at inner borders, tibial or femoral surface fibrillation, no tears. C) Grade 3: Partial substance tears (→), fraying, tibial or femoral side fibrillations. D) Grade 4: Full/complete substance tears, loss of tissue (⇐), tissue maceration. *Calcium deposition is marked in addition to the grade. Red marks in b – d indicate the degeneration pattern. Every location (A/F) posterior, (B/E) middle, (C/D) anterior can be given a grade 1–4.
Development of a histological scoring system
The grading system reported in this study was developed after reviewing slides from different healthy, aged, and diseased donors. For the histological evaluation of meniscus, criteria were selected that were significantly associated with major changes due to age and disease (Table 1). These criteria include i) tissue surface characteristics (smooth or degree of fibrillation, clefts and undulation) on the femoral, tibial side and inner border; ii) cellularity (normal, hypercellular, hypocellular, and acellular regions); iii) matrix and collagen fiber organization that included hyaline and mucoid degeneration, calcification, cyst formation, fraying and tears; iv) Safranin–O Fast Green matrix staining intensity. The presence or absence of cell clusters or calcium deposition was also recorded. Following evaluation of each category, a total score was calculated. Grade 1 represents normal tissue with scores ranging from 0–4. Grade 2 indicates early degeneration with scores ranging from 5–9. Moderate degeneration is seen in Grade 3 tissue (scores of 10–14), while Grade 4 represents the most severe degeneration (scores ranging from 15–18). Calcium deposits and cell clusters typically appeared in Grades 3–4. We propose a separate assessment of calcification and cluster formation to provide complete information concerning the various types of changes. Representative micrographs depicting the range of changes are presented in Figures 2–5.
Table 1. Criteria and scores for histological assessment of menisci.
The range of possible total scores is 0–18. This total score can be converted to a grade as follows: G1=0–4, G2=5–9, G3=10–14, G4=15–18. Grade 1 represents normal tissue, Grade 2 is mild degeneration, Grade 3 is moderate and Grade 4 is severe degeneration.
| I | SURFACE including lamellar layer: | |
| Score | ||
| I–I | FEMORAL SIDE: | |
| A Smooth | 0 | |
| B Slight fibrillation or slightly undulating | 1 | |
| C Moderate fibrillation or markedly undulating | 2 | |
| D Severe fibrillation or disruption | 3 | |
| I–II | TIBIAL SIDE: | |
| A Smooth | 0 | |
| B Slight fibrillation or slightly undulating | 1 | |
| C Moderate fibrillation or markedly undulating | 2 | |
| D Severe fibrillation or disruption | 3 | |
| I–III | INNER BORDER: | |
| A Smooth | 0 | |
| B Slight fibrillation or slightly undulating | 1 | |
| C Moderate fibrillation or markedly undulating | 2 | |
| D Severe fibrillation or disruption | 3 | |
| II | CELLULARITY | |
| A Normal | 0 | |
| B Diffuse hypercellularity | 1 | |
| C Diffuse hypo/acellular regions | 2 | |
| D Hypocellularity (empty lacuna, pycnotic cells) | 3 | |
| III | COLLAGEN ORGANIZATION/ALIGNMENT AND FIBER ORGANIZATION | |
| A Collagen fibers organized, homogenous eosinophilic staining of extracellular matrix | 0 | |
| B Collagen fibers organized, diffuse foci of hyaline or mucinous degeneration | 1 | |
| C Collagen fibers unorganized, confluent foci or bands of hyaline or mucinous degeneration, fraying | 2 | |
| D Collagen fibers unorganized, fibrocartilaginous separation (edema, cystic formation), severe fraying and tears | 3 | |
| IV | MATRIX STAINING (SAFRANIN O - FAST GREEN) | |
| A None | 0 | |
| B Slight | 1 | |
| C Moderate | 2 | |
| D Strong | 3 | |
| Cell clusters: | present +, ++, +++ | |
| Calcium deposition: | present +, ++, +++ | |
Fig. 2.
