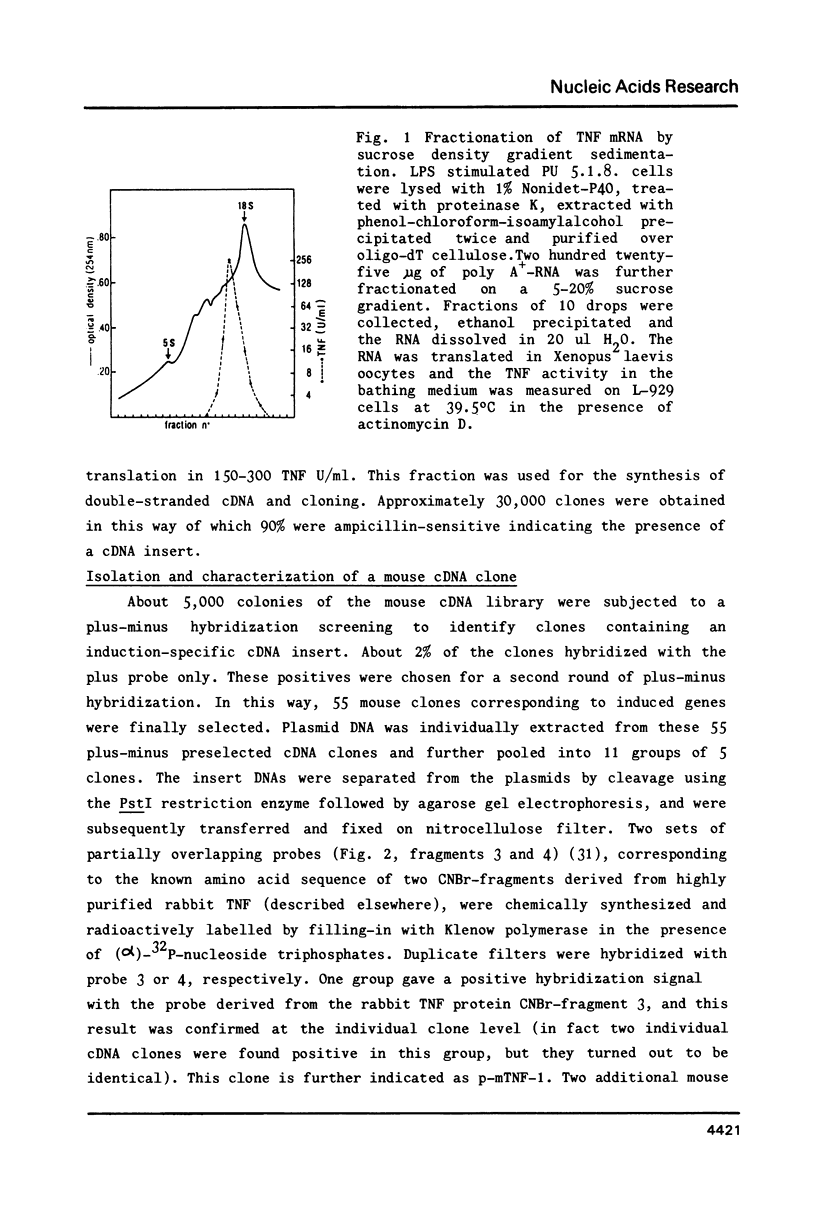
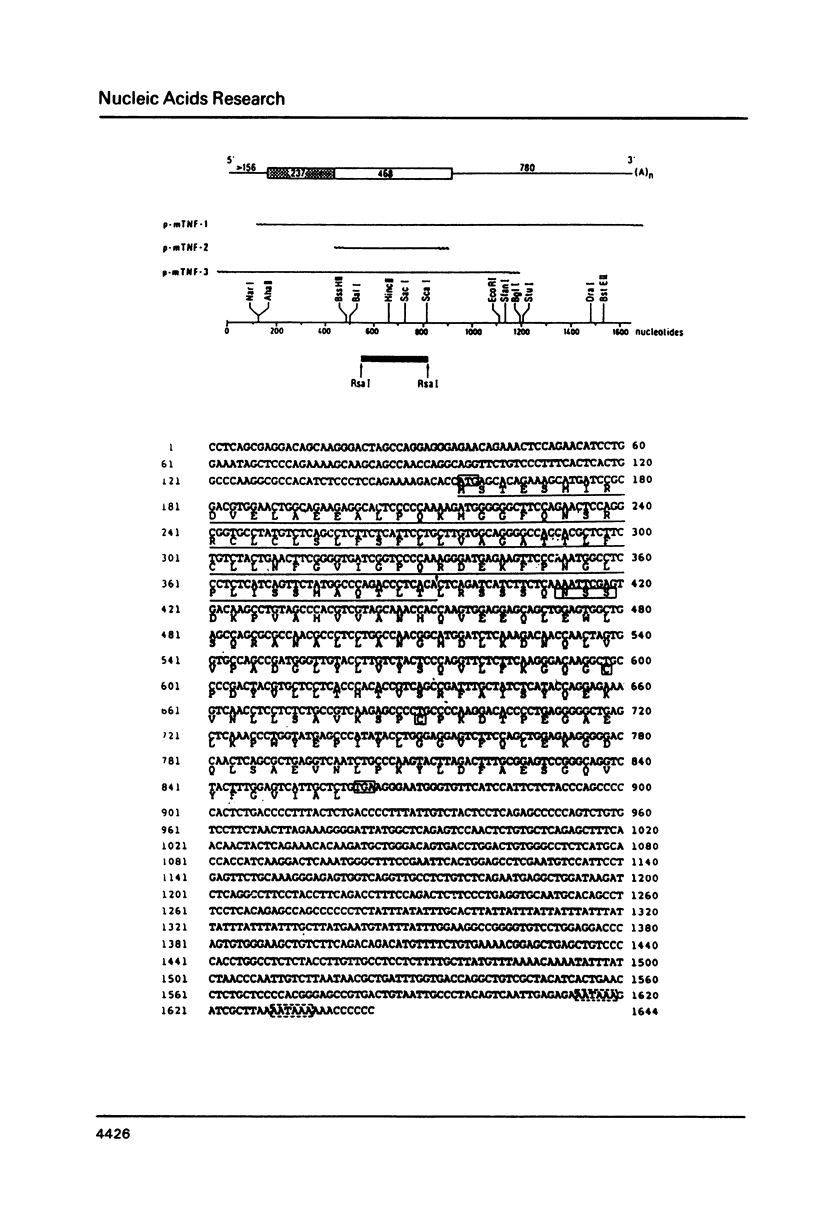
Abstract
Tumour necrosis factor (TNF), released by induced macrophages, causes tumour necrosis in animals and kills preferentially transformed cells in vitro. mRNA induced in the established mouse monocytic PU 5.1.8 cell line by lipopolysaccharide, was converted into double-stranded cDNA and cloned in the pAT153 vector. Recombinant plasmids were screened by plus-minus hybridization and TNF-specific oligonucleotide probes constructed on the basis of partial amino acid sequences of rabbit TNF. A series of TNF specific clones were identified and confirmed by hybrid selection of mouse TNF-specific mRNA. The sequence codes for a 235 amino acids long polypeptide, of which 156 amino acids presumably correspond to the mature product. It can be concluded that mature mouse TNF is a glycosylated dimer. Biologically active TNF was secreted by both Cos-I and CHO-cells transfected with the chimaeric expression vector pSV2d2-mTNF containing the coding region of the mouse TNF cDNA gene.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley W. B. II. Contribution to the Knowledge of Sarcoma. Ann Surg. 1891 Sep;14(3):199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R., Cheroutre H., Taya Y., Degrave W., Van Heuverswyn H., Fiers W. Molecular cloning of human immune interferon cDNA and its expression in eukaryotic cells. Nucleic Acids Res. 1982 Apr 24;10(8):2487–2501. doi: 10.1093/nar/10.8.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R., Opsomer C., Scahill S. J., Van der Heyden J., Fiers W. Purification of recombinant glycosylated human gamma interferon expressed in transformed Chinese hamster ovary cells. J Interferon Res. 1984 Fall;4(4):461–468. doi: 10.1089/jir.1984.4.461. [DOI] [PubMed] [Google Scholar]
