Abstract
The aim of the present study was to determine whether the risk of mortality associated with the concentration of soluble ST2 (sST2) differs in patients with acutely decompensated heart failure with preserved ejection fraction (HFpEF) compared to patients with systolic heart failure. We prospectively enrolled 447 patients with acutely decompensated heart failure. Blood samples were collected at presentation to determine the sST2 concentration. HFpEF was defined as symptoms or signs of acutely decompensated heart failure and left ventricular ejection fraction of ≥50% on the echocardiogram. The patients were followed up for 1 year, and the vital status was obtained for all. The sST2 concentrations were greater in the patients with systolic heart failure (n = 250) than in those with HFpEF (n = 197) at 0.55 versus 0.38 ng/ml (p <0.001). Receiver operating characteristic curve analyses showed different cutoff point values for sST2 for the prediction of 1-year mortality in patients with HFpEF (>0.35 ng/ml) and systolic heart failure (>0.56 mg//ml). These cutoff points had similar prognostic accuracy (area under the curve of 0.69 vs 0.73; p >0.05). In the adjusted analyses that included amino terminal B-type natriuretic peptide concentrations, elevated sST2 concentrations were associated with a greater mortality risk in both populations (HFpEF, per ng/ml, hazard ratio 1.41, 95% confidence interval 1.14 to 1.76, p = 0.002; and systolic heart failure, per ng/ml, hazard ratio 1.20, 95% confidence interval 1.10 to 1.32, p <0.001). The determination of the sST2 concentration improved the clinical risk prediction compared to amino terminal B-type natriuretic peptide, as assessed by both the improved C-statistic and an improvement in the net reclassification index and integrated discrimination improvement analyses. In conclusion, in the present multicenter study, sST2 concentrations were lower in patients with HfpEF; however, sST2 remained an independent predictor of mortality, regardless of the left ventricular ejection fraction.
In addition to the natriuretic peptides, other biochemical markers have been examined for prognostication in heart failure. Among these is the soluble form of the interleukin-1 receptor member, ST2. A biomarker suggested to potentially reflect ventricular remodeling and fibrosis,1–3 soluble ST2 (sST2) represents an attractive candidate marker for understanding heart failure biology. Moreover, concentrations of the marker represent a powerful prognostic variable in those with acutely decompensated heart failure.4–8 However, the value of sST2 as a biomarker of risk as a function of acutely decompensated heart failure with preserved ejection fraction (HfpEF) versus systolic heart failure has not been specifically examined. Thus, in a cohort of patients with acutely decompensated heart failure, we evaluated the effects of left ventricular function on the concentrations of sST2, the associations between the cardiac structure and function and sST2, and the prognostic meaning of sST2 in those with HFpEF versus systolic heart failure.
Methods
The study population consisted of subjects from 3 previously reported prospective clinical trials of acutely decompensated heart failure from Boston, Massachusetts, Linz, Austria, and Murcia, Spain.9–11 These trials had compatible inclusion/exclusion criteria and had used similar clinical and laboratory testing, including sST2, troponin T, amino terminal B-type natriuretic peptide (NT-proBNP), and C-reactive protein. For the purposes of the present study, 447 patients with acutely decompensated heart failure had data available and were considered (209 from Boston, Massachusetts; 131 from Linz, Austria; and 107 from Murcia, Spain). The ProBNP Investigation of Dyspnea in the Emergency Department study was a prospective, blinded study of NT-proBNP testing performed in Boston, Massachusetts, that examined 599 subjects with dyspnea in the emergency department. All patients with acutely decompensated heart failure from Boston were eligible for the present analysis. The Linz study had included 137 patients with dyspnea who had presented to the emergency department and had a final diagnosis of acutely decompensated heart failure. Of these patients, 131 had complete data and were included in the present study. The final source of data for the present analysis was a prospectively gathered group of subjects from a Spanish cohort study of patients with a diagnosis of acutely decompensated heart failure consecutively admitted to the University Hospital of Virgen de la Arrixaca from September 1, 2006 to February 28, 2009. During that period, 107 subjects with sST2 data on admission were available for analysis and were included in the present study. The patients from each study group were followed up for 1 year, and the vital status was obtained for all.
The patients were characterized as having HFpEF if their left ventricular ejection fraction was ≥50%, as estimated using echocardiography and Simpson’s biplane method.12,13 The concentrations of sST2 were measured using an enzyme-linked immunosorbent assay (Medical and Biological Laboratories, Woburn, Massachusetts) on blood specimens frozen at −80°C. In addition, NT-proBNP was measured using a validated, commercially available immunoassay (Elecsys ProBNP, Roche Diagnostics, Indianapolis, Indiana) using an established method.