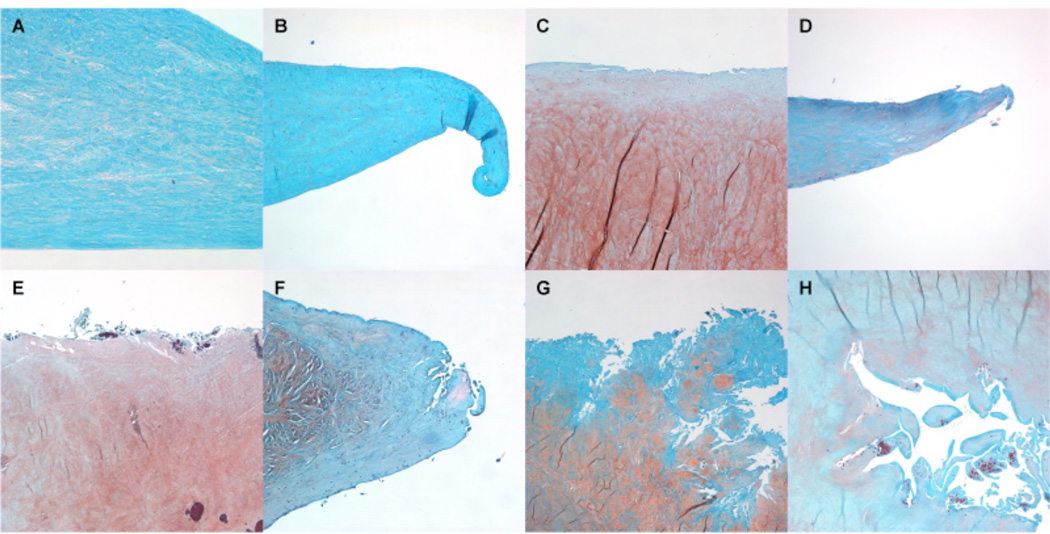
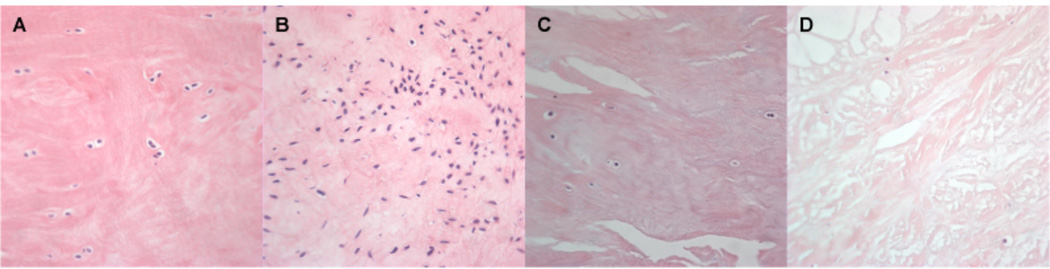
Histological assessment of meniscus integrity: (A, B) Normal meniscus: The meniscus surface is smooth, no fraying or surface fibrillation, score 0. (C, D) Mild changes: The meniscus surface shows slight fibrillation or undulation, score 1. (E, F) Moderate fibrillation, fraying and/or undulation, some clefts are present, score 2. (G, H) The meniscus shows severe fraying and tears, severe disruption, score 3. Safranin O - Fast Green, 4x.
Fig. 5.
Safranin O - Fast Green staining intensity: (A) No stain for Safranin O, score 0. (B) Slight staining intensity for Safranin O, score 1. (C) Moderate staining intensity for Safranin O, score 2. Strong staining intensity for Safranin O, score 3. Safranin O - Fast Green, 4x.
Validation of the grading systems
To validate the macroscopic grading system proposed in this study, 107 lateral and medial menisci were graded by 4 different readers for inter-reader agreement; and, 2 of the readers performed the grading twice, with a time difference of at least 3 weeks.
To validate the microscopic grading system proposed in this study, a set of 222 slides out of all the menisci in this study were collected covering all possible scores from 0–18. The slide collection was randomized and 3 readers performed the grading blind regarding donor age, gender and disease state to assess inter reader agreement. 2 of the readers performed the grading twice with a time difference of at least 3 weeks for intra-reader agreement.
Statistical analysis
We utilized intraclass correlation coefficients (ICCs) [28, 29] to summarize intra- and inter-rater agreement on the scales. We also used a nonparametric approach to compare raters, by calculating the proportions of rater differences greater than reference values. This is effectively a nonparametric form of Bland and Altman’s limits of agreement method [30]. Calculations were performed in Stata 9.2 (Statacorp, College Station, TX).
Results
Macroscopic scoring system for menisci
Macroscopic scoring for 107 pairs of menisci (lateral and medial) was performed independently by four readers. In addition, two readers undertook replicate scoring after a minimum time period of 3 weeks. Differences in replicate scores within readers were 1 unit or less in 96% to 98% of cases. Inter-reader differences were more widely dispersed than intra-reader differences (Supplementary Table 2A and 2B). Perfect agreement between readers dropped off, ranging from 37% to 70%. Nevertheless, relative to the 1 unit reference value, agreement remained high: scores from different readers were within 1 unit of one another in 84% to 95% of cases.
Intraclass correlation coefficients (ICCs) were uniformly high, ranging from 0.918 to 0.991 in medial scores, and from 0.923 to 0.994 in lateral scores. Intra-reader ICCs ranged from 0.969 to 0.994, and were somewhat larger than inter-reader ICCs [range 0.918 to 0.962], though differences were less than 0.1 in magnitude (Supplementary Table 4A and 4B).
Microscopic scoring system for menisci
Microscopic scoring was performed independently by three readers. In addition, two readers undertook replicate scoring. Two schemes are reported: total scores (0 to 18), and grades (1–4) (Score 0–4 = Grade 1; Score 5–9 = Grade 2; Score 10–14 = Grade 3; Score 15–18 = Grade 4). Frequency distributions of the intra- and inter-reader differences in scores among the 3 readers are given in Supplementary Table 3. In the replicate readings, perfect agreement with total scores (0–18) ranged from 73% to 87%, and from 94% to 98% with the Grades (1–4). For both readers, replicate scores were within 1 unit of one another in 97% to 100% of cases. Total scores from different readers were within 1 unit of one another in between 75% and 87% of cases; with the Grades (1–4), 100% of cases were scored within 1 unit of one another by different readers. ICCs were uniformly high: Intra-reader ICCs ranged from 0.972 to 0.997, and were somewhat higher than inter-reader ICCs [range 0.887 to 0.990] (Supplementary Table 5). These observations indicate that the new scoring system which covers a large number of important cell and extracellular matrix changes has a high degree of reproducibility within and between different readers.
Analysis of menisci in aged joints
To identify changes attributed primarily to aging, menisci from joints (age range = 23 to 92) with minimal changes in articular cartilage (macroscopically classified as Outerbridge Grade I or II) were analyzed and compared to young normal knees (Grade 1) and OA knees (Grade III or IV). Macroscopically, menisci with advancing age appeared more opaque with a yellowish color compared to healthy younger menisci with a translucent, smooth and glistening surface (Fig. 7A, C). Surface roughening without severe fibrillation was common in aged menisci.
