Abstract
The secondary structures of poly(dA-dC) X (dG-dT) were studied using CD and IR spectroscopies. We give spectroscopic evidence of secondary structure transitions of poly(dA-dC) X (dG-dT) from a B to a Z-like helix, induced by transition metal ions (Ni2+) in presence of high concentrations of Cs+ and Na+. In the presence of Na+, the B in equilibrium Z transition occurs at any temperature, whereas premelting conditions are required in presence of Cs+. For these two alkali ions the Z-like form is only induced by Ni2+ ions through their specific interactions at N7 of purines, under conditions of low water activity due to the high alkali salt concentration. We also show that the CD spectrum obtained in presence of Cs+ ions and characterized by a negative band at 275 nm, cannot be interpreted in terms of Z-like left-handed helix but reflects a modified B right-handed helix.
Full text
PDF

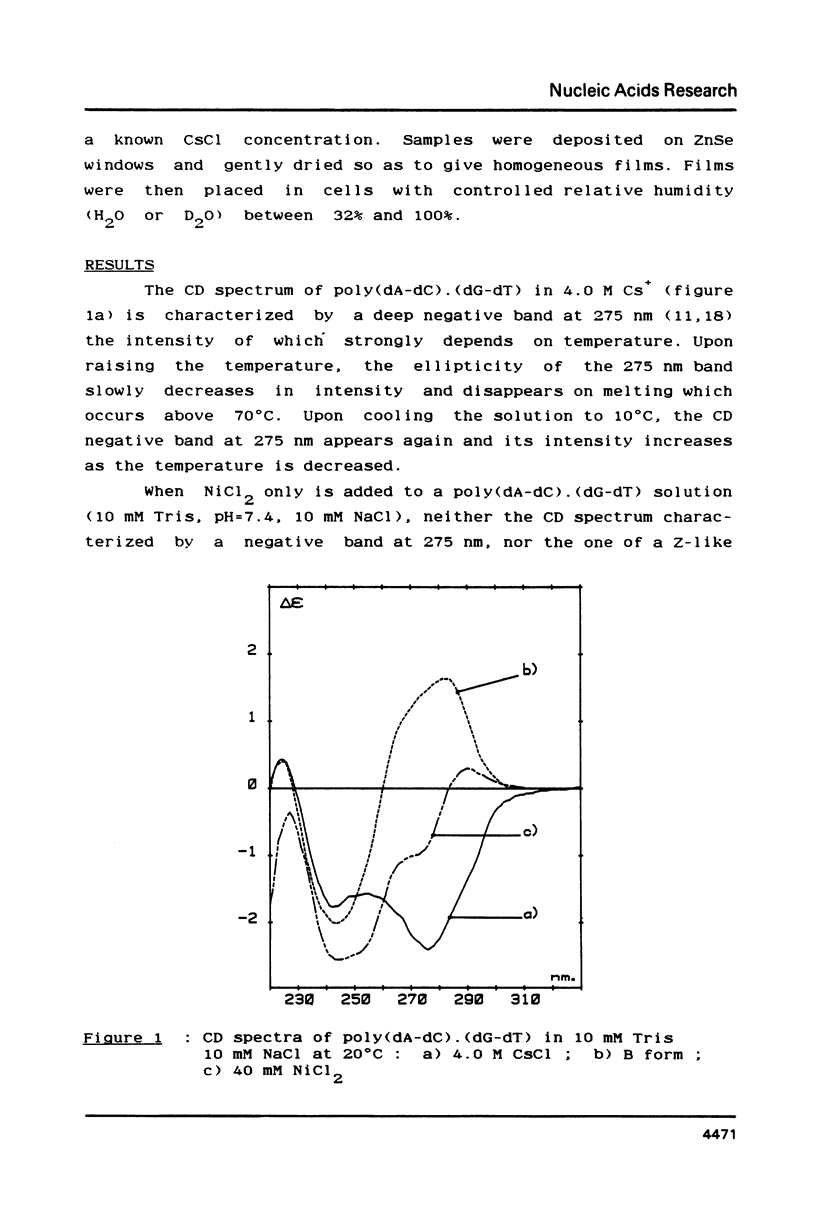







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baase W. A., Johnson W. C., Jr Circular dichroism and DNA secondary structure. Nucleic Acids Res. 1979 Feb;6(2):797–814. doi: 10.1093/nar/6.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms S., Vergne J., Brahms J. G., Di Capua E., Bucher P., Koller T. Natural DNA sequences can form left-handed helices in low salt solution under conditions of topological constraint. J Mol Biol. 1982 Dec 5;162(2):473–493. doi: 10.1016/0022-2836(82)90539-3. [DOI] [PubMed] [Google Scholar]
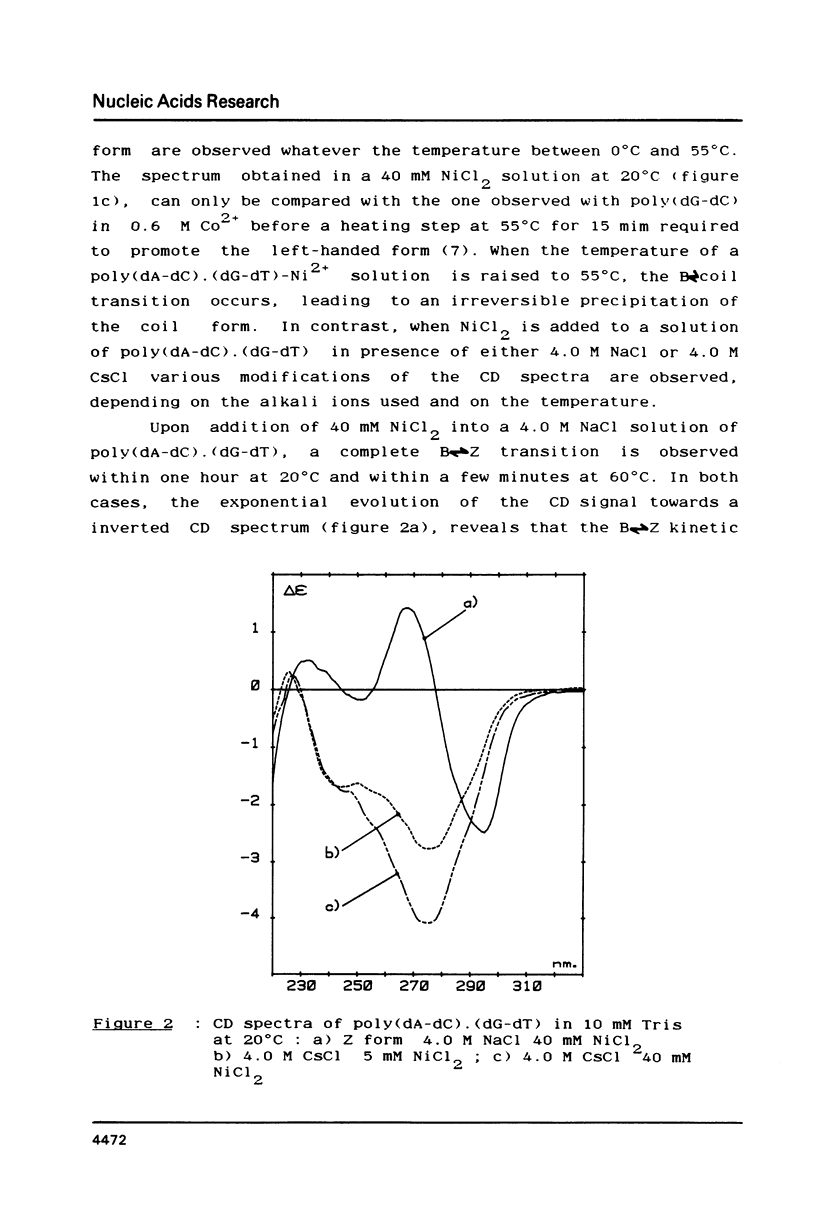
- Chan A., Kilkuskie R., Hanlon S. Correlations between the duplex winding angle and the circular dichroism spectrum of calf thymus DNA. Biochemistry. 1979 Jan 9;18(1):84–91. doi: 10.1021/bi00568a013. [DOI] [PubMed] [Google Scholar]
