Abstract
Background
Individual-based biophysical larval models, initialized and parameterized by observations, enable numerical investigations of various factors regulating survival of young fish until they recruit into the adult population. Exponentially decreasing numbers in Northeast Arctic cod and Norwegian Spring Spawning herring early changes emphasizes the importance of early life history, when ichthyoplankton exhibit pelagic free drift. However, while most studies are concerned with past recruitment variability it is also important to establish real-time predictions of ichthyoplankton distributions due to the increasing human activity in fish habitats and the need for distribution predictions that could potentially improve field coverage of ichthyoplankton.
Methodology/Principal Findings
A system has been developed for operational simulation of ichthyoplankton distributions. We have coupled a two-day ocean forecasts from the Norwegian Meteorological Institute with an individual-based ichthyoplankton model for Northeast Arctic cod and Norwegian Spring Spawning herring producing daily updated maps of ichthyoplankton distributions. Recent years observed spawning distribution and intensity have been used as input to the model system. The system has been running in an operational mode since 2008. Surveys are expensive and distributions of early stages are therefore only covered once or twice a year. Comparison between model and observations are therefore limited in time. However, the observed and simulated distributions of juvenile fish tend to agree well during early fall. Area-overlap between modeled and observed juveniles September 1st range from 61 to 73%, and 61 to 71% when weighted by concentrations.
Conclusions/Significance
The model system may be used to evaluate the design of ongoing surveys, to quantify the overlap with harmful substances in the ocean after accidental spills, as well as management planning of particular risky operations at sea. The modeled distributions are already utilized during research surveys to estimate coverage success of sampled biota and immediately after spills from ships at sea.
Introduction
Pelagic drift and environmental exposure of ichthyoplankton (egg, larvae and juvenile fish) of Northeast Arctic (NEA) cod and Norwegian spring-spawning (NSS) herring in relation to variability in recruitment indices (measured as survival until the 0-group stage, i.e. about 5 months old pelagic juveniles) have been the focus of many studies, e.g. [1], [2]. Particular emphasis has been put on early life history of fish due to the high mortality rates experienced by fish during this stage. Several hypothesis suggest key processes impacting survival in early stages of fish, e.g. match-mismatch [3], [4], bigger is better [5], and member-vagrant [6] hypotheses. However, none of these hypotheses, when considered alone, can explain the variability in recruitment in these fish stocks. [7] showed that modeled flow of Atlantic Water to the Barents Sea and modeled local primary production within the Barents Sea, partly representing several of the processes described above, accounted for 70% of the variability in cod with a 3-year lead. Hence, various, synergistic mechanisms may act in different ways to affect the feeding, growth and survival of early life stages as they drift from their spawning grounds along the Norwegian Coast to nursery areas located both along the Norwegian coast (herring) and into the Barents Sea (cod and herring). Along the drift routes the offspring need to find prey and avoid predators. Vertical positioning in the water column is important to the larvae as it affects the interactions with prey and predators and influences the drift routes [1], [8]. Circulation features affecting drift occur on many scales and it is still not clear what horizontal resolution is needed in numerical models to adequately resolve the ichthyoplankton drift routes. However, there seems to be a general agreement that it should at least be on the order of the baroclinic Rossby radius [9], about 5 km along the Norwegian Coast [10], though decreasing northwards with the increase in the Coriolis parameter.
The last decade of improvement in computer technology has enabled high temporal and spatial resolution in biophysical models which provide crucial information on how larval fish might interact with the environment. However, in addition to unveiling historic biophysical links it is also important to develop tools to report real-time distribution and abundance of the ichthyoplankton. Such short-term prediction systems were initially developed for reporting on real-time developments of the physical state of the ocean, e.g. wave-heights, currents and ice-drift, all of which are important for maritime safety. The systems were further developed to also report on biogeochemistry, e.g. algal blooms of critical knowledge to aquaculture. Currently, the European Union funded project MyOcean (www.myocean.eu) aims at integrating European efforts therein by building a pan-European capacity in operational oceanography including major centres involved in operational forecasting and monitoring. A system for operational assessment of ichthyoplankton distribution can be useful in many ways. Firstly, it can be consulted while surveys are ongoing to evaluate and modify the survey design. Secondly, if there are accidental spills of harmful substances in the ocean, an operational larval drift system can immediately report on area overlap with ichthyoplankton. Finally, such a system could be consulted when deciding on time and place for allowing particular risky operations at sea, which could result in increased mortality of ichthyoplankton.