Fig. 7.
Gross morphology: (A) Healthy meniscus with a smooth and glistening surface. The anterior horns are smaller and the posterior horns show a broad base. (B) A larger fiber bundle/connective tissue bundle penetrates through the vascular zone into the meniscus tissue (←). (C) Degenerated menisci with severe tissue disruption (medial), fraying, small tears and calcium deposition. (D) Tissue tag or scar tissue most likely due to a flap tear (*).
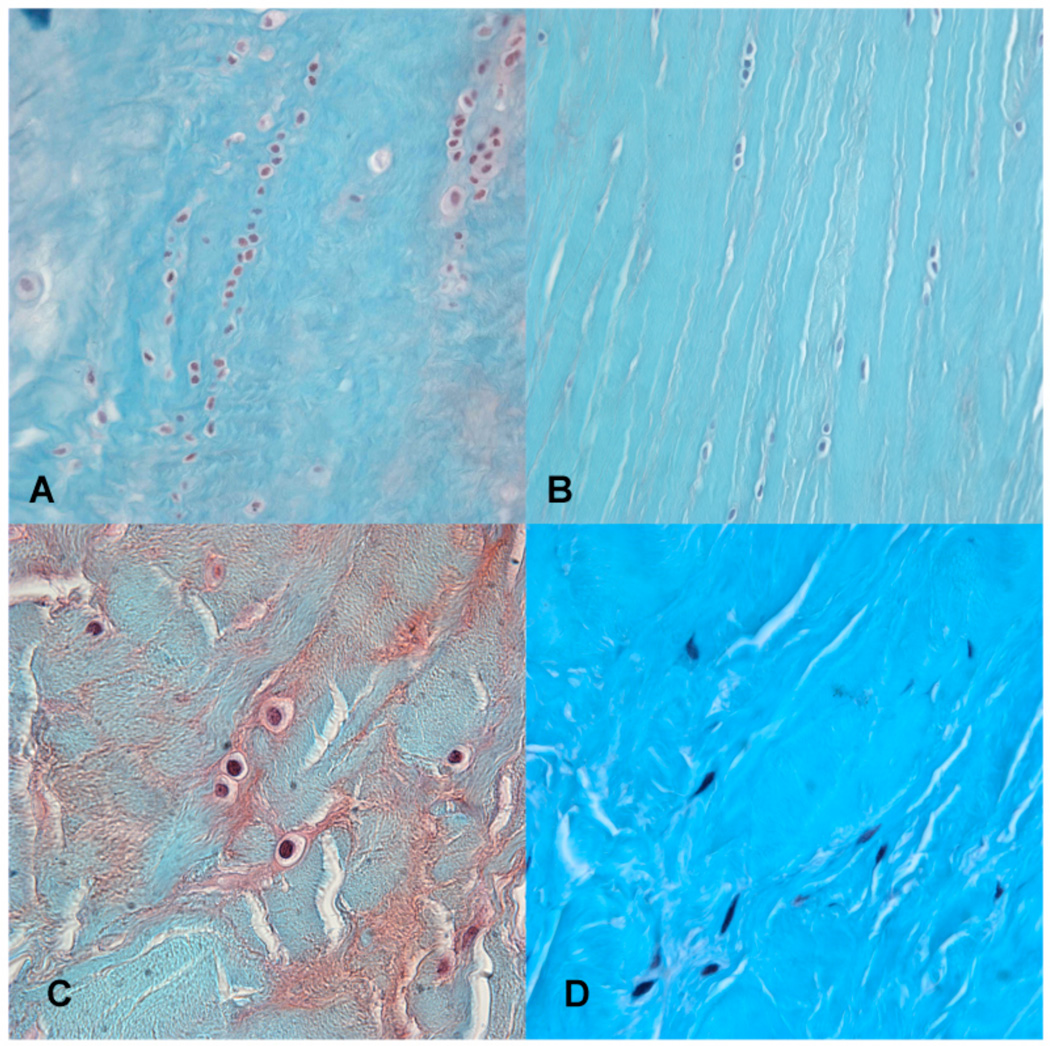
Microscopically, we analyzed changes in meniscus cellularity, surface integrity, matrix structure and Safranin-O staining intensity in two different planes including both the vascular and avascular regions. The major changes attributed to age included an increased Safranin-O staining intensity, which was evenly distributed within the central part of the avascular area. A decrease in cell density was noted and evidence of mucoid degeneration associated with some loss of collagen fiber organization (decreased birefringence under polarized light microscopy). Cell aggregates were observed, although not resembling the typical cell clusters described later that appear in osteoarthritic meniscus degeneration, which indicated a local fibro-chondrocyte proliferation or pseudo-cloning (Fig. 8a) reported before [31].
Fig. 8.
Cell arrangements: (A) Local fibro-chondrocyte proliferation or pseudo-cloning. (B) Cells appear more arranged between the fibers. (C) Ovoid to round cell shape; the so-called fibro-chondrocyte. (D) Fusiform, elongated cell shape, the so-called fibroblast like-cell. Safranin O-Fast Green, 40x (A, B), 100x (C, D).