The normally distributed data are presented as the mean ± SD and the non-normally distributed data as the median and interquartile range. Differences in the baseline characteristics were compared using Student’s t test for continuous variables and the chi-square test for categorical variables. The Mann-Whitney U test was used to compare continuous variables in states of non-normality. The Kruskal-Wallis test was performed to assess and compare the sST2 concentrations across the New York Heart Association (NYHA) functional class. The sST2 results were log-transformed to establish normality, and univariate Spearman correlation was used to evaluate the magnitude and significance of the relations among the continuous variables. To evaluate the characteristics of the sST2 concentrations as a predictor of death in patients with either HFpEF or systolic heart failure, several methods were used. The patients were grouped into tertiles, and the frequency of mortality relative to the increasing sST2 concentrations was calculated as a function of HFpEF and systolic heart failure. Receiver operating characteristic curve analyses with death at 1 year were also performed, and the area under the curve was estimated. The added predictive ability of sST2 compared to NT-proBNP for the detection of events was evaluated using the C-statistic, net reclassification improvement, and integrated discrimination improvement analyses. Net reclassification improvement and integrated discrimination improvement were performed with the biomarkers kept as dichotomous variables, as described by Pencina et al,14 such that the categories of probability for events are defined according to a prognostication scheme of the Heart Failure Survival Score.15 To identify the independent predictors of death at 1 year, we performed multivariate Cox proportional hazards analyses using forward stepping. Variables were retained if their univariate p value was <0.05 and were entered into a multivariate model. Only those variables with significant p values were retained in the final multivariate model. The cumulative incidence of death was estimated using the Kaplan-Meier method, and the log-rank statistic was used for comparisons. All p values <0.05 were accepted as statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences, version 15.0, for Windows (SPSS, Chicago, Illinois). Receiver operating characteristic curve analysis was performed using MedCalc statistical software, version 10.4, for Windows (MedCalc Software, Mariakerke, Belgium).
Results
A total of 447 subjects were included in the present analysis. The distribution of clinical characteristics and laboratory parameters as a function of left ventricular ejection fraction are listed in Table 1. The sST2 concentrations correlated with several clinical characteristics and laboratory parameters (Table 2). Moderate significant positive correlations were observed between sST2 and C-reactive protein, troponin T, and NT-proBNP (all p <0.001). The sST2 concentrations also correlated weakly with the left ventricular ejection fraction (r = −0.12; p = 0.01). Thus, patients with systolic heart failure had higher sST2 concentrations (median 0.55 ng/ml, interquartile range 0.30 to 1.03, vs median 0.38 ng/ml, interquartile range 0.26 to 0.79; p <0.001). When patients were categorized as a function of the NYHA functional class, the median sST2 concentrations were higher in those with worse