Such a system is now developed in a combined effort between the Institute of Marine Research (IMR) and the Norwegian Meteorological Institute (met.no), where met.no runs a version of the Princeton Ocean Model (MI-POM, [11]) and IMR utilizes the MI-POM two-day forecast to run an individual-based fish larvae model (IBM) for NEA cod and NSS herring. The outcome is numerically processed and made available online either as distribution maps (www.imr.no/larvedrift) or NetCDF files on request. The system has successfully been operating since 2008 and this paper describes the technical details of the model setup, biological constraints, and potential use. Furthermore, we evaluate the results against field observations to indicate the consistency between model predictions and observations of 0-group fish.
Methods
0-group data
The international 0-group fish survey in the Barents Sea is a pelagic juvenile fish survey where the fish species are sampled by the end of the period of pelagic free drift about 5 months after spawning. It has been carried out annually since 1965. In 1980 a standard trawling procedure was recommended by ICES [12], the International Council for the Exploration of the Sea, and has been used on both Norwegian and Russian vessels since then. Since 2003 it has been part of a Joint Norwegian-Russian ecosystem survey in the Barents Sea, designed and jointly carried out by IMR (Norway) and PINRO (Russia). The survey only covers the Barents Sea and therefore not the entire cod and herring 0-group, which can be distributed farther west in the Norwegian Sea and into the fjords along the Norwegian coast.
The standard gear is the “Harstad trawl”, a pelagic trawl with 20 by 20 m mouth opening, 7 panels and a cod end. The panels have mesh sizes varying from 100 mm in the first to 30 mm in the last [13]. The standard trawling procedure consists of predetermined tows at three or more depths, each of 0.5 nautical miles (nm), with the head-line at 0, 20 and 40 m and with a vessel speed of 3 knots. Additional tows at 60 and 80 m, also of 0.5 nm, were made where a dense concentration of fish was recorded deeper than 40 m depth on the echo-sounder.
The computation of abundance indices is made using the stratified sample mean method of swept area estimates [13], [14]. The fish abundance was estimated using only pelagic trawl (0–60 m) catches. For each trawl haul the fish abundance per nm2 was calculated based on catch and trawl data (depth intervals, effective opening and distance trawled).
Numerical ocean model and IBM
The ocean model used is MI-POM (Norwegian Meteorological Institute's version of the Princeton Ocean Model) described in e.g. [11], [15], [16], run operationally at met.no as their core ocean prediction model. The 4 km resolution domain covers the Nordic Seas, the North Sea and the Barents Sea and uses monthly mean climatological boundary conditions from [17] along its open boundaries. Atmospheric forcing is retrieved from met.no's operational Hirlam 8 km model. The heat flux formulations have been adjusted for Norwegian conditions [18] and the model also includes a simple nudging scheme to assimilate satellite SST products. Tides are included and described by the eight harmonic components (M2, S2, N2, K2, Q1, O1, P1 and K1) taken from a barotropic tidal model. The tidal forcing is applied at the lateral edges of the model. A daily forecast of the following two days is routinely made available.
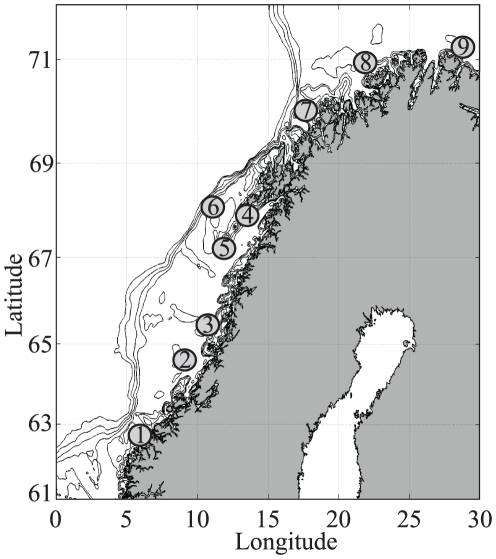
The larval IBM is a particle-tracking model Ladim [19] with a built-in behavioral algorithm for individual larval growth and vertical migration. Ladim reads the daily downloaded ocean forecast (daily averages) and updates the positions of NEA cod and NSS herring larvae using a 4′th order Runge-Kutta advection scheme. Larval growth is temperature dependent for cod with a growth function according to [20], while a fixed daily growth of 0.5 mm/day is applied for herring [21]. The growth affects the swimming capability and thereby the vertical distribution. It is assumed that larvae ascend during night and descend during day because they are visual feeders and dependent on light availability. Observations show that cod larvae are rarely found above 5 m depth and deeper than 40 m [22]. Upper and lower limits are therefore set to 5 and 40 m. A total of about 100 000 particles are initialized at spawning grounds representative for the most recent years during the spawning/hatching season which lasts from March until April [23], [24]. The relative importance of the different spawning grounds (Figure 1) is shown in Table 1.