In young normal tissue the transverse/horizontal plane showed cells in linear arrangement between collagen bundles (Fig. 8b). These same cells visualized in a cross section appeared as single evenly distributed cells. The cells were ovoid to round, resembling the so-called "fibro-chondrocyte" that is typically for young normal tissue (Fig. 8c). The cells close to the vascular zone were more fusiform and represent fibroblast-like cells (Fig. 8d) [32–34]. The earliest microscopic changes in the surface, cellularity and matrix organization were observed mainly along the inner rim.
Analysis of menisci in osteoarthritic joints
Knees with significant cartilage pathology (Outerbridge Grade III or Grade IV) contained degenerated menisci with a dark yellow to a light brown or reddish color. Calcium deposition on roughened and fibrillated surfaces was often present. Tears were frequently observed in both medial and lateral menisci (Fig. 7c). In a few menisci with more severe degeneration, a tissue tag or scar tissue most likely the result of a flap tear was seen (Fig. 7d). The posterior horn was the most affected region in the meniscus samples in this study, which is consistent with previous observations and severe degenerative lesions were typically horizontal cleavages [35–39]. Our findings of posterior horn degeneration, and the increasing prevalence with aging are also supported by a large-scale MRI study [40].
No meniscal ganglions or external cysts were observed in the current study. Histopathological evaluation of menisci from arthritic joints (Outerbridge Grade III or IV) revealed a more severe fibrocartilaginous disruption. The extracellular matrix features fine fibrillations and a loss of structure, most likely due to an edematous swelling of the tissue. The fusion of spaces originally occupied by matrix and meniscus cells created small, irregular, cyst-like cavities. These cavities appeared as irregular intra-meniscal pseudo-cysts that were lined by smooth collagenous fibers in which flattened cells (resembling fibrochondrocytes) can be seen (Fig. 8b). There was an extensive variability in the pattern of proteoglycan staining with areas of high intensity as well as areas devoid of staining. We observed large variation in cell distribution with hypercellular, hypocellular, and acellular areas as well as areas containing large and abundant cell clusters. Abnormal cell clusters were found close to the meniscus surface, typically associated with tears, and in frayed areas. These cell clusters were seen in both positive and negative Safranin O stained matrix, however, the matrix surrounding these clusters was typically acellular. Cells around frayed regions and tears were often larger in size compared to cells in normal regions and compared to normal menisci. Overall, the anterior horns of both medial and lateral menisci appeared to be least affected macroscopically as well as microscopically.
Discussion
Severe disruption and loss of articular cartilage is the structural hallmark of OA but OA is a whole-joint disorder involving other tissues, such as ligaments, menisci, subchondral bone, synovial membrane, and even muscle. The menisci play an important role in both tibiofemoral compartments through load distribution and shock absorption [7, 8, 41–43]. Our macroscopic and histopathologic analyses demonstrated a strong association between degenerated menisci and OA and support earlier findings that normally appearing menisci are rarely found in knees with OA [7, 8, 41–43]. The mechanistic relationship between meniscal damage and knee OA is not yet fully understood. An otherwise healthy knee may develop OA due to a meniscus tear and an intact meniscus can degenerate in an arthritic knee.
Experimental animal models have been used to investigate changes in menisci during the development of OA [9–15]. Human meniscal degeneration has also been described by several authors [18, 20, 21, 24, 25, 31, 32, 35–37, 39, 44–49]. However, most of the grading systems for meniscus pathology are MRI-based, and describe tears and grades of mucoid degeneration [19, 50–53]. In addition, little is known of meniscus aging, at macroscopic and histopathologic levels. Most studies of animal and human tissue developed specialized evaluation systems to capture specific features of interest such as cellularity and matrix organization [11, 18, 20, 24, 25].
We conducted a more systematic evaluation of changes in aging and OA menisci at a macroscopic and microscopic level. We developed and validated a more detailed grading system that is comprehensive and includes macroscopic, histopathologic and histochemical evaluation. The high intraclass correlation coefficients for the macroscopic and microscopic grading systems show that both grading systems are reproducible and can be readily learned.
Several characteristics appear to be specific to meniscus aging in the absence of significant OA. With aging in the absence of significant knee degeneration, the meniscal surface often remains intact while distinct changes in matrix stain and cellularity are observed within the meniscal substance. This is in direct contrast to degeneration in articular cartilage, which almost invariably progresses from the surface inward. Repetitive microtrauma, due to repeated exposure of the musculoskeletal tissue to low-magnitude forces, results in subclinical injury at the tissue level [54]. With aging there is a greater tendency for accumulation of repetitive microtrauma in the meniscus [55]. While we excluded donors with history or signs of major trauma, the aging-associated changes reported in this study might also represent a response to accumulated microtrauma.
In the early stages of OA, cartilage degeneration is seen typically at the surface as fibrillation of the superficial zone. Endogenous repair of articular cartilage is ineffective, extracellular matrix-degrading enzymes are released, and progressive loss of cartilage ensues. Our analyses indicate that degeneration of menisci initiates within the substance of the tissue rather than the surface. Tissue fibrillation and disruption is first seen at the inner rim, which spreads to the articular surfaces of the meniscus over time, and progresses to total disruption or loss of meniscus tissue, mainly in the avascular zone.
Safranin O staining has been used to analyze torn menisci or animal models of OA [15, 25, 56–58]. Little is known about changes in Safranin O staining associated with the degeneration seen with normal aging in the human meniscus. We analyzed changes in Safranin O staining in young normal, aged and degenerated menisci. The increased Safranin O staining with meniscus aging could represent a shift from a fibroblastic to chondrocytic phenotype during early degeneration and warrants further investigation. Our results along with biochemical data published by Herwig and Adams [15, 57] as well as gene expression studies [59] provide evidence for an accumulation of water-binding proteoglycans in aging and degenerating human menisci and these changes suggest an attempt at adaption or regeneration of the menisci [56, 58].