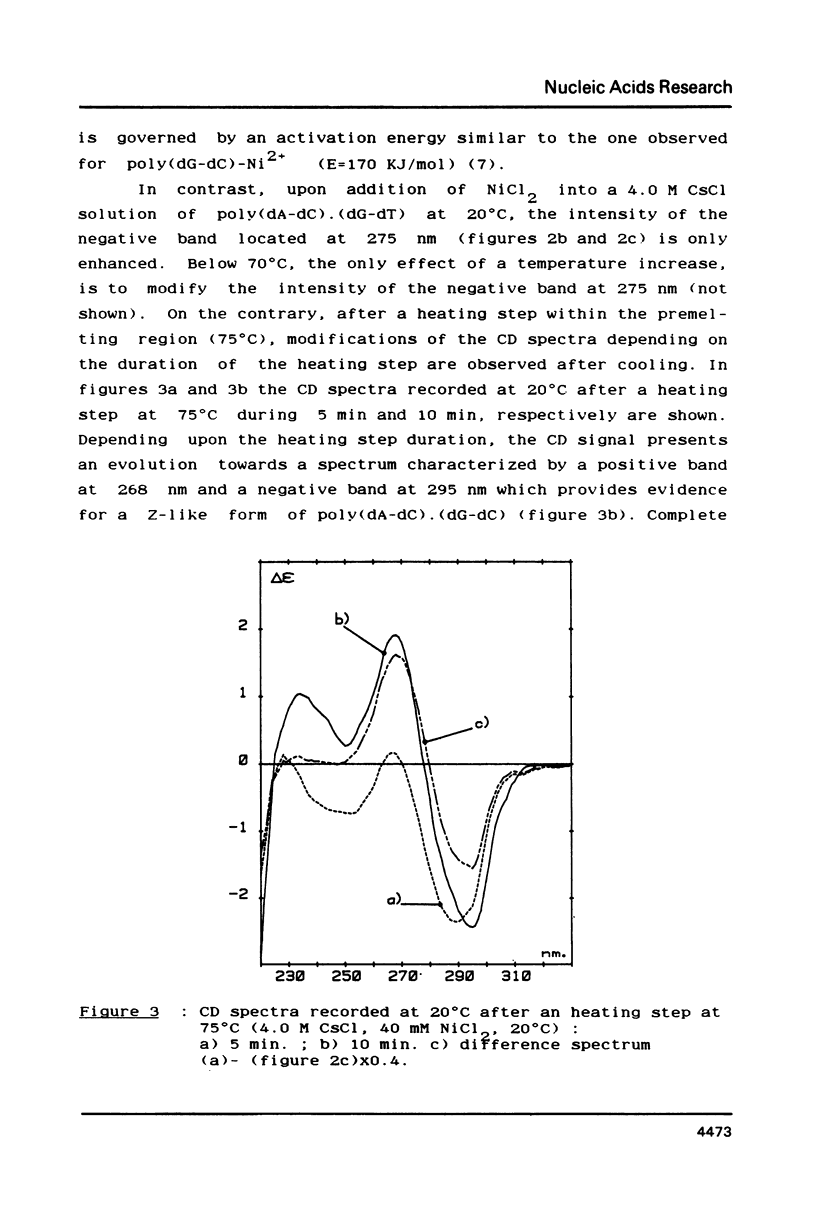
- Chen C. W., Cohen J. S. Salt- and sequence-dependence of the secondary structure of DNA in solution by 31P-NMR spectroscopy. Biopolymers. 1983 Mar;22(3):879–893. doi: 10.1002/bip.360220310. [DOI] [PubMed] [Google Scholar]
- Fazakerley G. V. Zinc Z-DNA. Nucleic Acids Res. 1984 Apr 25;12(8):3643–3648. doi: 10.1093/nar/12.8.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
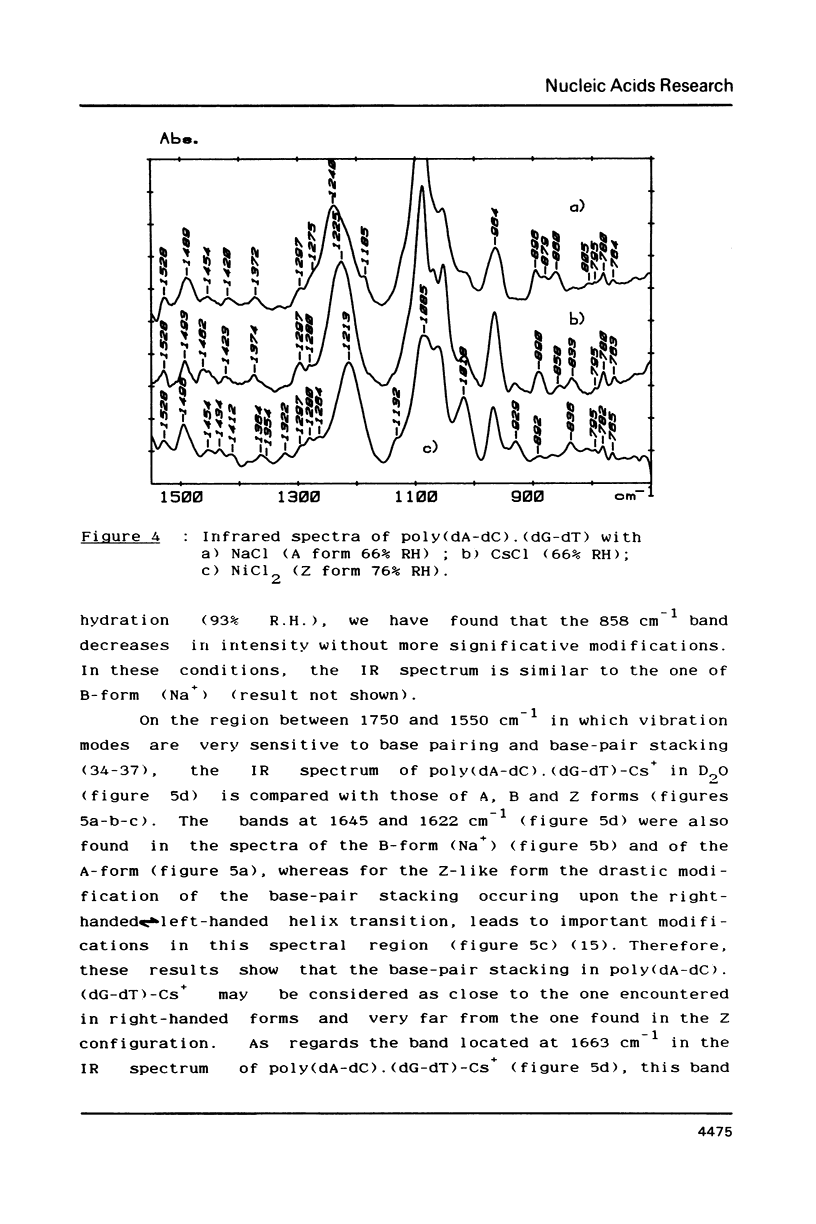
- Ghomi M., Taboury J. A., Taillandier E. Experimental and calculated study of the vibrational modes of poly(dG-dC).poly(dG-dC) in B and Z conformations. Biochimie. 1984 Feb;66(2):87–92. doi: 10.1016/0300-9084(84)90184-6. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Edmondson S. P., Lang D., Vaughan M. The circular dichroism and X-ray diffraction of DNA condensed from ethanolic solutions. Nucleic Acids Res. 1979;6(6):2089–2107. doi: 10.1093/nar/6.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism spectra of poly[d(AC):d(GT)], poly[r(AC):r(GU)], and hybrids poly[d(AC):r(GU)] and poly[r(AC):d(GT)] in the presence of ethanol. Biopolymers. 1975 Mar;14(3):487–498. doi: 10.1002/bip.1975.360140305. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minyat E. E. The transitions between left- and right-handed forms of poly(dG-dC). Nucleic Acids Res. 1981 Sep 25;9(18):4783–4798. doi: 10.1093/nar/9.18.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., McIntosh L. P., Arndt-Jovin D. J., Zarling D. A., Robert-Nicoud M., van de Sande J. H., Jorgenson K. F., Eckstein F. Left-handed DNA: from synthetic polymers to chromosomes. J Biomol Struct Dyn. 1983 Oct;1(1):21–57. doi: 10.1080/07391102.1983.10507425. [DOI] [PubMed] [Google Scholar]