Figure 1. Spawning ground distribution.
The spawning grounds used for initializing virtual ichthyoplankton are numbered from 1 to 9. Their relative importance is described in Table 1.
Table 1. Spawning grounds for cod and herring numbered from 1-9 in accordance with Figure 1, and the percentage of all ichthyoplankton initialized at the respective spawning grounds.
| Spawning ground | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| NEA cod (%) | 5 | 5 | 20 | 10 | 20 | 25 | 10 | 5 | |
| NSS herring (%) | 50 | 20 | 10 | 20 |
Particles are counted within each of the 4 by 4 km grid cells before filtering horizontally by convolution with a standard normal distribution covering 5 by 5 cells normalized to conserve mass. This is done to compensate for a relatively low number of particles and to smooth the noisy maps when displaying the results. However, the data behind the maps can also be made available on the web page for downloading. The concentrations are normalized according to the daily maximum value.
All subparts of the system (download the ocean forecast, update particle positions, process particle distribution and abundance into maps and Netcdf files, upload results to a ncWMS server) are written in Fortran or Matlab and integrated by a python script run regularly on an IMR server by a cron job. By maneuvering in the calendar on the web page one can display the distribution map of any day in the pelagic free drift period from March to September during the years 2008 until today.
Results
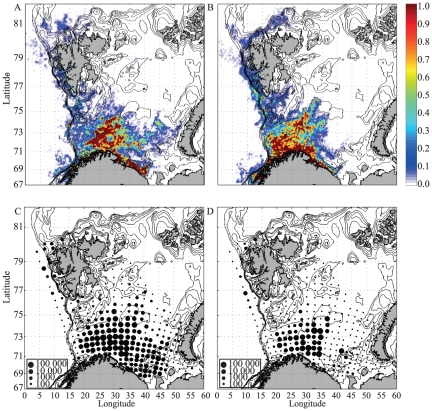
Figure 2 shows an example of modeled distribution maps for NEA cod (A) and NSS herring (B) along with the corresponding observations (C,D). In contrast to field data collected once or twice a year the modeled distributions are available on a daily basis. However, a systematic evaluation of the modeled distributions is necessary to determine their reliability. There is no single test to determine the reliability of the model output, one needs to evaluate both the different sub-models (ocean forecast, particle tracking algorithm and vertical migration scheme) and the final ichthyoplankton distribution. The MI-POM system has been thoroughly validated for the upstream domain of the North Sea ([16] and references therein), the southern part of the spawning grounds [25] and in the core of the drift area [26]. The particle tracking algorithm and the growth and vertical migration scheme has been thoroughly investigated in several studies [1], [2], [19], [20], [21]. We therefore focus on comparing the modeled 0-group distributions in early September with data from IMR surveys at the same time.
Figure 2. Modeled and observed distributions for NEA cod and NSS herring September 1st 2010.
Modeled distributions for NEA cod (A) and NSS herring (B) based on initialization of particles according to spawning grounds location (Figure 1) and relative importance (Table 1) September 1st 2010. Colors indicate abundance relative to maximum abundance for the given time on a logarithmic scale. The corresponding observed distributions for NEA cod (C) and NSS herring (D) where each dot indicates a station on the survey grid and the size of the dot indicate the abundance.