symptoms, regardless of their left ventricular ejection fraction. Thus, of the patients with an ejection fraction ≥50% (n = 197), those with NYHA class II had a median ST2 level of 0.28 ng/ml (interquartile range 0.17 to 0.36), NYHA class III, a median ST2 level of 0.43 ng/ml (interquartile range 0.30 to 0.84), and NYHA class IV, median 0.49 ng/ml (interquartile range 0.29 to 0.96 (p = 0.001). Of those with an ejection fraction <50% (n = 250), those with NYHA class II had a median ST2 level of 0.33 ng/ml (interquartile range 0.19 to 0.72), NYHA class III, median ST2 level of 0.59 ng/ml (interquartile range 0.31 to 1.01), and NYHA class IV, median ST2 level of 0.63 ng/ml (interquartile range 0.35 to 1.38; p <0.001).
Table 1.
Characteristics of study patients as function of left ventricular ejection fraction
| Variable | Overall (n = 447) |
Left Ventricular Ejection Fraction | p Value | |
|---|---|---|---|---|
| ≥50% (n = 197) |
<50% (n = 250) |
|||
| Age (year) | 73 ± 13 | 74 ± 12 | 72 ± 13 | 0.035 |
| Men | 290 (65%) | 83 (42%) | 207 (83%) | <0.001 |
| Body mass index (kg/m2) | 27 [24–31] | 28 [25–32] | 26 [23–30] | <0.001 |
| Systolic blood pressure (mm Hg) | 142 ± 32 | 149 ± 32 | 136 ± 31 | <0.001 |
| Diastolic blood pressure (mm Hg) | 80 ± 18 | 79 ± 17 | 80 ± 19 | 0.46 |
| Heart rate (beats/min) | 92 ± 27 | 88 ± 27 | 96 ± 26 | 0.002 |
| Hypertension | 306 (69%) | 148 (75%) | 158 (63%) | 0.007 |
| Diabetes mellitus | 183 (41%) | 79 (40%) | 104 (42%) | 0.75 |
| Coronary artery disease | 202 (45%) | 64 (33%) | 138 (55%) | <0.001 |
| Previous heart failure | 239 (54%) | 81 (41%) | 158 (63%) | <0.001 |
| Obstructive airway disease | 103 (23%) | 49 (25%) | 54 (22%) | 0.42 |
| Current smoking | 63 (14%) | 22 (11%) | 41 (16%) | 0.11 |
| Left ventricular ejection fraction (%) | 46 (32–60) | 60 (55–65) | 34 (25–42) | <0.001 |
| Admission New York Heart Association functional class | 0.46 | |||
| II | 102 (23%) | 45 (23%) | 57 (23%) | |
| III | 156 (35%) | 63 (32%) | 93 (37%) | |
| IV | 189 (42%) | 89 (45%) | 100 (40%) | |
| Atrial fibrillation/flutter | 189 (42%) | 82 (42%) | 107 (43%) | 0.80 |
| Medication | ||||
| β Blocker | 233 (52%) | 105 (53%) | 128 (51%) | 0.66 |
| Angiotensin-converting enzyme | 208 (47%) | 71 (36%) | 137 (55%) | <0.001 |
| Angiotensin-receptor blocker | 57 (13%) | 34 (17%) | 23 (9%) | 0.011 |
| Digoxin | 104 (23%) | 31 (16%) | 73 (29%) | 0.01 |
| Loop diuretic | 309 (69%) | 124 (63%) | 185 (74%) | 0.012 |
| Hemoglobin (g/dl) | 12.7 ± 2.2 | 12.1 ± 2.3 | 13.1 ± 2.1 | <0.001 |
| Leukocytes (per 103) | 8.7 (7.0–10.9) | 8.6 (7.1–10.6) | 8.7 (6.7–11.1) | 0.79 |
| Creatinine (mg/dl) | 1.10 (0.83–1.50) | 1.10 (0.82–1.49) | 1.14 (0.88–1.56) | 0.53 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 63 (43–86) | 61 (40–83) | 65 (45–90) | 0.029 |
| Blood urea nitrogen (mg/dl) | 25 (18–34) | 24 (18–33) | 25 (18–35) | 0.36 |
| C-reactive protein (mg/dl) | 3.5 (0.9–16.3) | 5.2 (1–22) | 2.65 (0.80–9.95) | 0.013 |
| Troponin T (ng/ml) | 0.01 (0.01–0.04) | 0.01 (0.01–0.037) | 0.016 (0.01–0.062) | 0.004 |
| Plasma amino terminal B-type natriuretic peptide (pg/ml) | 3,558 (1,646–9,250) | 2,749 (1,344–6,634) | 4,709 (2099–11,159) | <0.001 |
| Soluble ST2 (ng/ml) | 0.47 (0.28–0.94) | 0.38 (0.26–0.79) | 0.55 (0.30–1.03) | <0.001 |
Data are presented as mean ± SD, median (quartiles), or n (%).
Table 2.
Correlations between soluble ST2 (sST2) and continuous covariates as function of left ventricular ejection fraction