- Kypr J., Vorlícková M., Budesinský M., Sklenár V. Strange double helix of poly (dA-dT) in high-salt solution. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1257–1264. doi: 10.1016/0006-291x(81)90755-5. [DOI] [PubMed] [Google Scholar]
- Leslie A. G., Arnott S., Chandrasekaran R., Ratliff R. L. Polymorphism of DNA double helices. J Mol Biol. 1980 Oct 15;143(1):49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- Lezius A. G., Gottschalk E. M. Uber eine reversible kooperative Konformationsumwandlung einer synthetischern DNA unter dem Einfluss hoher Salzkonzentrationen. Hoppe Seylers Z Physiol Chem. 1970 Mar;351(3):413–416. [PubMed] [Google Scholar]
- Liquier J., Bourtayre P., Pizzorni L., Sournies F., Labarre J. F., Taillandier E. Spectroscopic studies of conformational transitions in double stranded DNAs in the presence of carcinogenic nickel compounds and an antitumoral drug (SOAZ). Anticancer Res. 1984 Jan-Apr;4(1-2):41–44. [PubMed] [Google Scholar]
- Maestre M. F. Circular dichroism of DNA films: reversibility studies. J Mol Biol. 1970 Sep 28;52(3):543–556. doi: 10.1016/0022-2836(70)90418-3. [DOI] [PubMed] [Google Scholar]
- McIntosh L. P., Grieger I., Eckstein F., Zarling D. A., van de Sande J. H., Jovin T. M. Left-handed helical conformation of poly[d(A-m5C).d(G-T)]. Nature. 1983 Jul 7;304(5921):83–86. doi: 10.1038/304083a0. [DOI] [PubMed] [Google Scholar]
- Miles H. T., Frazier J. Infrared spectroscopy of polynucleotides in the carbonyl region in H2O solution: A.U systems. Biochemistry. 1978 Jul 11;17(14):2920–2927. doi: 10.1021/bi00607a034. [DOI] [PubMed] [Google Scholar]