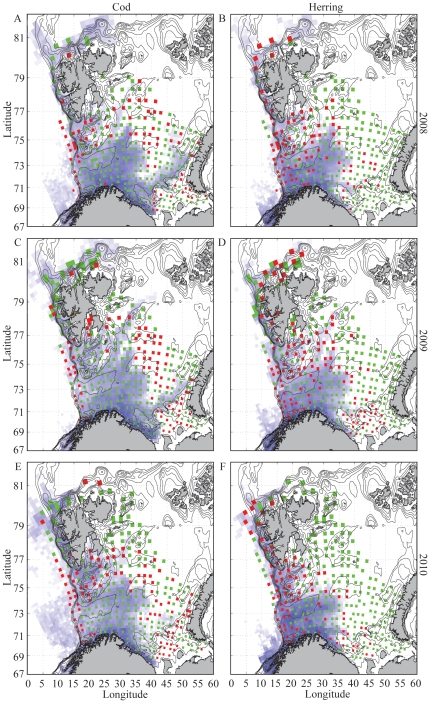
The 4 km model grid resolution is significantly higher than the horizontal sampling grid from the surveys. In order to compare the overlap between modeled and observed juveniles we interpolate both to a 25 by 25 km grid previously defined by [27]. Figure 3 shows the resulting modeled (dm) and observed (do) 0-group distributions of NEA cod (A,C,E) and NSS herring (B,D,F) for 2008 (A,B), 2009 (C,D) and 2010 (E,F). Shades of blue indicate cells occupied by modeled particles but where there are no observations. Dark blue indicate relatively higher concentrations than lighter blue. Observations are only available within cells colored by green and red. Green cells show that modeled and observed concentrations are consistent, i.e. either 0-group fish are present in both or in none of them. Red cells show that modeled and observed concentrations are inconsistent, i.e. either 0-group fish are present in the model but not in the observations, or the other way around. We quantify the overlap in percent as the sum of grid cells where either (do, dm) >0 or (do, dm) = 0 (i.e. green cells) divided by the total number of grid cells with observations (i.e. green and red cells). Cells where the model indicate juveniles but there are no observations are left out of the overlap estimates, as we cannot determine the quality of the model prediction. The numbers are reported in Table 2. In addition, we partitioned the observed and modeled abundance as either high or low (depending on whether they were above or below median) and weighted the overlap estimate. These results are reported in parenthesis in Table 2.
Figure 3. Quantification of percentage area overlap and abundance weighted area overlap between modeled and observed distribution September 1st 2008–2010.
Modeled and observed juveniles are interpolated to a 25 by 25 km grid [27] for cod and herring in 2008 (A,B), 2009 (C,D) and 2010 (E,F). Blue cells show concentrations of modeled particles where there are no observations (dark blue indicate relatively higher concentrations than lighter blue). Green cells show that there are observations available and that they are consistent with the model, i.e. either 0-group fish are present in both or in none of them. Red cells show that there are observations available but that they are inconsistent with the model, i.e. either 0-group fish are present in the model but not in observations, or the other way around.
Table 2. Percentage overlap varies between years from 61 to 73%.
| Year | 2008 | 2009 | 2010 |
| NEA cod (%) | 73 (66) | 73 (67) | 69 (71) |
| NSS herring (%) | 68 (64) | 61 (63) | 61 (61) |
Numbers in parenthesis are percentage overlap when weighted with concentrations characterized by above or below the median.
The percentage overlap varies between years from 61 to 73%. When weighted with concentrations, effectively posing a stronger criterion for overlap, the percentage decreases in 2008 for NEA cod and NSS herring and 2009 for NEA cod (Fig.3A,B,C) by 7, 4 and 6% respectively. However, for NSS herring in 2009 and NEA cod in 2010 (Fig.3D,E) the overlap in fact increases by 2%, indicating that the area overlap fits even better when taking concentrations into consideration. The area overlaps are higher for cod than herring in all years. The differences are less when weighted with concentrations in 2008 and 2009, though opposite in 2010.
In general the main features of the modeled distributions tend to compare well with the observations (Fig. 2); i) they are limited in the west by the shelf edge, ii) they are limited by the polar front in the northeastern Barents Sea [28], iii) most particles have been advected into the Barents Sea while some are advected to the west and north of Spitsbergen, and iv) NEA cod are distributed farther east then NSS herring.
Discussion
How can the model system be utilized?
This study describes a numerical system used to predict real-time ichthyoplankton distributions of NEA cod and NSS herring based on a two-day ocean forecast by the national meteorological institute of Norway. Although the system has only been running for the previous four years, it has already proven useful in situations with urgent requirement for updated ichthyoplankton distributions. One such application addressed the need to know whether the same larval patches were sampled during two subsequent days. Another application was a sinking vessel, and subsequent fuel spill, at a bank structure in the typical drift paths of ichthyoplankton and the need for a preliminary assessment of possible overlap. However, there are a number of potential benefits of a model system for operational larval drift.