| Variable | Left Ventricular Ejection Fraction | |||||
|---|---|---|---|---|---|---|
| Overall (n = 447) |
≥50% (n = 197) |
<50% (n = 250) |
||||
| r | p Value | r | p Value | r | p Value | |
| Age (years) | — | 0.69 | — | 0.65 | 0.10 | 0.12 |
| Body mass index (kg/m2) | — | 0.23 | — | 0.94 | — | 0.42 |
| Systolic blood pressure (mm Hg) | −0.10 | 0.040 | — | 0.47 | −0.10 | 0.11 |
| Diastolic blood pressure (mm Hg) | — | 0.68 | −0.11 | 0.13 | — | 0.59 |
| Heart rate (beats/min) | 0.20 | <0.001 | — | 0.19 | 0.25 | <0.001 |
| Hemoglobin (g/dl) | −0.11 | 0.018 | −0.12 | 0.09 | −0.26 | 0.006 |
| Leukocytes | 0.23 | <0.001 | 0.19 | 0.01 | 0.26 | <0.001 |
| Creatinine (mg/dl) | 0.25 | <0.001 | 0.18 | 0.015 | 0.30 | <0.001 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | −0.23 | <0.001 | −0.18 | 0.01 | −0.30 | <0.001 |
| Blood urea nitrogen (mg/dl) | 0.26 | <0.001 | 0.13 | 0.08 | 0.36 | <0.001 |
| C-reactive protein (mg/dl) | 0.40 | <0.001 | 0.36 | <0.001 | 0.47 | <0.001 |
| Plasma amino terminal B-type natriuretic peptide (pg/ml) | 0.41 | <0.001 | 0.35 | <0.001 | 0.43 | <0.001 |
| Troponin T (ng/ml) | 0.31 | <0.001 | 0.25 | 0.001 | 0.34 | <0.001 |
| Left ventricular end-systolic diameter (mm) | 0.15 | 0.033 | — | 0.l6 | 0.23 | 0.034 |
| Left ventricular end-diastolic diameter (mm) | — | 0.382 | — | 0.49 | — | 0.39 |
| Right ventricular systolic pressure (mm Hg) | 0.22 | <0.001 | 0.16 | 0.08 | 0.27 | <0.001 |
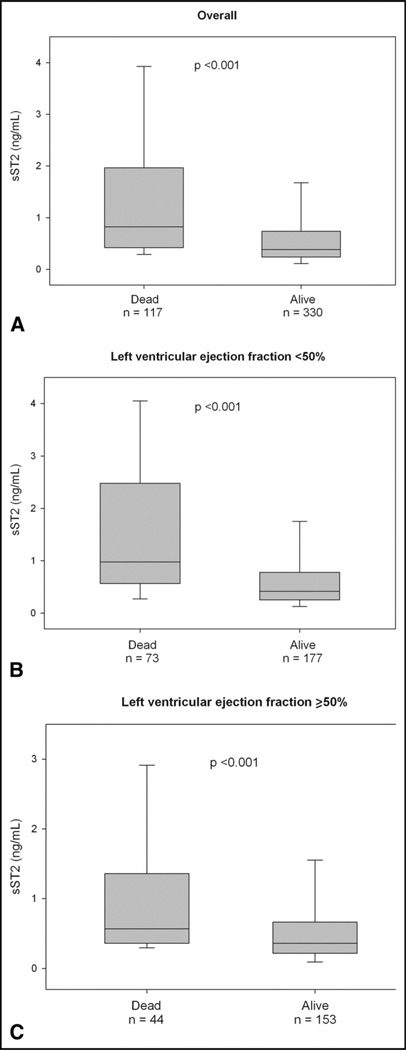
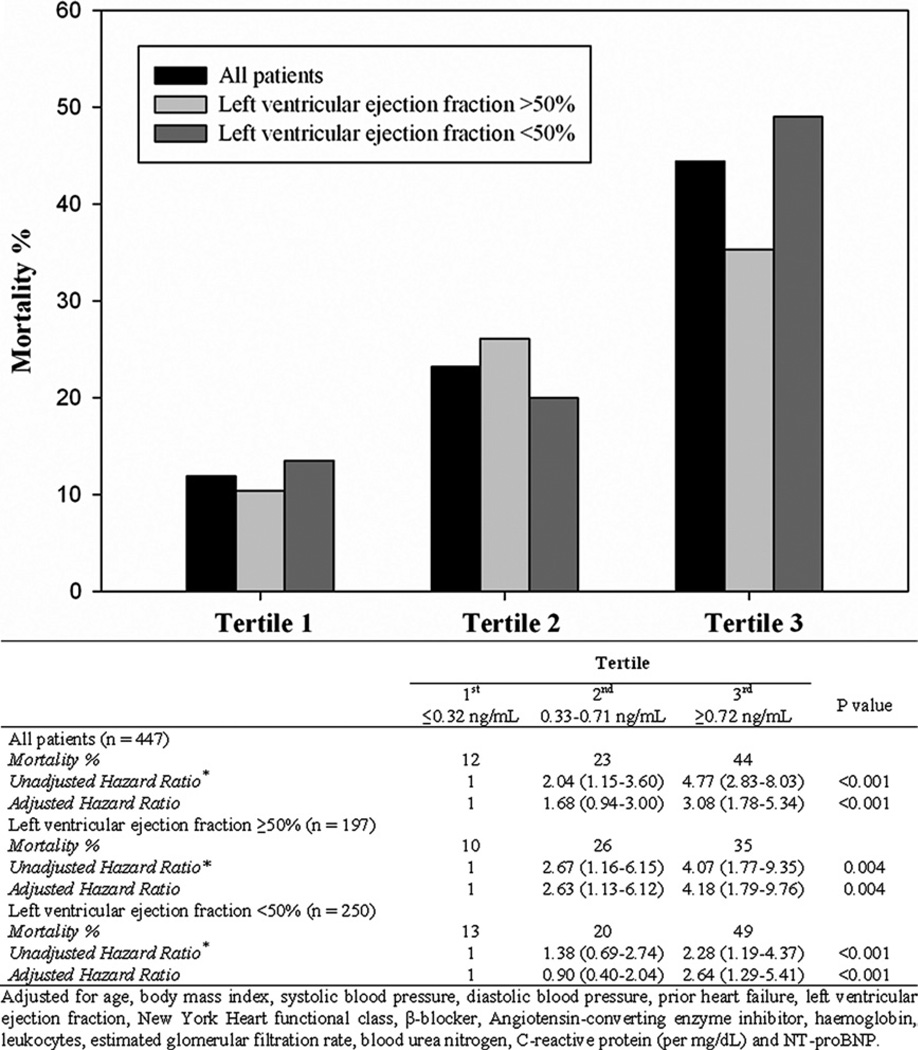
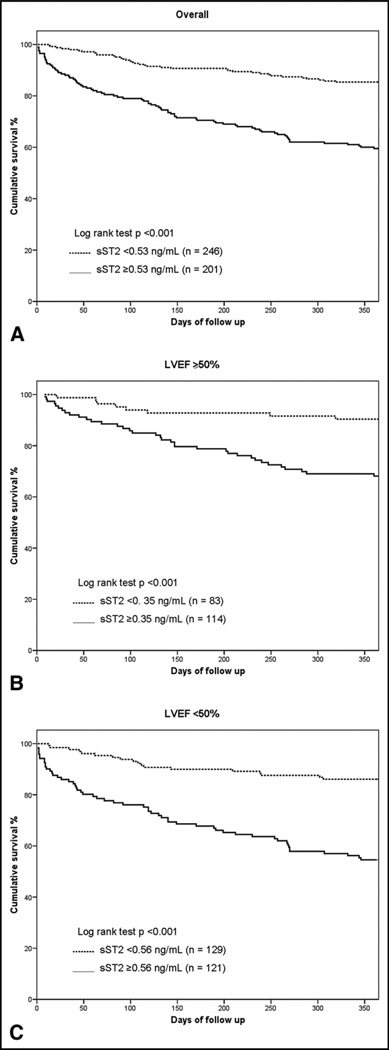
During the 1 year of follow-up, 117 patients (26%) died. The median concentration of sST2 were significantly greater among the deceased than among the survivors (median 0.80 ng/ml, interquartile range 0.42 to 1.83, vs median 0.38 ng/ml, interquartile range 0.24 to 0.72; p <0.001). This pattern of higher ST2 concentrations in those who died remained for patients with HFpEF (median 0.57 ng/ml, interquartile range 0.26 to 1.28, vs 0.35 ng/ml, interquartile range 0.22 to 0.66; p <0.001) and those with systolic heart failure (median 0.98 ng/ml, interquartile range 0.57 to 2.48, vs median 0.42 ng/ml, interquartile range 0.26 to 0.78; p <0.001; Figure 1). To evaluate the optimal prognostic accuracy of the sST2 concentrations for the prediction of 1-year mortality, we performed receiver operating characteristic curve analyses as a function of the left ventricular ejection fraction. The sST2 concentrations had an area under the curve for patients with HFpEF that was comparable to that for patients with systolic heart failure (Table 3). On multivariate Cox regression analysis, we found that elevated sST2 levels, as a quantitative variable, were associated with a greater risk of 1-year mortality for patients with HFpEF (per ng/ml, hazard ratio 1.41, 95% confidence interval 1.14 to 1.76, p = 0.002) and for those with systolic heart failure (per ng/ml, hazard ratio 1.20, 95% confidence interval 1.10 to 1.32, p <0.001; Table 4). In addition, tertile analyses of sST2 concentrations revealed that a graded increase occurred in the 1-year mortality rate with increasing concentrations of sST2 in both groups of acutely decompensated heart failure (Figure 2). The Kaplan-Meier survival analysis showed an early diverging rate of mortality according to sST2 cutoff values throughout the 1 year of follow-up for the entire cohort (sST2 ≥0.53 ng/ml [n = 201], 35% vs sST2 <0.53 ng/ml [n = 246], 12%; log-rank test, p <0.001), as well as after stratification by left ventricular ejection fraction (HFpEF, sST2 ≥0.35 ng/ml [n = 114], 31% vs sST2 <0.35 ng/ml [n = 83], 9.6%; log-rank test, p <0.001; and systolic heart failure, sST2 ≥0.56 ng/ml [n = 121], 37% vs sST2 <0.56 ng/ml [n = 129], 14%; log-rank test, p <0.001).