- Narasimhan V., Bryan A. M. Temperature-induced perturbations in the circular dichroic spectrum of the synthetic polymer poly[d(G-C)]. Biochim Biophys Acta. 1976 Jul 16;435(4):433–437. doi: 10.1016/0005-2787(76)90209-4. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Rhodes N. J., Mahendrasingam A., Pigram W. J., Fuller W., Brahms J., Vergne J., Warren R. A. The C conformation is a low salt form of sodium DNA. Nature. 1982 Mar 18;296(5854):267–269. doi: 10.1038/296267a0. [DOI] [PubMed] [Google Scholar]
- Sarma M. H., Gupta G., Sarma R. H. Solution structure of poly(dA-dT).poly(dA-dT) in low and high salt: a 500 MHz 1H NMR study using one-dimensional NOE. J Biomol Struct Dyn. 1984 Jun;1(6):1423–1455. doi: 10.1080/07391102.1984.10507529. [DOI] [PubMed] [Google Scholar]
- Simion F. A., Fleischer B., Fleischer S. Subcellular distribution of bile acids, bile salts, and taurocholate binding sites in rat liver. Biochemistry. 1984 Dec 18;23(26):6459–6466. doi: 10.1021/bi00321a028. [DOI] [PubMed] [Google Scholar]
- Studdert D. S., Patroni M., Davis R. C. Circular dichroism of DNA: temperature and salt dependence. Biopolymers. 1972;11(4):761–779. doi: 10.1002/bip.1972.360110404. [DOI] [PubMed] [Google Scholar]
- Sutherland J. C., Griffin K. P., Keck P. C., Takacs P. Z. Z-DNA: vacuum ultraviolet circular dichroism. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4801–4804. doi: 10.1073/pnas.78.8.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboury J. A., Adam S., Taillandier E., Neumann J. M., Tran-Dinh S., Huynh-Dinh T., Langlois d'Estaintot B., Conti M., Igolen J. The B----Z transition in two synthetic oligonucleotides: d(C-2-amino-ACGTG) and d(m5CGCAm5CGTGCG) studied by IR, NMR and CD spectroscopies. Nucleic Acids Res. 1984 Aug 10;12(15):6291–6305. doi: 10.1093/nar/12.15.