Firstly, a real-time modeling system can be consulted while surveys are ongoing to evaluate and modify the survey design. As there are fundamental limitations to the model predictions we know that it may never be able to represent the ichthyoplankton distribution exactly, but it may still be able to indicate whether the survey is covering the main parts of the distribution. Additionally, it may prove important while conducting dedicated process studies (in addition to the 0-group surveys) when knowledge of day-to-day dispersal may impose restrictions on the validity of a study.
Secondly, potential contamination of marine habitats by accidental spills and long-term introduction of harmful substances motivate the creation of an operational larval drift system that can report on potential overlap with ichthyoplankton in real-time. Also, it may be necessary to prioritize the protection of certain areas due to limitations in resources (e.g. personnel, vessels, chemicals) and a system for real-time surveillance may guide decision-makers in taking the right decisions.
Finally, the system enables monitoring of ichthyoplankton exposure to long-term contamination such as radioactivity, agriculture pollution through freshwater runoff along the coast, and waste-water from petroleum industry. Similarly, the system may be consulted when conducting particularly risky operations at sea where spatiotemporal distribution of ichthyoplankton may be taken into consideration to limit potential risk.
However, while utilizing such a tool it is important to be aware of the limitations. Sources of errors relevant to this study can be divided into those resulting in erroneous model predictions and uncertainties in the field data used for evaluating the model result. The bulk part of the errors in the first category is due to poorly understood processes, such as the motivation for vertical migration in fish and lack of data on prey and predator distributions causing area-specific and time-specific mortality of the offspring. We have therefore tried to keep the model code as simple as possible in order to avoid complex algorithms representing poorly understood processes.
Sources of errors in the model predictions
We assume that the spawning distribution is similar to the most recent years and that we have a fairly good knowledge of what the spawning distribution has been. A herring larvae survey is conducted in March/April each year to estimate the abundance and distribution of newly hatched larvae. This gives a good indication of the spawning distribution. However, hatching time varies throughout the survey area and hatching may occur after the survey. Also, larvae have been subject to various duration of dispersal dependent on age and may have drifted away from the spawning areas. However, we assume this drift is limited since larvae are mostly caught soon after hatching.
Further, we assume for simplicity that herring eggs hatch at 15 m depth while in nature they are demersal and hatch at the seabed of up to 250 m depth [29]. Insufficient knowledge of the rising velocities is why we have omitted this, though we know it may take some days to ascend to the upper water column after hatching. Contrary, cod eggs have an initial pelagic drift phase of about three weeks where the depth distribution is dependent on the individual egg buoyancy distribution [30]. Algorithms for implementing such a dynamic vertical distribution is described in [31] and utilized in [32], [33] but not yet incorporated here.
Both cod and herring perform a diel vertical migration constrained by an upper and lower boundary and available light at their individual respective depths. Diel migration is here limited by individual length but not dependent on the presence of prey and predators, which is likely to affect vertical habitat selection [1], [8], [34]. The upper and lower boundaries are based on qualitative descriptions from different historic surveys [35], and should be considered approximate as the process of vertical migration is poorly sampled and not well understood.
The ocean forecast by met.no is modeled with MI-POM on a grid with a horizontal resolution of 4 by 4 km. Important small-scale dynamics that will clearly affect dispersal are therefore not included [36]. Because of computational costs it is not possible to both resolve all scales of importance while at the same cover the area of concern. Important effects of this are that dispersal on scales less than about twice the grid resolution is truncated and resulting trajectories are smoother than in reality and that the modeled spread of particles is likely underestimated. Specifically, we see in these particular results that trajectories tend to divert towards shore and overestimate concentrations close to the coast. This might be a result of misrepresentation of sub-grid scale processes or suboptimal handling of coastal boundaries in the particle tracking. In addition, air-sea-wave interactions including Stokes drift are not included in the ocean forecast and are therefore also a source of error in the modeled drift. We are addressing these challenges in ongoing studies.
Finally, natural mortality is not included since spatiotemporal variation therein is one of the main knowledge gaps of early life history in fish. Natural cod egg mortality was studied by [37] and they found that the increased egg mortality in 2001 was connected to age, size and condition of the spawning cod females, and increasing fraction of first time spawners may negatively influence both egg and larval survival. Additionally, both eggs and fish larvae are common prey for several species. Also, studies have revealed indications of spatiotemporal variations in natural survival in ichthyoplankton [38], [39], but details on how this vary through the season are complex and remain a continuous area of research. Our approach is therefore to leave this out completely and acknowledge the limitation this has on the predictive capabilities of the model system.