Figure 1.
Soluble ST2 values as function of 1-year mortality in (A) all patients and patients with (B) preserved and (C) reduced left ventricular ejection fraction.
Table 3.
Performance of soluble ST2 (sST2) values for prediction of mortality at 1 year
| Variable | AUC | 95% CI | Cutpoint (ng/ml) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| All patients (n = 447) | 0.71 | 0.67–0.76 | 0.53 | 0.69 | 0.64 | 0.41 | 0.85 |
| Left ventricular ejection fraction ≥50% (n = 197) | 0.69 | 0.62–0.75 | 0.35 | 0.82 | 0.49 | 0.37 | 0.88 |
| Left ventricular ejection fraction <50% (n = 250) | 0.73 | 0.67–0.79 | 0.56 | 0.76 | 0.62 | 0.42 | 0.88 |
AUC = area under the curve; CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value.
Table 4.
Univariate and multivariate Cox proportional hazards for soluble ST2 (sST2) as predictor of 1-year mortality
| Variable | All Patients (n = 447) |
Left Ventricular Ejection Fraction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥50% (n = 197) |
<50% (n = 250) |
||||||||||
| Univariate | Multivariate | Multivariate | Multivariate | ||||||||
| Hazard Ratio | p Value | Hazard Ratio | p Value | Hazard Ratio | p Value | Hazard Ratio | p Value | ||||
| Age (years) | 1.006 (1.004–1.008) | <0.001 | 1.05 (1.03–1.07) | <0.001 | 1.07 (1.03–1.11) | <0.001 | 1.03 (1.008–1.06) | 0.008 | |||
| Body mass index (kg/m2) | 0.94 (0.90–0.97) | 0.001 | |||||||||
| Systolic blood pressure (mm Hg) | 0.985 (0.979–0.992) | <0.001 | 0.988 (0.982–0.994) | <0.001 | 0.986 (0.977–0.995) | 0.002 | |||||
| Diastolic blood pressure (mm Hg) | 0.981 (0.971–0.992) | 0.001 | |||||||||
| Previous heart failure | 1.72 (1.18–2.50) | 0.005 | |||||||||
| New York Heart Association functional class | 1.43 (1.12–1.82) | 0.004 | |||||||||
| β Blocker | 0.68 (0.48–0.98) | 0.038 | 0.66 (0.45–0.97) | 0.035 | 0.56 (0.34–0.91) | 0.019 | |||||
| Angiotensin-converting enzyme inhibitor | 0.64 (0.44–0.93) | 0.018 | |||||||||
| Hemoglobin (g/dl) | 0.87 (0.80–0.94) | <0.001 | |||||||||
| Leukocytes (U) | 1.07 (1.02–1.03) | 0.003 | 1.13 (1.03–1.24) | 0.008 | |||||||
| Creatinine (mg/dl) | 1.74 (1.43–2.12) | <0.001 | |||||||||
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 0.985 (0.979–0.992) | <0.001 | 0.98 (0.97–0.99) | 0.012 | |||||||
| Blood urea nitrogen (mg/dl) | 1.02 (1.01–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 | 1.03 (1.01–1.04) | <0.001 | |||||
| C-reactive protein (mg/dl) | 1.006 (1.004–1.008) | <0.001 | |||||||||
| Plasma amino terminal B-type natriuretic peptide (100 pg/ml) | 1.003 (1.002–1.004) | <0.001 | 1.002 (1.001–1.003) | <0.001 | 1.002 (1.001–1.003) | <0.001 | |||||
| Soluble ST2 (ng/ml) | 1.74 (1.43–2.12) | <0.001 | 1.23 (1.13–1.34) | <0.001 | 1.37 (1.11–1.68) | 0.003 | 1.20 (1.10–1.32) | <0.001 | |||
| Soluble ST2 greater than cutoff | |||||||||||
| All patients (>0.53 mg/ml) | 3.31 (2.23–4.89) | <0.001 | 2.43 (1.60–3.69) | <0.001 | |||||||
| Left ventricular ejection fraction ≥50% (>0.35 mg/ml) | 3.26 (1.50–7.05) | 0.003 | |||||||||
| Left ventricular ejection fraction <50% (>0.56 mg/ml) | 2.94 (1.66–5.19) | <0.001 | |||||||||