In the presence of moderate or severe OA there was severe matrix disruption in one or more of the meniscal regions. Macroscopically the posterior horn was most commonly affected. From a biomechanical perspective, during knee flexion, the femoral condyles roll back on the tibial plateau and more forces are transmitted to the posterior region of the meniscus often subluxing the posterior horn of the lateral meniscus in deep flexion [15, 56–58]. On the other hand, the anterior horn was consistently less affected. This suggests that the anterior horn could be more resistant to degeneration or that the anterior horn is exposed to less damaging biomechanical loading. By macroscopic and histopathologic examination, the inner rim of both medial and lateral menisci have the tendency to degenerate before the femoral and tibial surface.
Abnormal cell clusters, reminiscent of those found in osteoarthritic cartilage were found in regions of severe meniscus matrix disruption. These cells were associated with frayed edges and tears, and with superficial areas of degeneration. Single cells and cells in hypercellular regions around these frayed edges appeared larger in size than the more typical fibrochondrocyte seen in normal menisci. The association of abnormal cell clusters and hypertrophic single cells with increased Safranin O stain indicates a phenotypic transition to a chondrocytic appearance and the increase in cell size could represent hypertrophic differentiation.
We studied menisci from almost equal numbers of male and female donors. We did note higher scores for the females compared to males but we did not detect statistically significant gender differences in macroscopic and histologic scores and grades, or in Safranin O staining intensity.
In summary, we present a comprehensive meniscus grading system that is reliably and reproducibly sensitive to macroscopic and histologic changes of aging and osteoarthritic degeneration. We found several differences that distinguish aging from osteoarthritic meniscus degeneration. The initiation and progression of meniscus degeneration differs in many aspects from that occurring in articular cartilage.
Our ongoing studies include characterization of the phenotypically different cells in the different regions of the meniscus and the change in cell function that is reflected in the macroscopic and microscopic changes of degeneration. Our findings support the notion that the meniscus plays an important role in the initiation and progression of knee OA. A deeper understanding of meniscus function is crucial to gain therapeutic insights into this disease process.
Supplementary Material
Fig. 3.
Histological assessment of meniscus cellularity: (A) Normal cell distribution, score 0. (B) Diffuse hypercellularity, score 1. (C) Meniscus tissue shows hypo- to acellular regions, score 2 (D) Hypocellular meniscus tissue, score 3. Hematoxylin & Eosin, 40x.
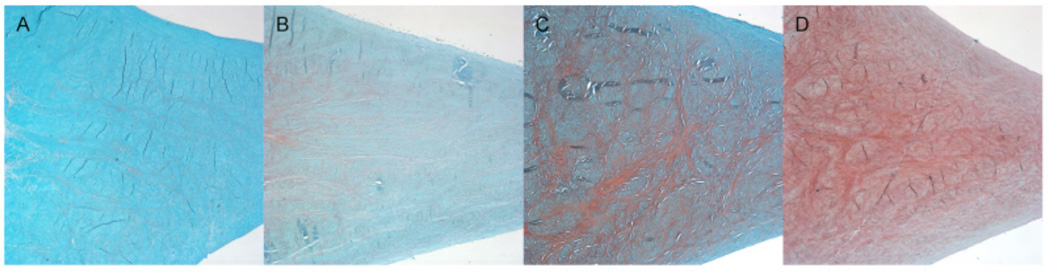
Fig. 4.
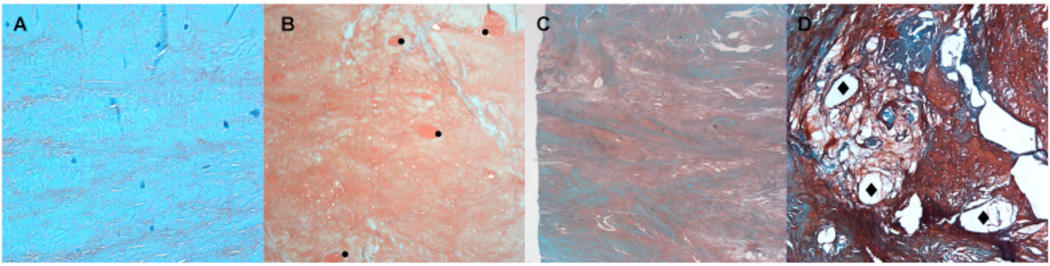
Meniscus morphology and collagen fiber organization: (A) Normal appearance of extracellular matrix and collagen fiber organization, score 0. (B) Diffuse foci (•) of degenerated extracellular matrix (hyalin and/or mucoid), most of the collagen fibers are organized, score 1. (C) Bands or confluent foci of degenerated extracellular matrix substance (hyalin and/or mucoid), most of the collagen fibers appear unorganized, score 2. (D) Fibrocartilaginous separation (edema, hyaline and/or mucoid degeneration, cyst formation (♦), tears) with unorganized collagen fibers, score 3. Safranin O - Fast Green, 4x.
Fig. 6.