The present system is run without any kind of data assimilation. Assimilation of data is in principle easy with such a system, as a particle distribution may be modified and the application restarted without much harm to the model dynamics. However, there is little information available. For herring the distribution may only be reinitialized after the larvae survey in March/April, shortly after hatching. For NEA cod no such data is available.
Sources of errors in the observational data
The 0-group survey has been carried during August-September, and is clearly not synoptic. This has not been accounted for in abundance estimations as drift paths and swimming behavior of the fish during this period are not well known. The capture efficiency of the sampling trawl differs between species and decreases with decreasing 0-group length [40], [41]. Hence, a correction factor was therefore included during the original storage of the data to avoid underestimates in abundance [14]. In addition, the transition in cod from pelagic free drifting to bottom settled is a rather prolonged process occurring gradually in September-October in the Spitsbergen area and October-November in the Southern Barents Sea [42]. This may lead to an underestimate of abundance and distribution as juveniles may escape the sampling volume and an evaluation of the model prediction on false premises. Herring, on the other hand, does not settle to the bottom.
We compared the model predictions for early September with observations of 0-group cod and herring from the same time, though the surveys typically span several weeks. Hence, there is a chance for repeated sampling of a juvenile patch or missing patches as they ‘slip through’ survey masks while we steam for the next sampling station. However, increasing the sampling frequency can compensate for such events, although it is still debated what resolution is required to obtain a representative description of juvenile distribution. One argument is that surveys need to resolve the main physical features of the ocean investigated to ensure a representative estimate of abundance [9], [43]. To a first guess this is likely to be represented by the baroclinic Rossby radius. Whether this is true can be checked by oversampling the area during a few test surveys. The September surveys of cod and herring in the Barents Sea are not close to such sampling rates. Here the distance between stations are more on the order of 25–35 nautical miles.
Survey design is made in advance, but may be modified during the cruise to reach zero-levels of abundance to ensure that the entire distribution is covered. However, this is not always the case, either because there is not enough time to explore the outskirts of the distribution area or because low levels of abundance may falsely be misinterpreted as the boundary. Incomplete coverage will anyhow complicate the evaluation of model predictions, and in this study we have therefore chosen to not let model predictions outside the surveyed area affect the overlap estimate.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Vikebø F, Jørgensen C, Kristiansen T, Fiksen Ø. Drift, growth and survival of larval Northeast Arctic cod with simple rules of behavior. Marine Ecology Progress Series Vol. 2007;347:207–219. [Google Scholar]
- 2.Vikebø F, Husebø Å, Slotte A, Stenevik EK, Lien V. Impact of hatching date, vertical distribution and inter-annual variation in physical forcing on northward displacement and temperature conditions of Norwegian spring-spawning herring larvae. ICES Journal of Marine Science. 2010;67:1948–1956. [Google Scholar]
- 3.Hjort J. Fluctuations in the great fisheries of northern Europe viewed in light of biological research. Rapports et Procès-verbaux des Réunions Conseil international pour l'Exploration de la Mer. 1914;19:1–228. [Google Scholar]
- 4.Cushing D. Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Advances in Marine Biology. 1990;26:249–293. [Google Scholar]
- 5.Leggett WC, DeBlois E. Recruitment In marine fishes: is it regulated by starvation and predation in the egg and larval stages. Neth J Sea Res. 1994;32:119–134. [Google Scholar]
- 6.Iles TD, Sinclair M. Atlantic herring: stock discreteness and abundance. Science. 1982;215:627–633. doi: 10.1126/science.215.4533.627. [DOI] [PubMed] [Google Scholar]
- 7.Svendsen E, Skogen M, Budgell P, Huse G, Stiansen JE, et al. An ecosystem approach to predicting cod recruitment. Deep-Sea Research II. 2007;54:2810–2821. [Google Scholar]
- 8.Fiksen Ø, Jørgensen C, Kristiansen T, Vikebø F, Huse G. Linking behavioural ecology and oceanography: larval behaviour determines growth, mortality and dis- persal. Marine Ecology Progress Series. 2007;347:195–205. [Google Scholar]
- 9.Pepin P, Helbig JA. Sampling variability of ichthyoplankton surveys – exploring the roles of scale and resolution on uncertainty. Fisheries Research. 2011. Doi: 10.1016/j.fishres.2011.07.004.