Figure 2.
Tertile analysis comparing concentrations of sST2 relative to outcome in all subjects and those with preserved or impaired left ventricular ejection fraction. Univariate and multivariate Cox proportional hazard ratios for sST2 tertiles as predictors of 1-year mortality depicted.
The added predictive ability of sST2 compared to NT-proBNP for the detection of events was evaluated using the C-statistic, net reclassification improvement, and integrated discrimination improvement analyses. The addition of the sST2 concentration to NT-proBNP improved the C-statistic and both net reclassification improvement and integrated discrimination improvement, regardless of the left ventricular ejection fraction (Table 5).
Table 5.
Soluble ST2 (sST2) concentration improved C-statistic and net reclassification improvement and integrated discrimination improvement
| Variable | All Patients (n = 447) |
Left Ventricular Ejection Fraction <50% (n = 250) |
Left Ventricular Ejection Fraction ≥50% (n = 197) |
|---|---|---|---|
| Incremental value of biomarkers for detection of 1-year mortality assessed by C-statistic improvement | |||
| C-statistic (model 1) | 0.64 | 0.65 | 0.60 |
| C-statistic (model 2) | 0.71 | 0.74 | 0.68 |
| Differences in C-statistic (95% CI) | 0.07 (0.02–0.11) | 0.08 (0.03–0.14) | 0.08 (0.02–0.15) |
| p Value | 0.002 | 0.08 | 0.01 |
| Evaluating added predictive ability of model 2 plus model 1 for detection of events using net reclassification and integrated discrimination indexes | |||
| Net reclassification improvement (95% CI) | 0.56 (0.39–0.73) | 0.71 (0.49–0.92) | 0.47 (0.22–0.71) |
| Events correctly reclassified (%) | 9% | 26% | 16% |
| Nonevents correctly reclassified (%) | 48% | 45% | 31% |
| p Value | <0.0001 | <0.0001 | 0.0002 |
| Evaluating added predictive ability of model 2 plus model 1 for detection of events using net reclassification and integrated discrimination indexes | |||
| Integrated discrimination improvement (95% CI) | 0.04 (0.02–0.06) | 0.07 (0.04–0.10) | 0.04 (0.01–0.07) |
| Events change probability | 3% | 5% | 3% |
| Nonevents change probability | −1.1% | −2.1% | −0.9% |
| p Value | <0.0001 | <0.0001 | 0.004 |
Model 1 included NT-proBNP and model 2 included NT-proBNP and sST2.
CI = confidence interval.
Discussion
In the present study, we have provided novel data on the relation between the sST2 concentrations and the clinical and biochemical characteristics of hospitalized patients with acutely decompensated heart failure considered as a function of preserved versus impaired left ventricular ejection fraction. The rationale for such an analysis was the biologic and clinical significance of sST2 as a potential marker of ventricular remodeling and prognosis in patients with acutely decompensated heart failure.1–8,16 Basic science studies have suggested a pivotal biologic role for sST2 in the process of ventricular remodeling in the context of ventricular pressure or volume overload. In addition, the concentrations of sST2 appear to predict a clinical phenotype vulnerable to remodeling, and are prognostically meaningful in the context of acutely decompensated heart failure.1–8,17 Because remodeling is a meaningful process in most forms of heart failure,18 our hypothesis was that if the sST2 values reflect the risk of remodeling, they would be—as a consequence—prognostically important across the wide spectrum of left ventricular function in our cohort.