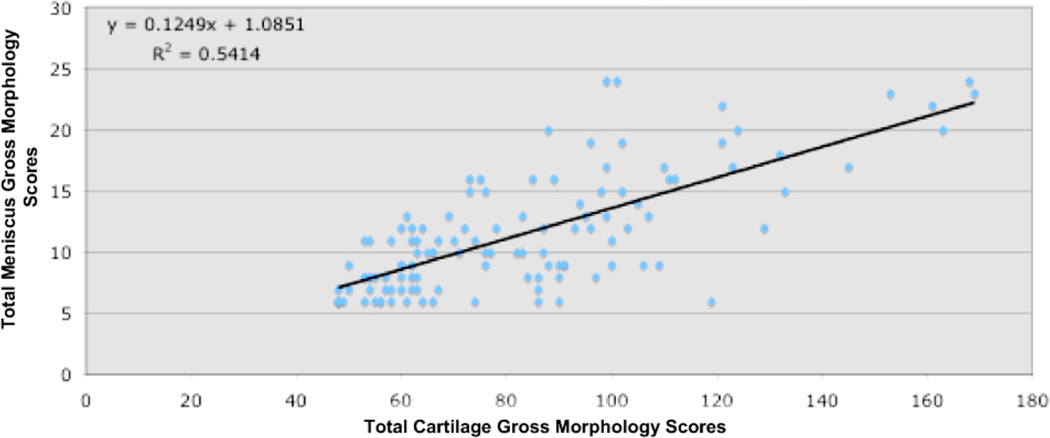
Relationship between meniscus and cartilage gross morphology. Correlation between total cartilage scores (condyles, tibia, trochlea and patella) compared to the total menisci scores. Cartilage scores were assessed according the mapping system recommended by the International Cartilage Repair Society (ICRS) and by using a modified Outerbridge system. Macroscopic grades for both menisci were obtained by using the new proposed grading systems in this study for each location (anterior, middle and posterior).
Fig. 9.
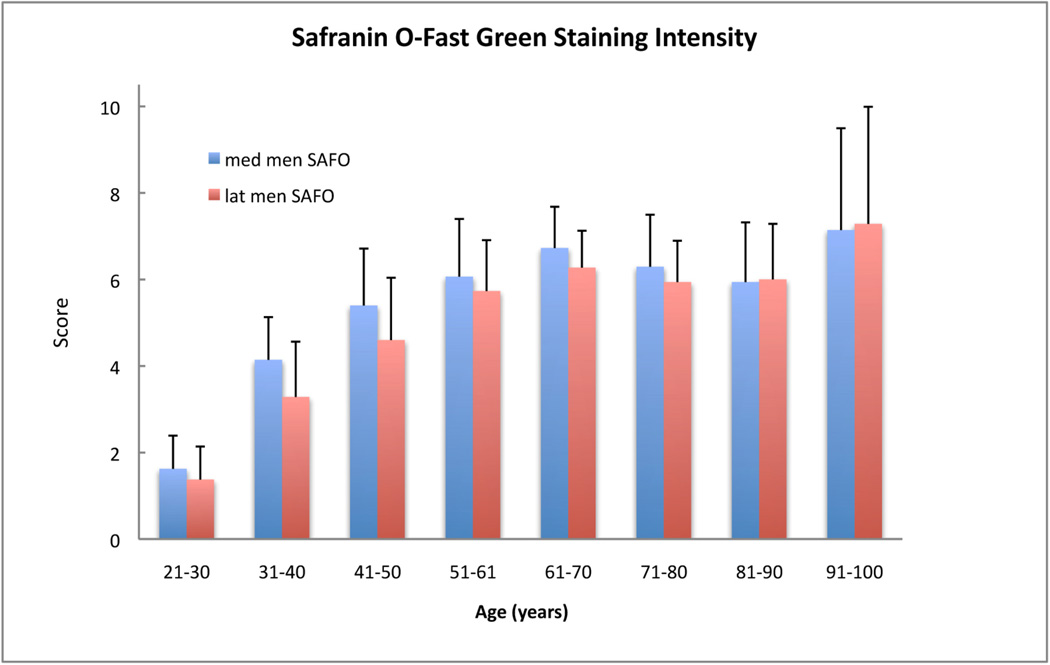
Scores for Safranin O staining intensity of both genders among age groups. In the age groups over 30 years Safranin O - Fast Green staining intensity is increased. Per age group we analyzed 7–17 menisci (n=7–17). The error bars represent the 95% confidence interval.
Acknowledgments
We are grateful to Kimberly Winsor and Brandon Richland for testing the reproducibility of the macroscopic and microscopic grading; and Lilo Creighton, Margaret Chadwell and Anita San Soucie for histologic processing of the specimens. This study was supported by the National Institutes of Health (AG007996), by Donald and Darlene Shiley, the Arthritis Foundation and the Sam and Rose Stein Endowment Fund.
Footnotes
Author contribution
All authors have made substantial contributions to the completion of this study. Shantanu Patil contributed to the conception of the macroscopic grading system. Shawn Grogan contributed to the drafting and critically reviewing of the manuscript. Statistical analysis was guided by Jim Koziol.
Chantal Pauli contributed to the study design, data acquisition, to the conception of the microscopic grading system, analysis and interpretation of data, and drafting the manuscript. Shuhei Otsuki and Akihiko Hasegawa contributed to specimen acquisition. Martin Lotz contributed to study design and manuscript preparation.
Darryl D’Lima contributed to study design and to the drafting and critically reviewing of the manuscript.
Conflict of interest
No author has any conflict of interest related to this work.
