Abstract
Introduction
We have developed a mouse model to examine the effects of host exposure (ie, hematopoietic system) to secreted HIV-1 Nef or peptides derived from Nef.
Methods
We used a combination of terminal uridine deoxynucleotidyl transferase (dUTP) nick end labeling (TUNEL) assays and CD4+ cell counts to assess the status of circulating immune cells in mice treated with Nef-derived proteins.
Results
Mice treated with peptides derived from HIV-1 Nef protein displayed significant increases in apoptotic CD4+ lymphocytes and thymus cells and significant decreases in the numbers of circulating CD4+ lymphocytes. No effects were observed in mice treated with controls. There was a clear dose- and time-response relationship between cell changes and the amount of protein or peptide. Induction of multiple markers of apoptosis such as DNA laddering and caspase 3 activation was observed during dose- or time-response experiments. Cell death and lymphocyte depletion were blocked by induction of a humoral response to the HIV Nef apoptotic epitope.
Conclusions
Extracellular Nef can induce apoptosis and lymphocyte depletion in vivo. Appropriate antibody response can block these effects, but the apoptotic motifs in Nef are thought to be poorly immunogenic.
Keywords: HIV-1 Nef, Apoptotic Motifs, CD4+ or CD8+ T Lymphocytes, CXCR4, in vivo, Mouse
Introduction
Previous studies show that Nef is apoptotic and is secreted in concentrations that could contribute to CD4+ lymphocyte depletion.1–4 In addition, the accumulated evidence from studies on human subjects, primates, and transgenic animals suggests that soluble Nef could cause pathogenic effects, including T-cell depletion. An analysis of data describing Nef cytotoxic T lymphocyte and antibody epitopes (HIV Molecular Immunology Database, http://hiv-web.lanl.gov/content/immunology) suggests that the immune response to the Nef apoptotic motifs is at best weak and probably nonexistent in primates and mice.1,5–7 Yet, other epitopes from Nef induce relatively strong immune responses. This evidence supports a second key aspect of Nef-induced pathogenesis: that the host raises a weak (or no) immune response to the specific epitopes that induce apoptosis. This level of response may not be sufficient to block soluble Nef-induced apoptosis, which may be an important factor in allowing Nef-induced bystander effects that ultimately lead to HIV pathogenesis.
A clear connection between soluble Nef, Nef-induced apoptosis, and in vivo lymphocyte depletion has not yet been established. To help establish this connection, we have developed a mouse model and examined lymphocyte depletion by Nef. Specifically, we hypothesized that the Nef apoptotic motif 1 (NefM1) peptide alone is sufficient to drive depletion of peripheral blood lymphocytes in mice, and NefM1-induced lymphocyte depletion can be blocked by induction of a specific antibody response to the apoptotic peptide.
Methods
Proteins and Antibodies
Stromal cell-derived factor 1α was obtained from Chemicon (Temecula, Calif). The following antibodies were used: phycoerythrin (PE)-conjugated monoclonal rat anti-mouse CD4 (eBioscience, San Diego, Calif), PE-conjugated rat anti-mouse CD8 (eBioscience), allophycocyanin-conjugated rat anti-mouse CD8 (Invitrogen Laboratories, Carlsbad, Calif), rat anti-mouse CD4 purified antibody (Invitrogen Laboratories), goat anti-rat immunoglobulin G (IgG) (H+L) fluorescein isothiocyante (FITC) conjugate (Invitrogen), goat anti-rat IgG (H+L) PE conjugate (Invitrogen), rabbit anti- HIV-1 motif 1 (M1) peptide antibody (Genemed Synthesis Inc, San Antonio, Texas), polyclonal rabbit anti mouse CXCR4 antibody (Abcam, Cambridge, Mass), mouse anti-caspase-3 antibody (Abcam), goat anti-mouse IgG (H+L) label horseradish peroxidase (Pierce, Rockford, Ill), and goat anti-rabbit IgG (H+L) label horseradish peroxidase (Pierce). Peptides and ceramide use were described previously.2
Animals
CD1 male mice, 3–4 weeks old (Charles Rivers Laboratories, Raleigh, NC) and BC57BL6 male mice, 6–8 weeks old (Charles Rivers Laboratories, Kingston, NY) were kept under specific-pathogen-free conditions and used under conditions approved by the Ethics Review Committees for Animal Experimentation of Morehouse School of Medicine.