Consistent with our primary hypothesis, in the present multinational pooled analysis, regardless of the left ventricular ejection fraction status, we found that the sST2 concentrations correlated significantly with several prognostically and biologically meaningful biomarkers involved in deleterious remodeling. Clinically, the sST2 values were associated with heart failure symptom severity. Furthermore, the sST2 concentration was an independent predictor of 1-year mortality in patients with acutely decompensated heart failure from either HFpEF or systolic heart failure, irrespective of the presence of natriuretic peptides in the analysis. With sST2 in the model, NT-proBNP was not a predictor of death in those with HFpEF.
Cardiac remodeling is a common mechanism for the progression of heart failure and involves multiple deleterious changes in the myocardium, including cardiomyocyte loss (by necrosis or apoptosis), left ventricular dilation, cardiomyocyte hypertrophy, fibroblast proliferation, and collagen accumulation.19 Remodeling has been associated with a greater rate of adverse outcomes among patients with heart failure and has been a prime target for therapeutic strategies to reduce the risk in affected patients.20–23 In addition to those with systolic heart failure, it has been well established that cardiac remodeling plays a crucial role in the pathophysiology and complications of HFpEF, including having an important effect on myocardial relaxation abnormalities.24,25
It is in this context that measurement of sST2 is relevant. Several experimental and clinical studies have demonstrated sST2 to be a biomarker of mechanical stress with a pivotal role in myocardial fibrosis. sST2 has a broad role in the body, including in inflammatory responses, atherosclerosis, autoimmunity, and cardiac remodeling.1–4,17,26 Recently, interleukin-33 was identified as the ligand for sST2. Interleukin-33/ST2 signaling protects the myocardium under mechanical strain and acts as a biomechanically activated fibroblast-cardiomyocyte paracrine system to prevent cardiac hypertrophy and fibrosis. Sanada et al1 recently suggested that sST2 abrogates this adaptative response in a dose-dependent manner by binding interleukin-33 and preventing signaling through the ST2 ligand. Moreover, Weinberg et al2 showed that derangement of sST2 signaling leads to a phenotype quite consistent with myocardial remodeling. More recently, Weir et al3 showed a relation between the sST2 levels and the cardiac remodeling parameters, including left ventricular ejection fraction, left ventricular end-diastolic volume, and myocardial infarct size, as well as with the plasma aldosterone level (which itself has strong profibrotic effects on the heart).
In a previous study,5 we observed the important prognostic associations between sST2 and heart failure. However, because HFpEF could fundamentally differ from systolic heart failure, we wished to extend our initial observations by examining sST2 as a function of the left ventricular ejection fraction. We found—similar to findings reported with the natriuretic peptides—that the concentrations of sST2 were lower in those with HFpEF compared with those with systolic heart failure. This might be related to differences in wall stress—the trigger for both natriuretic peptide and sST2 release. Nonetheless, similar to natriuretic peptides, we found sST2 to be prognostically meaningful in those with HfpEF. Given the universally deleterious nature of remodeling in heart failure, our results lend internal consistency to the potential value of sST2 for directed antiremodeling therapies in those with heart failure, as has been previously suggested.3
The limitations of our study included that, as a pooled multinational analysis, it lacked predefined end points, despite the similar designs and goals of the respective data sources. Another consideration was the timing of sample collection. A predischarge sST2 value might have added stronger prognostic information; nonetheless, our results remain significant. Also, we lacked complete echocardiographic data for each subject. Although not easily feasible in such a large analysis, such data would have provided important correlates of cardiac structure and function with respect to the sST2 concentrations. We recently reported that the sST2 values correlate with important echocardiographic measures of remodeling, including myocardial relaxation abnormalities.8 It also remains unclear whether the described association between the plasma sST2 concentrations, cardiac function parameters, and the prognosis of patients with heart failure reflects what is occurring at a cardiac level, or whether the sST2 concentrations reflect other pathologic processes, independent of cardiac function, such as pulmonary disease, as we have shown.27 Mechanistic studies of myocardial expression and the secretion of sST2 are needed. We did not simultaneously measure interleukin-33 and therefore could not comment on the interleukin-33/sST2 ratio; however, this is a clear focus for future research of both heart failure and myocardial infarction. (Figure 3).
Figure 3.
Kaplan-Meier survival curves for 1-year mortality according to sST2 cutoff point values in (A) all patients and patients with (B) preserved and (C) reduced left ventricular ejection fraction.
Acknowledgments
Dr. Januzzi received grant support from Roche Diagnostics, Siemens, and Critical Diagnostics. Dr. Pascual-Figal received grant support from Roche Diagnostics. Dr. Truong received grant support from NIH K23HL098370.
