Abstract
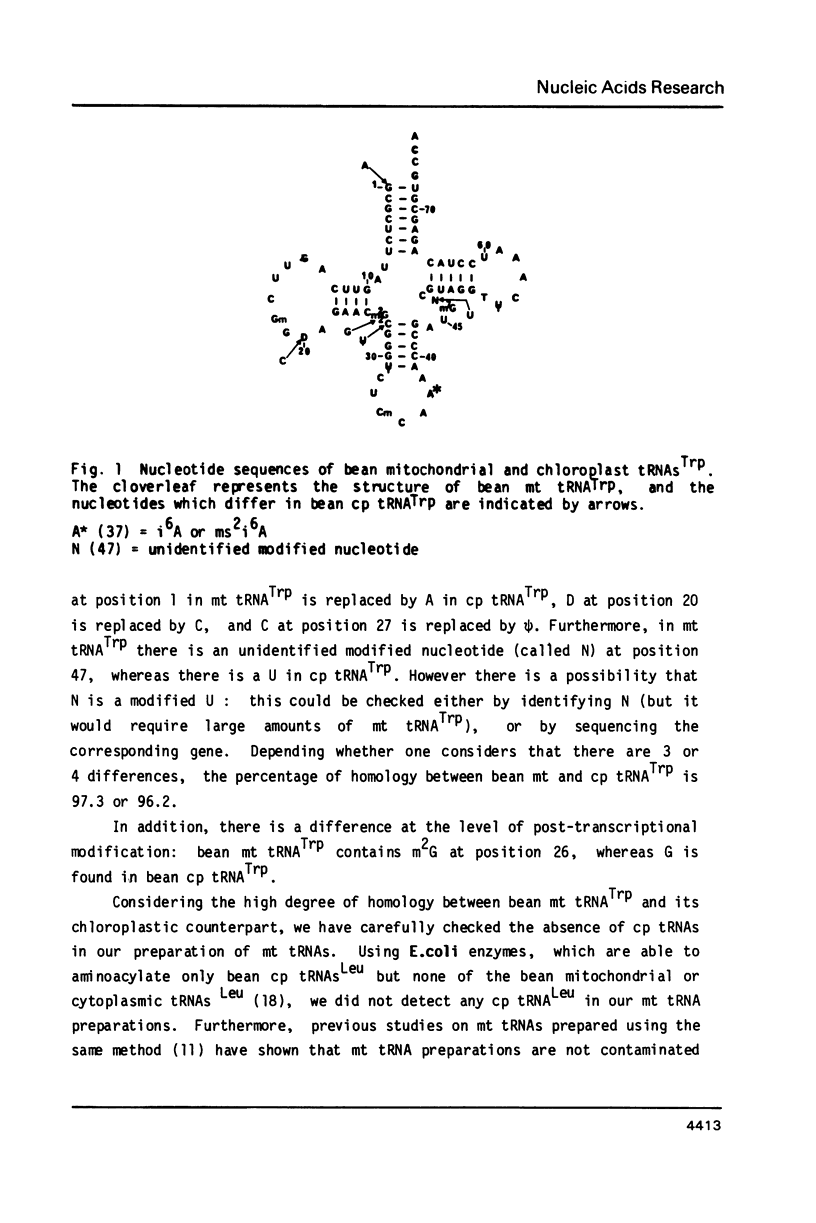
Bean mitochondria and chloroplast tRNAsTrp, purified by RPC-5 chromatography and two-dimensional gel electrophoresis, have been sequenced using post-labeling techniques. The high degree of sequence homology between bean mitochondria and chloroplast tRNAsTrp shows that these two tRNAs are coded for by closely related genes which have probably evolved from a common ancestor gene. The anticodon of bean mitochondria tRNATrp is CmCA, which can recognize UGG (the codon for tryptophan in the universal code) and is complementary neither to UGA (which codes for tryptophan in mammalian and yeast mitochondria) nor to CGG (which could be a tryptophan codeword in plant mitochondria).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonen L., Boer P. H., Gray M. W. The wheat cytochrome oxidase subunit II gene has an intron insert and three radical amino acid changes relative to maize. EMBO J. 1984 Nov;3(11):2531–2536. doi: 10.1002/j.1460-2075.1984.tb02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaday J., Guillemaut P., Gloeckler R., Weil J. H. The nucleotide sequence of spinach chloroplast tryptophan transfer RNA. Nucleic Acids Res. 1981 Jan 10;9(1):47–53. doi: 10.1093/nar/9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P. Structure and function of tryptophan tRNA from wheat germ. Nucleic Acids Res. 1984 Jun 25;12(12):4997–5003. doi: 10.1093/nar/12.12.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemaut P., Steinmetz A., Burkard G., Weil J. H. Aminoacylation of tRNA-Leu species from Escherichia coli and from the cytoplasm, chloroplasts and mitochondria of Phaseolus vulgaris by homologous and heterologous enzymes. Biochim Biophys Acta. 1975 Jan 6;378(1):64–72. doi: 10.1016/0005-2787(75)90137-9. [DOI] [PubMed] [Google Scholar]
- Hiesel R., Brennicke A. Cytochrome oxidase subunit II gene in mitochondria of Oenothera has no intron. EMBO J. 1983;2(12):2173–2178. doi: 10.1002/j.1460-2075.1983.tb01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA's. J Mol Biol. 1971 Jun 14;58(2):459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Jeannin G., Burkard G., Weil J. H. Aminoacylation of Phaseolus vulgaris cytoplasmic, chloroplastic and mitochondrial tRNAsPro and tRNAsLys by homologous and heterologous enzymes. Biochim Biophys Acta. 1976 Aug 2;442(1):24–31. doi: 10.1016/0005-2787(76)90171-4. [DOI] [PubMed] [Google Scholar]
- Kelmers A. D., Heatherly D. E. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal Biochem. 1971 Dec;44(2):486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- Mubumbila M., Gordon K. H., Crouse E. J., Burkard G., Weil J. H. Construction of the physical map of the chloroplast DNA of Phaseolus vulgaris and localization of ribosomal and transfer RNA genes. Gene. 1983 Mar;21(3):257–266. doi: 10.1016/0378-1119(83)90009-4. [DOI] [PubMed] [Google Scholar]
- Ohme M., Kamogashira T., Shinozoki K., Sugiura M. Locations and sequences of tobacco chloroplast genes for tRNAPro(UGG), tRNATrp, tRNAfMet and tRNAGly(GCC): the tRNAGly contains only two base-pairs in the D stem. Nucleic Acids Res. 1984 Sep 11;12(17):6741–6749. doi: 10.1093/nar/12.17.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks T. D., Dougherty W. G., Levings C. S., Timothy D. H. Identification of two methionine transfer RNA genes in the maize mitochondrial genome. Plant Physiol. 1984 Dec;76(4):1079–1082. doi: 10.1104/pp.76.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay D. T., Guillemaut P., Weil J. H. Nucleotide sequences of three soybean chloroplast tRNAsLeu and re-examination of bean chloroplast tRNA2Leu sequence. Nucleic Acids Res. 1984 Mar 26;12(6):2997–3001. doi: 10.1093/nar/12.6.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1984;12 (Suppl):r1–57. [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]


