Abstract
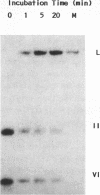
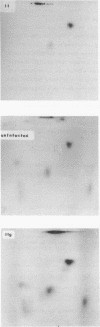
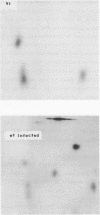
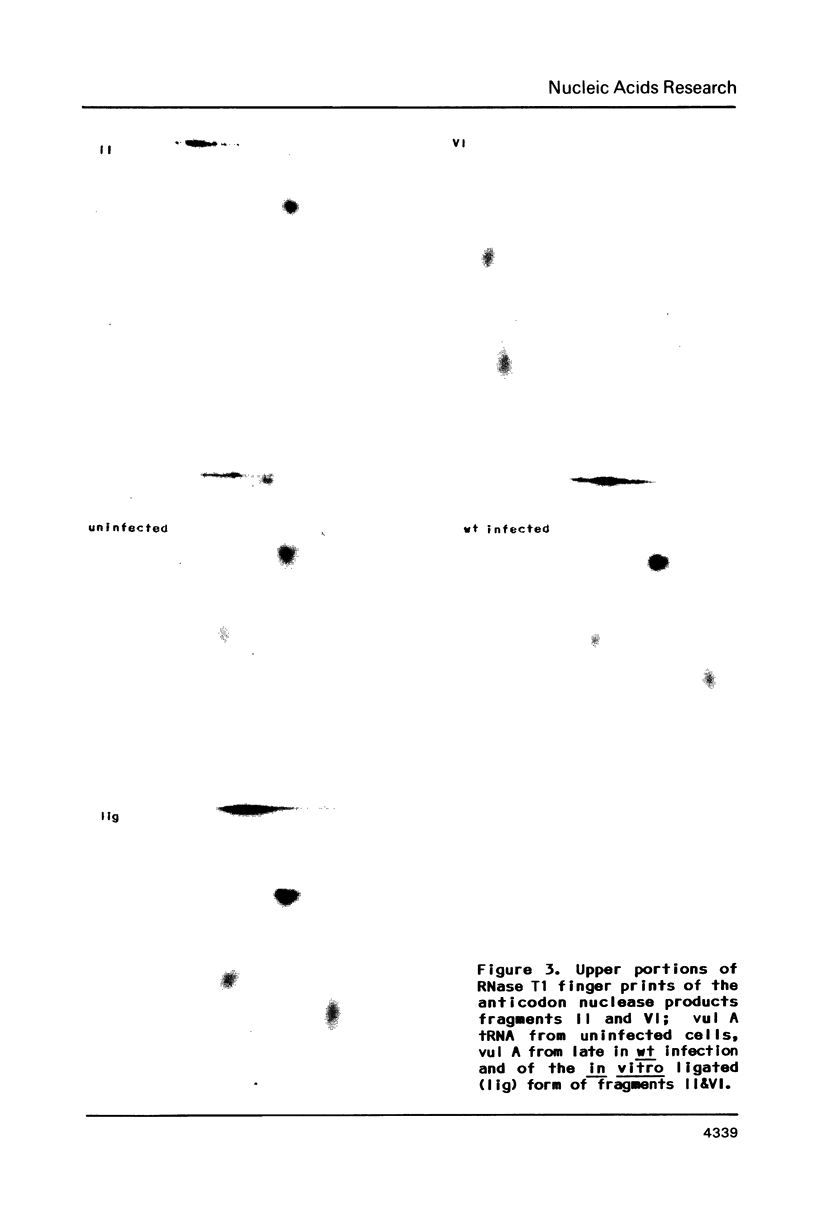
T4 mutants lacking polynucleotide kinase (pnk-) or RNA ligase (rli-) do not grow on E. coli CTr5x. During the abortive infections there accumulate host tRNA fragments that match into two species severed 3' to the anticodon. The CTr5x-specific fragments appear only transiently with wt phage, implicating the affected enzymes in phosphoryl group rearrangement and religation [David et al. (1982) Virol. 123, 480]. In a search for the vulnerable host tRNAs and putative religation products, tRNA ensembles from uninfected E. coli CTr5x or cells infected with various phage strains were fractionated and compared. A tRNA species absent from rli- infected cells but present in uninfected cells or late in wt infection was thus detected. RNase T1 finger prints of this species, isolated before or after wt infection, were compared with that of an in vitro ligated pair of CTr5x-specific fragments. The results indicated that this tRNA is cleaved upon infection and later on restored to it's original or to a very similar form, by polynucleotide kinase and RNA ligase reactions. It is suggested that depletion of such vulnerable host tRNA species underlies the restriction of pnk- or rli- phage on E. coli CTr5x.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu F. K., Maley G. F., Maley F., Belfort M. Intervening sequence in the thymidylate synthase gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1984 May;81(10):3049–3053. doi: 10.1073/pnas.81.10.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Borasio G. D., Kaufmann G. Bacteriophage T4-induced anticodon-loop nuclease detected in a host strain restrictive to RNA ligase mutants. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7097–7101. doi: 10.1073/pnas.79.23.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Borasio G. D., Kaufmann G. T4 bacteriophage-coded polynucleotide kinase and RNA ligase are involved in host tRNA alteration or repair. Virology. 1982 Dec;123(2):480–483. doi: 10.1016/0042-6822(82)90284-7. [DOI] [PubMed] [Google Scholar]