- 10.Hansen C, Samuelsen A. Influence of horizontal model grid resolution on the simulated primary production in an embedded primary production model in the Norwegian Sea. Journal of Marine Systems. 2009;75(1-2):236–244. [Google Scholar]
- 11.Blumberg A, Mellor GL. A description of a three-dimensional coastal ocean circulation model. In: Heaps NS, editor. In Three-Dimensional Coastal Ocean Models. American Geophysical Union, Washington, DC; 1987. pp. 1–16. [Google Scholar]
- 12.Anon Preliminary report of the international 0-group fish survey in the Barents Sea and adjacent waters in August–September 1980. Annales biologiques, Conseil international pour l'exploration de la mer. 1980;37:259–266. [Google Scholar]
- 13.Eriksen E, Prozorkevich DV, Dingsør GE. An evaluation of 0-group abundance indices of the Barents Sea fish stocks. The Open Fish Science Journal. 2009;2:6–14. [Google Scholar]
- 14.Dingsør GE. Estimating abundance indices from the international 0-group fish survey in the Barents Sea. Fisheries Research. 2005;72:205–218. [Google Scholar]
- 15.Engedahl H. 1995. Implementation of the Princeton ocean model (POM/ECOM-3D) at the Norwegian Meteorological Institute (DNMI), Research Report 5, Norwegian Meteorological Institute, P.O.Box 43, Blindern, 0313 OSLO, NORWAY.
- 16.Albretsen J, Røed LP. Decadal long simulations of mesoscale structures in the North Sea and Skagerrak using two ocean models. Ocean Dynamics. 2010;60:933–955. doi: 10.1007/s10236-010-0296-0. [Google Scholar]
- 17.Engedahl H, Ådlandsvik B, Martinsen EA. Production of monthly mean climatological archives. Journal of Marine System. 1998;14:1–26. [Google Scholar]
- 18.Røed LP, Debernard J. 2004. Description of an integrated flux and sea-ice model suitable for coupling to an ocean and atmosphere model, met.no report no. 4/2004, Norwegian Meteorological Institute, POBox 43 Blindern, 0313 Oslo, Norway.
- 19.Ådlandsvik B, Sundby S. Modelling the transport of cod larvae from the Lofoten area. ICES Marine Science Symposium. 1994;198:379–392. [Google Scholar]
- 20.Folkvord A. Comparison of size-at-age of larval cod (Gadus morhua L.) from different populations based on size- and temperature-dependent models. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:1037–1052. [Google Scholar]
- 21.Stenevik EK, Fossum P, Johannessen A, Folkvord A. Identification of Norwegian spring spawning herring (Clupea harengus L.) larvae from spawning grounds off western Norway applying otolith microstructure analysis. Sarsia. 1996;80:285–292. [Google Scholar]
- 22.Ellertsen B, Fossum P, Solemdal P, Sundby S, Tilseth S. A case study on the distribution of cod larvae and availability of prey organisms in relation to physical processes in Lofoten. In: The propagation of cod. Flødevigen rapportserie 1. ISSN. 1984;0333-2594:453–478. [Google Scholar]
- 23.Sundby S, Nakken O. Spatial shifts in spawning habitats of Arcto-Norwegian cod related to multidecdal climate oscillations and climate change. ICES Journal of Marine Science. 2008;65:953–962. [Google Scholar]
- 24.ICES. ICES CM 2011/ACOM. 2011;15:624. Report of the Working Group on Widely Distributed Stocks (WGWIDE), 23 - 29 August 2011, ICES Headquarters, Copenhagen, Denmark. [Google Scholar]
- 25.LaCasce JH, Røed LP, Bertino L, Ådlandsvik B. 2007. CONMAN Technical Report no. 2: analysis of model results, met.no Report 5/2007, Norwegian Meteorological Institute, P.O.Box 43, Blindern, 0313 Oslo, Norway. http://met.no/Forskning/Publikasjoner/Publikasjoner_2007/filestore/met.no_report_5-2007.pdf.