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboury J. A., Bourtayre P., Liquier J., Taillandier E. Interaction of Z form poly(dG-dC).poly(dG-dC) with divalent metal ions: localization of the binding sites by I.R. spectroscopy. Nucleic Acids Res. 1984 May 25;12(10):4247–4258. doi: 10.1093/nar/12.10.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboury J. A., Taillandier E. Polymorphisme de l'hélice gauche (forme Z) du poly(dG-dC).poly(dG-dC) induit par le MgCl2 étudié par spectroscopie infrarouge. Magnesium. 1984;3(3):152–158. [PubMed] [Google Scholar]
- Taillandier E., Taboury J. A., Adam S., Liquier J. Left-handed helical structure of poly[d(A-C)].poly[d(G-T)] studied by infrared spectroscopy. Biochemistry. 1984 Nov 20;23(24):5703–5706. doi: 10.1021/bi00319a007. [DOI] [PubMed] [Google Scholar]
- Taillandier E., Taboury J., Liquier J., Sautière P., Couppez M. Structural transitions in DNAs and nucleohistones studied by I.R. spectroscopy. Biochimie. 1981 Nov-Dec;63(11-12):895–898. doi: 10.1016/s0300-9084(82)80282-4. [DOI] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Kleinwächter V., Palecek E. Salt-induced conformational changes of poly(dA-dT). Nucleic Acids Res. 1980 Sep 11;8(17):3965–3973. doi: 10.1093/nar/8.17.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Sklenár V. Salt-induced conformational transition of poly[d(A-T)] X poly[d(A-T)]. J Mol Biol. 1983 May 5;166(1):85–92. doi: 10.1016/s0022-2836(83)80052-7. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Sedlácek P., Kypr J., Sponar J. Conformational transitions of poly(dA-dT)poly(dA-dT) in ethanolic solutions. Nucleic Acids Res. 1982 Nov 11;10(21):6969–6979. doi: 10.1093/nar/10.21.