- Depew R. E., Cozzarelli N. R. Genetics and physiology of bacteriophage T4 3'-phosphatase: evidence for involvement of the enzyme in T4 DNA metabolism. J Virol. 1974 Apr;13(4):888–897. doi: 10.1128/jvi.13.4.888-897.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer C. L., Javor B., Abelson J. RNA ligase in bacteria: formation of a 2',5' linkage by an E. coli extract. Cell. 1983 Jul;33(3):899–906. doi: 10.1016/0092-8674(83)90032-6. [DOI] [PubMed] [Google Scholar]
- Jabbar M. A., Snyder L. Genetic and physiological studies of an Escherichia coli locus that restricts polynucleotide kinase- and RNA ligase-deficient mutants of bacteriophage T4. J Virol. 1984 Aug;51(2):522–529. doi: 10.1128/jvi.51.2.522-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaine B. P., Gupta R., Woese C. R. Putative introns in tRNA genes of prokaryotes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3309–3312. doi: 10.1073/pnas.80.11.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano-Sueoka T., Sueoka N. Characterization of a modified leucyl-tRNA of Escherichia coli after bacteriophage T2 infection. J Mol Biol. 1968 Nov 14;37(3):475–491. doi: 10.1016/0022-2836(68)90116-2. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels J. M., Soltis D., Hey T., Snyder L. Genetic and physiological studies of the role of the RNA ligase of bacteriophage T4. J Mol Biol. 1982 Jan 15;154(2):273–286. doi: 10.1016/0022-2836(82)90064-x. [DOI] [PubMed] [Google Scholar]
- Silber R., Malathi V. G., Hurwitz J. Purification and properties of bacteriophage T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3009–3013. doi: 10.1073/pnas.69.10.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Sirotkin K., Cooley W., Runnels J., Snyder L. R. A role in true-late gene expression for the T4 bacteriophage 5' polynucleotide kinase 3' phosphatase. J Mol Biol. 1978 Aug 5;123(2):221–233. doi: 10.1016/0022-2836(78)90322-4. [DOI] [PubMed] [Google Scholar]
- Yudelevich A. Specific cleavage of an Escherichia coli leucine transfer RNA following bacteriophage T4 infection. J Mol Biol. 1971 Aug 28;60(1):21–29. doi: 10.1016/0022-2836(71)90444-x. [DOI] [PubMed] [Google Scholar]