- Devos R., van Emmelo J., Contreras R., Fiers W. Construction and characterization of a plasmid containing a nearly full-size DNA copy of bacteriophage MS2 RNA. J Mol Biol. 1979 Mar 15;128(4):595–619. doi: 10.1016/0022-2836(79)90295-x. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Fiers W. Expression and excretion of human fibroblast beta 1 interferon in monkey cells after transfection with a recombinant SV40 plasmid vector. J Mol Appl Genet. 1982;1(5):385–394. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Aggarwal B. B., Benton C. V., Bringman T. S., Henzel W. J., Jarrett J. A., Leung D. W., Moffat B., Ng P., Svedersky L. P. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984 Dec 20;312(5996):721–724. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- Green S., Dobrjansky A., Carswell E. A., Kassel R. L., Old L. J., Fiore N., Schwartz M. K. Partial purification of a serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1976 Feb;73(2):381–385. doi: 10.1073/pnas.73.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Dobrjansky A., Chiasson M. A., Carswell E., Schwartz M. K., Old L. J. Corynebacterium parvum as the priming agent in the production of tumor necrosis factor in the mouse. J Natl Cancer Inst. 1977 Nov;59(5):1519–1522. doi: 10.1093/jnci/59.5.1519. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T. [Interaction of receptors on parietal cells at time of the vagal nerve stimulation with 2-deoxy-D-glucose]. Nihon Shokakibyo Gakkai Zasshi. 1984 Jun;81(6):1355–1364. [PubMed] [Google Scholar]