Thymus Immunostaining and Terminal Uridine Deoxynucleotidyl Transferase (dUTP) Nick End Labeling (TUNEL) Assay
Thymus glands were removed from mice and fixed in 10% formalin. Embedding and sectioning were performed commercially (Histology Services Co., Stone Mountain, Ga). Subsequently, the sections were assayed for TUNEL as described previously1 and were washed with phosphate-buffered saline (PBS) three times and then incubated with anti-mouse CD4 monoclonal antibody (1:25) and the second antibody, anti-mouse IgG Texas Red (1:25), for 1hour at room temperature. The sections were prepped for fluorescent microscopic analysis.
Flow Cytometry
Peripheral blood lymphocytes (1 × 105) were fixed in 4% paraformaldehyde, washed with PBS, blocked with 2% goat serum in PBS, incubated with the primary antibody 1:25 dilution of rat anti-mouse CD8 purified antibody or rat anti-mouse CD4 purified antibody overnight at 4°C, and the secondary tagged antibody goat anti-rat IgG (H+L) FITC conjugate. The stained cells were washed and counted by flow cytometric analysis by using BD FACScalibur (BD Biosciences, San Jose, Calif), with gating performed to exclude debris and cell clumps.
Data Analysis
SigmaPlot 9 (Systat Software, Inc., San Jose, Calif) was used for the numerical and graphical analyses of all data obtained.
Results
Treatment with Nef-Derived Peptide Causes Lymphocyte Depletion and Thymus Cell Apoptosis
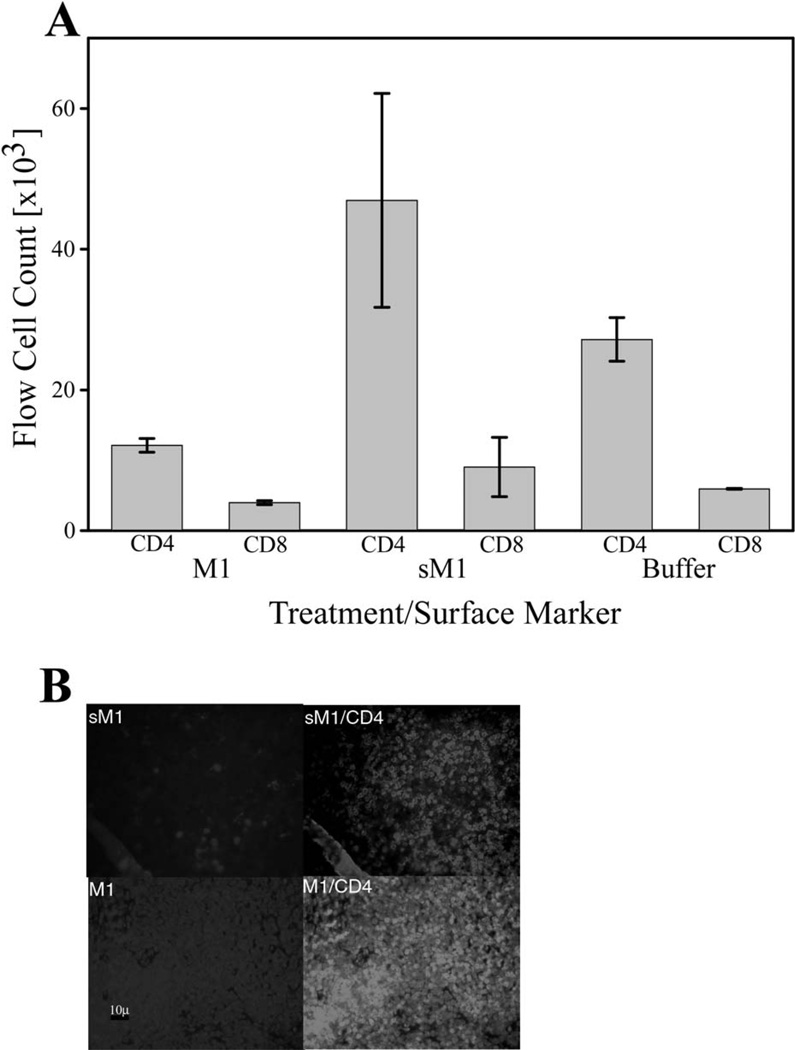
Mice treated for four weeks with physiologic saline buffer, physiologic saline buffer containing NefM1, or the scrambled Nef apoptotic motif (NefsM1) were sacrificed, and the total number of either CD4+ or CD8+ peripheral lymphocytes were assayed by flow cytometry (Figure 1A). A significant depletion of CD4+ lymphocytes was observed in NefM1-treated mice (Figure 1A, bar M1/CD4 vs bar buffer/CD4), while no lymphocyte depletion was observed in NefsM1-treated mice (Figure 1A, bar sM1/CD4 vs bar buffer/CD4). No significant depletion of CD8+ lymphocytes was observed in NefM1-treated mice (Figure 1A, bar M1/CD8 vs bar buffer/CD8).