References
- 1.Song Y, Greve JM, Carter DR, Giori NJ. Meniscectomy alters the dynamic deformational behavior and cumulative strain of tibial articular cartilage in knee joints subjected to cyclic loads. Osteoarthritis Cartilage. 2008;16:1545–1554. doi: 10.1016/j.joca.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Seedholm B. Part II. Transmission of the load in the knee with special reference to the role of the meniscus. Eng Med. 1979;8:221–228. [Google Scholar]
- 3.Seedholm B. Part I. Transmission of the load in the knee with special reference to the role of the meniscus. Eng Med. 1979;9:207–221. [Google Scholar]
- 4.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 5.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7:526–532. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 6.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B:664–670. [PubMed] [Google Scholar]
- 7.Englund M, Guermazi A, Lohmander LS. The meniscus in knee osteoarthritis. Rheum Dis Clin North Am. 2009;35:579–590. doi: 10.1016/j.rdc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Englund M. Meniscal tear -- a common finding with often troublesome consequences. J Rheumatol. 2009;36:1362–1364. doi: 10.3899/jrheum.090335. [DOI] [PubMed] [Google Scholar]
- 9.Hellio Le Graverand MP, Vignon E, Otterness IG, Hart DA. Early changes in lapine menisci during osteoarthritis development: Part I: cellular and matrix alterations. Osteoarthritis Cartilage. 2001;9:56–64. doi: 10.1053/joca.2000.0350. [DOI] [PubMed] [Google Scholar]
- 10.Gigante A, Specchia N, Greco F. Age-related distribution of elastic fibers in the rabbit knee. Clin Orthop Relat Res. 1994:33–42. [PubMed] [Google Scholar]
- 11.Copenhaver W, Kelley D, Wood R. Bailey's textbook of histology. Baltimore: Williams & Wilkins; 1978. [Google Scholar]
- 12.Cook JL, Tomlinson JL, Kreeger JM, Cook CR. Induction of meniscal regeneration in dogs using a novel biomaterial. Am J Sports Med. 1999;27:658–665. doi: 10.1177/03635465990270051901. [DOI] [PubMed] [Google Scholar]
- 13.Cook JL, Fox DB, Malaviya P, Tomlinson JL, Kuroki K, Cook CR, et al. Long-term outcome for large meniscal defects treated with small intestinal submucosa in a dog model. Am J Sports Med. 2006;34:32–42. doi: 10.1177/0363546505278702. [DOI] [PubMed] [Google Scholar]
- 14.Chiari C, Koller U, Dorotka R, Eder C, Plasenzotti R, Lang S, et al. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthritis Cartilage. 2006;14:1056–1065. doi: 10.1016/j.joca.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Adams ME, Billingham ME, Muir H. The glycosaminoglycans in menisci in experimental and natural osteoarthritis. Arthritis Rheum. 1983;26:69–76. doi: 10.1002/art.1780260111. [DOI] [PubMed] [Google Scholar]
- 16.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 17.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Rodeo SA, Seneviratne A, Suzuki K, Felker K, Wickiewicz TL, Warren RF. Histological analysis of human meniscal allografts. A preliminary report. J Bone Joint Surg Am. 2000;82-A:1071–1082. doi: 10.2106/00004623-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Raunest J, Hotzinger H, Burrig KF. Magnetic resonance imaging (MRI) and arthroscopy in the detection of meniscal degenerations: correlation of arthroscopy and MRI with histology findings. Arthroscopy. 1994;10:634–640. doi: 10.1016/s0749-8063(05)80061-1. [DOI] [PubMed] [Google Scholar]
- 20.Krenn V, Kurz B, Krukemeyer MG, Knoess P, Jakobs M, Poremba C, et al. Histopathological degeneration score of fibrous cartilage. Low- and high-grade meniscal degeneration. Z Rheumatol. 2010;69:644–652. doi: 10.1007/s00393-010-0609-1. [DOI] [PubMed] [Google Scholar]
- 21.Krenn V, Knoss P, Ruther W, Jakobs M, Otto M, Krukemeyer MG, et al. Meniscal degeneration score and NITEGE expression : immunohistochemical detection of NITEGE in advanced meniscal degeneration. Orthopade. 2010;39:475–485. doi: 10.1007/s00132-010-1606-4. [DOI] [PubMed] [Google Scholar]
- 22.Verdonk PC, Forsyth RG, Wang J, Almqvist KF, Verdonk R, Veys EM, et al. Characterisation of human knee meniscus cell phenotype. Osteoarthritis Cartilage. 2005;13:548–560. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Adams J, Athanasiou A. The Knee Meniscus: A Complesx Tissue of Diverse Cells. Cellular and Molcular Bioengineering. 2009;2:332–340. [Google Scholar]
- 24.Mesiha M, Zurakowski D, Soriano J, Nielson JH, Zarins B, Murray MM. Pathologic characteristics of the torn human meniscus. Am J Sports Med. 2007;35:103–112. doi: 10.1177/0363546506293700. [DOI] [PubMed] [Google Scholar]
- 25.Meister K, Indelicato PA, Spanier S, Franklin J, Batts J. Histology of the torn meniscus: a comparison of histologic differences in meniscal tissue between tears in anterior cruciate ligament-intact and anterior cruciate ligament-deficient knees. Am J Sports Med. 2004;32:1479–1483. doi: 10.1177/0363546503262182. [DOI] [PubMed] [Google Scholar]
- 26.Outerbridge R. The Etiology of Chondromalacia Patellae. THE JOURNAL OF BONE AND JOINT SURGERY. 1961;43 B:752–756. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- 28.Schuster C. A note on the interpretation of weighted kappa and its relations to other rater agreement statistics for metric scales. Educational and Psychological Measurement. 2004;64:243–253. [Google Scholar]