- Kull F. C., Jr, Cuatrecasas P. Preliminary characterization of the tumor cell cytotoxin in tumor necrosis serum. J Immunol. 1981 Apr;126(4):1279–1283. [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Watkins J. F. Tumour-necrosis factor from the rabbit. I. Mode of action, specificity and physicochemical properties. Br J Cancer. 1978 Aug;38(2):302–309. doi: 10.1038/bjc.1978.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Männel D. N., Moore R. N., Mergenhagen S. E. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. doi: 10.1128/iai.30.2.523-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrove J. M., Gifford G. E. Stimulation of RNA synthesis in L-929 cells by rabbit tumor necrosis factor. Proc Soc Exp Biol Med. 1979 Mar;160(3):354–358. doi: 10.3181/00379727-160-40449. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D., Michalak M., Daisley S. L., Glick R. A method for the purification of E. coli plasmid DNA by homogeneous lysis and polyethylene glycol precipitation. Mol Biol Rep. 1983 Aug;9(3):191–195. doi: 10.1007/BF00775367. [DOI] [PubMed] [Google Scholar]
- Scahill S. J., Devos R., Van der Heyden J., Fiers W. Expression and characterization of the product of a human immune interferon cDNA gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4654–4658. doi: 10.1073/pnas.80.15.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Yamaguchi H., Ito H., Todd C. W., Wallace R. B. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. 1985 Feb 28-Mar 6Nature. 313(6005):803–806. doi: 10.1038/313803a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson B. D., Carswell E. A., Rubin B. Y., Prendergast J. S., Old L. J. Human tumor necrosis factor produced by human B-cell lines: synergistic cytotoxic interaction with human interferon. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5397–5401. doi: 10.1073/pnas.80.17.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]