Fig 1.
In vivo NefM1-induced changes in CD4+ PBLs. Mice were treated intraperitoneally with Buffer (Buffer, bar represents 2 mice), or 1 µg NefM1 (M1; bar represents 4 mice) or NefsM1 (sM1; bar represents 2 mice) peptide three times a week for 4 weeks. The mice were then sacrificed, total blood was harvested, and peripheral blood lymphocytes (PBLs) collected by gradient isolation. (A) PBLs were fixed, and stained for CD3, CD4, CD8 and analyzed by Fluorescence-activated cell sorting, (FACS) for total CD3+/CD4+, or total CD3+/CD8+ cells. Error bars represent the standard error of measurement. (B) Thymus was harvested, fixed, sectioned, and CD4+/TUNEL stained. Each panel is a representative section image for each category of treatment. The top panels are TUNEL (Fluorescein isothiocyanate, FITC) staining of a thymus section from a NefsM1 treated mouse (sM1; left) or a TUNEL/CD4+ stained (Texas Red) image of the same thymus section (right). The bottom panels are a TUNEL stained thymus section from a NefM1 treated mouse (M1; left); or a transposed TUNEL/CD4+ image of the same thymus section (right). The scale bar at the bottom left represents 10 µ
Thymus glands from treated mice were harvested, fixed, sectioned, and stained for CD4/TUNEL stained (Figure 1B). Representative NefsM1-treated mouse sections (top panels) and NefM1 treated mouse sections (bottom panels) are shown. Representative sections display TUNEL staining alone (left panels, FITC, green) and transposed TUNEL and CD4 (Texas Red, red) staining (right panels). Bright green TUNEL labeling (FITC) in apoptotic cells was observed in the NefM1-treated mouse section (Figure 1B, M1 TUNEL vs sM1 TUNEL). A high prevalence of yellow fluorescing cells (simultaneous CD4 and TUNEL staining) were observed (Figure 1B, M1 TUNEL/CD4 panel vs sM1 TUNEL/CD4 panel) indicative of significant amounts of apoptosis in CD4+ cells in the thymus of NefM1-treated mice.