- 26.Hackett B, Røed LP. Numerical modeling of the Halten Bank area: a validation study. Tellus. 1994;46A:113–133. [Google Scholar]
- 27.Westgård T, Johansen GO, Kvamme C, Ådlandsvik B, Stiansen JE. A framework for storing, retrieving and analysing marine ecosystem data of different origin with variable scale and distribution in time and space. GIS/Spatial Analyses in Fishery and Aquatic sciences. 2010;4:417–432. [Google Scholar]
- 28.Loeng H. Features of the physical oceanographic conditions of the Barents Sea. Polar Research. 1991;10:5–18. [Google Scholar]
- 29.Slotte A. Factors influencing location and time of spawning in Norwegian spring spawning herring: an evaluation of different hypotheses. In: Funk F, Blackburn J, Hay D, Paul A J, Stephenson R, Toresen R, Witherell D, editors. Herring: Expectations for a New Millennium. Fairbanks: 18th Lowell Wakefield Fisheries Symposium, Anchorage, Alaska, 23–26 February 2000. University of Alaska; 2001; 2001. pp. 255–278. 800. [Google Scholar]
- 30.Sundby S. Turbulence and ichthyoplankton: Influence on vertical distributions and encounter rates. Scientia Marina. 1997;61:159–176. [Google Scholar]
- 31.Thygesen UH, Ådlandsvik B. Simulating Vertical Turbulent Dispersal with Finite Volumes and Binned Random Walks. Marine Ecology Progress Series. 2007;347:145–153. [Google Scholar]
- 32.Myksvoll M, Sundby S, Ådlandsvik B, Vikebø FB. Retention of coastal cod eggs in a fjord caused by interactions between egg buoyancy and circulation pattern. Marine and Coastal Fisheries. 2011;3:279–294. [Google Scholar]
- 33.Opdal AF, Vikebø FB, Fiksen Ø. Parental migration, climate and thermal exposure of larvae: spawning in southern regions gives cod a warm start. Marine Ecology Progress Series. 2011;439:255–262. Doi: 10.3354/meps09335. [Google Scholar]
- 34.Kristiansen T, Jørgensen C, Lough RG, Vikebø F, Fiksen Ø. Modeling rule-based behavior: habitat selection and the growth-survival trade-off in larval cod. Behavioral Ecology. 2009;20:490–500. [Google Scholar]
- 35.Ferreira A, Stenevik EK, Vollset KW, Folkvord A. Marine Biology Research; (Conditionally accepted) Vertical migration of Norwegian spring spawning herring larvae in relation to predator or prey distribution. [Google Scholar]
- 36.North EW, Gallego A, Petitgas P, editors. Manual of recommended practices for modelling physical–biological interactions during fish early life. ICES Cooperative Research Report No. 2009;295:111. [Google Scholar]
- 37.Makhotin V, Solemdal P, Korsbrekke K, Salthaug A. ICES CM 2001/N; 2001. Types and frequency of malformations and mortality in eggs of Arcto-Norwegian cod: A field study.12 [Google Scholar]
- 38.Ciannelli L, Dingsør GE, Bogstad B, Ottersen G, Chan KS, et al. Spatial anatomy of species survival: effects of predation and climate-driven environmental variability. Ecology. 2007;88:635–645. doi: 10.1890/05-2035. [DOI] [PubMed] [Google Scholar]
- 39.Garrison LP. Diets of five hake species in the northeast United States continental shelf ecosystem. Marine Ecology Progress Series. 2000;204:243. [Google Scholar]
- 40.Godø OR, Valdemarsen JV, Engas A. Comparison of efficiency of standard and experimental juvenile gadoid sampling trawls. ICES Marine Science Symposium. 1993;196:196–201. [Google Scholar]
- 41.Hylen A, Korsbrekke K, Nakken O, Ona E. Comparison of capture eflciency of pelagic trawls for 0-group fish. In: Hylen A, editor. : Precision and relevance of pre-recruit studies for fishery management related to fish stocks in the Barents Sea and adjacent waters. Proceedings of the sixth IMR-PINRO symposium. Bergen, 14-17 June 1994. Institute of Marine Research, Bergen, Norway; 1995. 145-156 in. [Google Scholar]
- 42.Boitsov VD, Lebed NI, Ponomarenko VP, Ponomarenko IY, Tereshchenko VV, et al. Murmansk, PINRO Press; 1996. The Barents Sea cod (biology and fisheries outline).296 [Google Scholar]
- 43.Stenevik EK, Nash R, Vikebø FB, Fossum P, Bakkeplass K. Journal of Marine Systems; (Conditionally accepted) The effects of survey design and circulation pattern on the perceived abundance of herring larvae: A case study for Norwegian Spring Spawning herring (Clupea harengus). [Google Scholar]