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlíckovă M., Kypr J., Stokrová S., Sponar J. A Z-like form of poly(dA-dC).poly(dG-dT) in solution? Nucleic Acids Res. 1982 Feb 11;10(3):1071–1080. doi: 10.1093/nar/10.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Miglietta J. J., Kłysik J., Larson J. E., Stirdivant S. M., Zacharias W. Spectroscopic studies on acetylaminofluorene-modified (dT-dG)n . (dC-dA)n suggest a left-handed conformation. J Biol Chem. 1982 Sep 10;257(17):10166–10171. [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Klysik J., Stirdivant S. M., Wells R. D. Conditions which cause the right-handed to left-handed DNA conformational transitions. Evidence for several types of left-handed DNA structures in solution. J Biol Chem. 1982 Mar 25;257(6):2775–2782. [PubMed] [Google Scholar]
- Zarlenga D. S., Halsall H. B., Day R. A. A polycationic amine that induces unique conformational changes in poly(dA-dT) in low salt. Nucleic Acids Res. 1984 Aug 10;12(15):6325–6335. doi: 10.1093/nar/12.15.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Tymen S., Marck C., Guschlbauer W. Conformational transitions of poly(dA-dC).poly(dG-dT) induced by high salt or in ethanolic solution. Nucleic Acids Res. 1982 Feb 11;10(3):1081–1091. doi: 10.1093/nar/10.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., McIntosh L. P., Jovin T. M. Mn2+ and other transition metals at low concentration induce the right-to-left helical transformation of poly[d(G-C)]. EMBO J. 1982;1(7):777–782. doi: 10.1002/j.1460-2075.1982.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