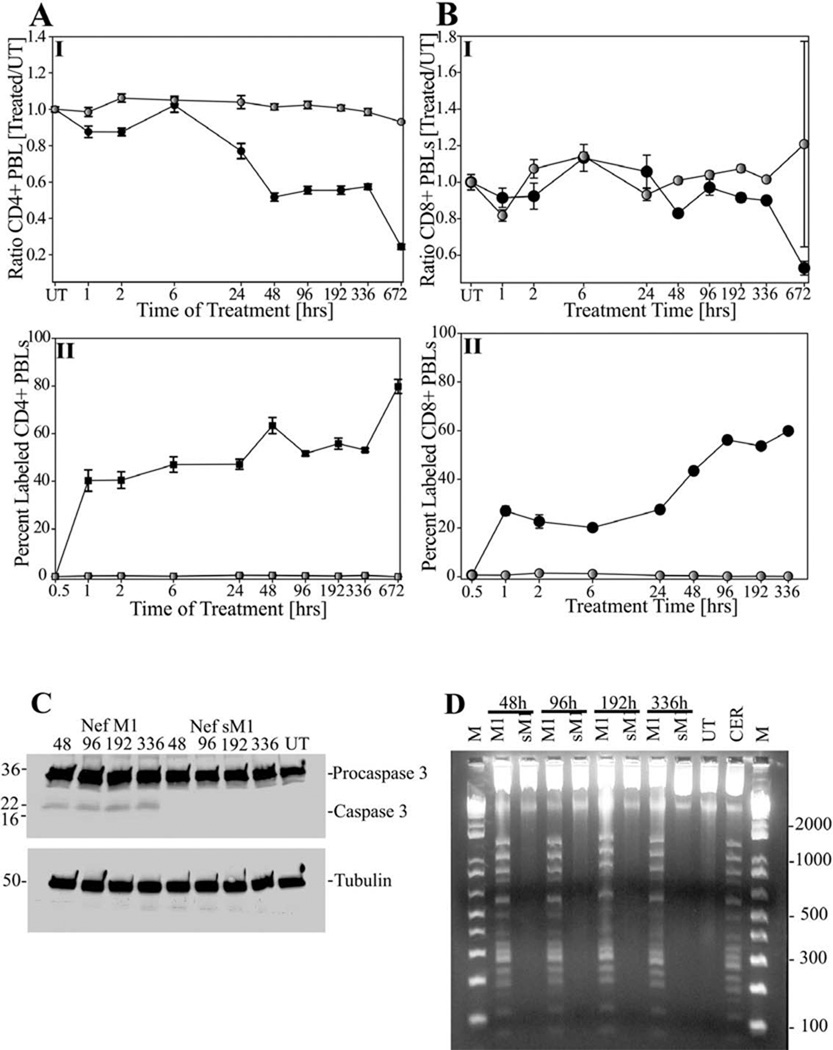
Time Course of Mouse Peripheral Blood Lymphocyte (PBL) Depletion
Cells from mice treated for time periods from one hour to four weeks with NefM1 or NefsM1 were determined by microscopy for the total number of either CD4+ or CD8+ peripheral lymphocytes or by TUNEL for the level of apoptosis in the same cell populations (Figure 2). Decreased numbers of CD4+ PBLs were observed as a function of treatment time in mice treated with NefM1 (Figure 2, panel AI, black circles vs NefsM1, panel AI, grey circles). Increased levels of CD4+ PBL apoptosis were observed as a function of NefM1 treatment (Figure 2, panel AII, black circles vs NefsM1, panel AII, grey circles). These observations suggest that NefM1 kills CD4+ PBLs through apoptosis and decreases the numbers of CD4+ cells in the peripheral blood, which affects the organism’s ability to replenish this cell type.
Fig 2.