- 29.Fleiss J. Measuring nominal scale agreement among many raters. Psychological Bulletin. 1971;76:378–382. [Google Scholar]
- 30.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 31.Fisseler-Eckhoff A, Muller KM. Histopathological meniscus diagnostic. Orthopade. 2009;38:539–545. doi: 10.1007/s00132-008-1401-7. [DOI] [PubMed] [Google Scholar]
- 32.Ghadially FN, Lalonde JM, Wedge JH. Ultrastructure of normal and torn menisci of the human knee joint. J Anat. 1983;136:773–791. [PMC free article] [PubMed] [Google Scholar]
- 33.Day B, Mackenzie WG, Shim SS, Leung G. The vascular and nerve supply of the human meniscus. Arthroscopy. 1985;1:58–62. doi: 10.1016/s0749-8063(85)80080-3. [DOI] [PubMed] [Google Scholar]
- 34.Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 35.Noble J. Lesions of the menisci. Autopsy incidence in adults less than fifty-five years old. J Bone Joint Surg Am. 1977;59:480–483. [PubMed] [Google Scholar]
- 36.Ferrer-Roca O, Vilalta C. Lesions of the meniscus. Part I: Macroscopic and histologic findings. Clin Orthop Relat Res. 1980:289–300. [PubMed] [Google Scholar]
- 37.Ferrer-Roca O, Vilalta C. Lesions of the meniscus. Part II: Horizontal cleavages and lateral cysts. Clin Orthop Relat Res. 1980:301–307. [PubMed] [Google Scholar]
- 38.Campo-Ruiz V, Patel D, Anderson RR, Delgado-Baeza E, Gonzalez S. Evaluation of human knee meniscus biopsies with near-infrared, reflectance confocal microscopy. A pilot study. Int J Exp Pathol. 2005;86:297–307. doi: 10.1111/j.0959-9673.2005.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burman MS, Sutro C. A study of degenerative changes of the menisci of the knee joint, and the clinicl significance thereof. J Bone Joint Surg Am. 1933;15:835–861. [Google Scholar]
- 40.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Englund M. The role of the meniscus in osteoarthritis genesis. Med Clin North Am. 2009;93:37–43. doi: 10.1016/j.mcna.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Ding C, Martel-Pelletier J, Pelletier JP, Abram F, Raynauld JP, Cicuttini F, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol. 2007;34:776–784. [PubMed] [Google Scholar]
- 44.Petersen W, Tillmann B. Collagenous fibril texture of the human knee joint menisci. Anat Embryol (Berl) 1998;197:317–324. doi: 10.1007/s004290050141. [DOI] [PubMed] [Google Scholar]
- 45.Papadopoulos A, Kirkos JM, Kapetanos GA. Histomorphologic study of discoid meniscus. Arthroscopy. 2009;25:262–268. doi: 10.1016/j.arthro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Noble J, Hamblen DL. The pathology of the degenerate meniscus lesion. J Bone Joint Surg Br. 1975;57:180–186. [PubMed] [Google Scholar]
- 47.Merkel KH. The surface of human menisci and its aging alterations during age. A combined scanning and transmission electron microscopic examination (SEM, TEM) Arch Orthop Trauma Surg. 1980;97:185–191. doi: 10.1007/BF00389725. [DOI] [PubMed] [Google Scholar]
- 48.Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage. 2010;18 Suppl 3:S53–S65. doi: 10.1016/j.joca.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 49.Hopker WW, Angres G, Klingel K, Komitowski D, Schuchardt E. Changes of the elastin compartment in the human meniscus. Virchows Arch A Pathol Anat Histopathol. 1986;408:575–592. doi: 10.1007/BF00705337. [DOI] [PubMed] [Google Scholar]
- 50.Rosas HG, De Smet AA. Magnetic resonance imaging of the meniscus. Top Magn Reson Imaging. 2009;20:151–173. doi: 10.1097/RMR.0b013e3181d657d1. [DOI] [PubMed] [Google Scholar]
- 51.Roemer FW, Guermazi A, Hunter DJ, Niu J, Zhang Y, Englund M, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage. 2009;17:748–753. doi: 10.1016/j.joca.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85-A:4–9. doi: 10.2106/00004623-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Kannus P. Etiology and pathophysiology of chronic tendon disorders in sports. Scand J Med Sci Sports. 1997;7:78–85. doi: 10.1111/j.1600-0838.1997.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 55.Callaghan J, Rosenberg A, Rubash H, Simonian P. The Adult Knee. The Adult Knee. 2003;vol. 1:343–351. [Google Scholar]
- 56.Nakano T, Dodd CM, Scott PG. Glycosaminoglycans and proteoglycans from different zones of the porcine knee meniscus. J Orthop Res. 1997;15:213–220. doi: 10.1002/jor.1100150209. [DOI] [PubMed] [Google Scholar]
- 57.Herwig J, Egner E, Buddecke E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43:635–640. doi: 10.1136/ard.43.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheung HS. Distribution of type I, II, III and V in the pepsin solubilized collagens in bovine menisci. Connect Tissue Res. 1987;16:343–356. doi: 10.3109/03008208709005619. [DOI] [PubMed] [Google Scholar]
- 59.Katsuragawa Y, Saitoh K, Tanaka N, Wake M, Ikeda Y, Furukawa H, et al. Changes of human menisci in osteoarthritic knee joints. Osteoarthritis Cartilage. 2010;18:1133–1143. doi: 10.1016/j.joca.2010.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.