References
- 1.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;3:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, McInnes IB, Dargie HJ, McMurray JJ. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. 2010;55:243–250. doi: 10.1016/j.jacc.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 5.Januzzi JL, Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, O’Donoghue M, Sakhuja R, Chen AA, van Kimmenade RR, Lewandrowski KB, Lloyd-Jones DM, Wu AH. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Mueller T, Dieplinger B, Gegenhuber A, Poelz W, Pacher R, Haltmayer M. Increased plasma concentrations of soluble ST2 are predictive for 1-year mortality in patients with acute destabilized heart failure. Clin Chem. 2008;54:752–756. doi: 10.1373/clinchem.2007.096560. [DOI] [PubMed] [Google Scholar]
- 7.Rehman SU, Mueller T, Januzzi JL., Jr Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–1465. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 8.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ Heart Fail. 2009;2:311–319. doi: 10.1161/CIRCHEARTFAILURE.108.833707. [DOI] [PubMed] [Google Scholar]
- 9.Mueller T, Gegenhuber A, Poelz W, Haltmayer M. Diagnostic accuracy of B type natriuretic peptide and amino terminal proBNP in the emergency diagnosis of heart failure. Heart. 2005;91:606–612. doi: 10.1136/hrt.2004.037762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Januzzi JL, Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, Lloyd-Jones DM, Brown DF, Foran-Melanson S, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB. The N-terminal pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Manzano-Fernandez S, Boronat-Garcia M, Baladejo-Oton MD, Pastor P, Garrido IP, Pastor-Perez FJ, Martinez-Hernandez P, Valdes M, Pascual-Figal DA. Complementary prognostic value of cystatin C, N-terminal pro-B-type natriuretic peptide and cardiac troponin T in patients with acute heart failure. Am J Cardiol. 2009;103:1753–1759. doi: 10.1016/j.amjcard.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez-Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie MR, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Garcia MA, Dickstein K, Albuquerque A, Conthe P, Crespo-Leiro M, Ferrari R, Follath F, Gavazzi A, Janssens U, Komajda M, Morais J, Moreno R, Singer M, Singh S, Tendera M, Thygesen K. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 13.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 14.Pencina MJ, D’Agostino RB, Sen, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 15.Zugck C, Kruger C, Kell R, Korber S, Schellberg D, Kubler W, Haass M. Risk stratification in middle-aged patients with congestive heart failure: prospective comparison of the heart failure survival score (HFSS) and a simplified two-variable model. Eur J Heart Fail. 2001;3:577–585. doi: 10.1016/s1388-9842(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 16.Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, Vazquez R, Puig T, Valdes M, Cinca J, de Luna AB, Bayes-Genis A. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. 2009;54:2174–2179. doi: 10.1016/j.jacc.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 17.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2:684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 18.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 19.Takano H, Hasegawa H, Nagai T, Komuro I. Implication of cardiac remodeling in heart failure: mechanisms and therapeutic strategies. Intern Med. 2003;42:465–469. doi: 10.2169/internalmedicine.42.465. [DOI] [PubMed] [Google Scholar]
- 20.Konstam MA, Kronenberg MW, Rousseau MF, Udelson JE, Melin J, Stewart D, Dolan N, Edens TR, Ahn S, Kinan D. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (Studies of Left Ventricular Dysfunction) investigators. Circulation. 1993;88:2277–2283. doi: 10.1161/01.cir.88.5.2277. [DOI] [PubMed] [Google Scholar]
- 21.Doughty RN, Whalley GA, Gamble G, MacMahon S, Sharpe N. Australia-New Zealand Heart Failure Research Collaborative Group. Effects of carvedilol on left ventricular regional wall motion in patients with heart failure caused by ischemic heart disease. J Card Fail. 2000;6:11–18. doi: 10.1016/s1071-9164(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 22.Colucci WS, Kolias TJ, Adams KF, Armstrong WF, Ghali JK, Gottlieb SS, Greenberg B, Klibaner MI, Kukin ML, Sugg JE. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the REversal of VEntricular Remodeling with Toprol-XL (REVERT) trial. Circulation. 2007;116:49–56. doi: 10.1161/CIRCULATIONAHA.106.666016. [DOI] [PubMed] [Google Scholar]
- 23.Waagstein F, Stromblad O, Andersson B, Bohm M, Darius M, Delius W, Goss F, Osterziel KJ, Sigmund M, Trenkwalder SP, Wahlqvist I. Increased exercise ejection fraction and reversed remodeling after long-term treatment with metoprolol in congestive heart failure: a randomized, stratified, double-blind, placebo-controlled trial in mild to moderate heart failure due to ischemic or idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2003;5:679–691. doi: 10.1016/s1388-9842(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 24.Sugihara N, Genda A, Shimizu M, Suematsu T, Kita Y, Minamoto M, Kawagoshi H, Umeda K, Chin S, Takeda R. [Diastolic dysfunction and its relation to myocardial fibrosis in essential hypertension] J Cardiol. 1988;18:353–361. [PubMed] [Google Scholar]
- 25.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 26.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Rumayor A, Camargo CA, Green SM, Baggish AL, O’Donoghue M, Januzzi JL. Soluble ST2 plasma concentrations predict 1-year mortality in acutely dyspneic emergency department patients with pulmonary disease. Am J Clin Pathol. 2008;130:578–584. doi: 10.1309/WMG2BFRC97MKKQKP. [DOI] [PubMed] [Google Scholar]