Time Response Analysis of mouse peripheral blood lymphocyte (PBL) population treated in vivo. Mice were treated intraperitoneally with NefM1 (closed circle graphs) or NefsM1 (open circle graphs) for various periods of time. Those mice treated for less than 48 hours were injected once at T = 0. Those treated for more than 48 hours were injected every 48 hours. Then either cell numbers were measured as # treated cells (1–672 hrs)/# untreated cells (UT; A-I, B-I) or percent apoptosis in each PBL population (panel A-II, B-II). Panel A-I and A-II display CD4+ results, and Panels B-I and B-II display CD8+ results. The x-axis displays the treatment time in hours, each point represents a minimum of two mice, and the error bars represent the standard error of measurement. (C) Caspase 3 activation was assayed by Western blot analysis. 20 µg of total protein was loaded per lane. Displayed are PBLs from mice treated with NefM1 for 48, 96, 192, and 336 hours respectively (1–4), or NefsM1 for 48, 96, 192, and 336 hours respectively (5–8), with the last lane being PBLs from untreated mice (UT). Procaspase 3 is the high molecular weight band (32 kD), the large catalytic Caspase 3 active subunit being 17 kD, Tubulin (50 kD) used as the gel-loading control, and prestrained SDS-PAGE Standards (broad range) (BIO-RAD Labs, Hercules, CA) used as molecular weight markers. (D) DNA from PBLs was analyzed for apoptosis characteristic laddering as previously described. From left to right on the image, DNA markers [lane M], NefM1/48 hour treated mice [lane 48h-M1]; NefsM1/48 hour treated mice [lane 48h-sM1]; NefM1/96 hour treated mice [lane 96h-M1]; NefsM1/96 hour treated mice [lane 96h-sM1]; NefM1/192 hour treated mice [lane 192h-M1]; NefsM1/192 hour treated mice [lane 192h-sM1]; NefM1/384 hour treated mice [lane 336h-M1]; NefsM1/384 hour treated mice [lane 336h-sM1]; untreated mice [lane UT]; Ceramide treated Jurkat cell cultures as a positive control [lane CER; 23.4 µM]; markers [lane M]. Each lane is a representation of a minimum of two mice
Although increased levels of NefM1-induced apoptosis were observed in CD8+ PBLs as a function of treatment time (Figure 2, panel BII, black circles vs NefsM1, panel BII, grey circles), no change in the total count of CD8+ PBLs from NefM1-treated mice was observed as a function of treatment (Figure 2, panel BI, black circles vs NefsM1, panel BI, grey circles). This suggests that NefM1 can kill CD8+ PBLs through apoptosis but does not affect the total CD8+ cell numbers, which indicates that the CD8 population continues to be replenished.
Caspase 3 activation (Figure 2C) and DNA laddering (Figure 2D) were used to confirm apoptosis induction in the PBL cell population from NefM1- or NefsM1-treated mice over time. The active caspase 3 isoform was observed in all samples treated with NefM1 (Figure 2C, lanes NefM1 48hr–336hr), but not in any NefsM1-treated samples (Figure 2C, lanes NefsM1 48hr–336hr). Similar results were observed using the DNA laddering assay (Figure 2D, M1 lanes vs sM1 lanes or UT lane).
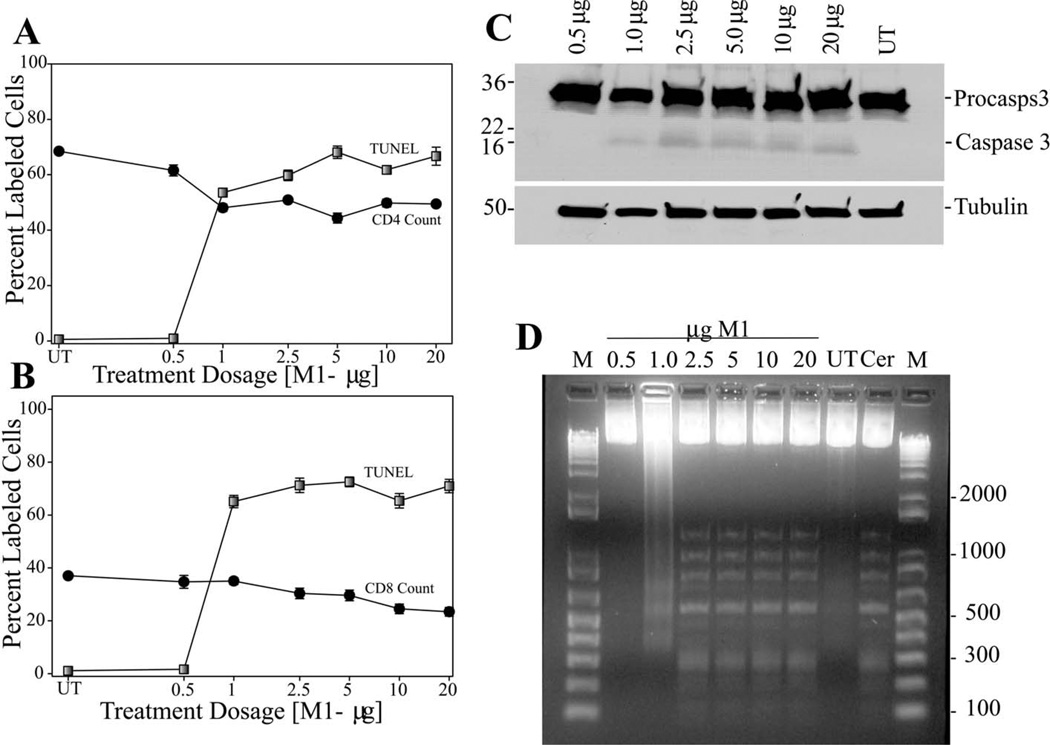
Dose Response of Mouse PBL Population to Nef Peptides
PBLs collected from mice untreated or treated for 1 week with varying dosages of NefM1 were analyzed for the total number of either CD4+ or CD8+ peripheral lymphocytes or the level of apoptosis in the same cell populations (Figure 3). A relatively sharp 25% decrease in cell count (Figure 3A, black circles), and a corresponding 80% increase in TUNEL labeling (Figure 3A, grey circles) of CD4+ cells was observed between 0 and 1 µg/mouse, with no further change between 1 and 20 µg/mouse. A slow 25% decrease in CD8+ cells (Figure 3B, black circles) over a broad dosage range (0 to 20 µg/mouse) was observed linked to a discordant 80% increase in TUNEL labeling of the CD8+ cell population (Figure 3B, grey circles). The active form of caspase 3 (Figure 3C) and DNA laddering (Figure 3D) were observed in all samples collected from mice treated with dosages ≥1.0 µg. Neither was observed in the .5 µg/mouse dosage or in untreated controls.
Fig 3.
Dose Response Analysis of mouse peripheral blood lymphocyte (PBL) population treated in vivo. Mice were treated IP every 48 hours with NefM1 for one week at various dosages. Untreated mice [UT]; mice were treated with: 0.5 µg NefM1/mouse, 1.0 µg NefM1/mouse, 2.5 µg NefM1/mouse, 5 µg NefM1/mouse, 10 µg NefM1/mouse, 20 µg NefM1/mouse. Harvested PBLs were fixed and stained for CD3, CD4, and CD8 and analyzed by microscopic examination. (A) PBL populations were assayed for CD4+ cells (black circles), and the CD4+ cells were assayed for apoptosis by TUNEL (grey squares). (B) PBLs population was assayed for CD8+ cells (black circles), and the CD8+ cells were assayed for apoptosis by TUNEL (grey squares). The percentage of labeled cells (either TUNEL labeled, or CD4+ labeled) was plotted as a function of the treatment dosage. Each point represents a minimum of two treated mice, and the error bars represent the standard error of measurement. The identification of the hallmarks of apoptosis as a function of treatment dosage. (C) Caspase 3 activation was assayed by Western analysis with 20 µg of total protein loaded per lane. From left to right on the image: 0.5 µg NefM1/mouse, 1.0 µg NefM1/mouse, 2.5 µg NefM1/mouse, 5 µg NefM1/mouse, 10 µg NefM1/mouse, 20 µg NefM1/mouse, and untreated mice (UT). Procaspase 3 is the high molecular weight band (32 kD), the large catalytic Caspase 3 active subunit being 17 kD, Tubulin (50 kD) used as the gel-loading control, and prestrained SDS-PAGE Standards (broad range) (BIO-RAD Labs, Hercules, CA) used as molecular weight markers. (D) DNA from PBLs was analyzed for apoptosis characteristic laddering as described previously. From left to right, DNA markers [lane M]; 0.5 µg NefM1/mouse, 1.0 µg NefM1/mouse; 2.5 µg NefM1/mouse; 5 µg NefM1/mouse; 10 µg NefM1/mouse; 20 µg NefM1/mouse; untreated mice [lane UT]; Ceramide treated Jurkat cell cultures as a positive control [lane CER; 23.4 µM]; markers [lane M]. Each lane is a representation of a minimum of two mice
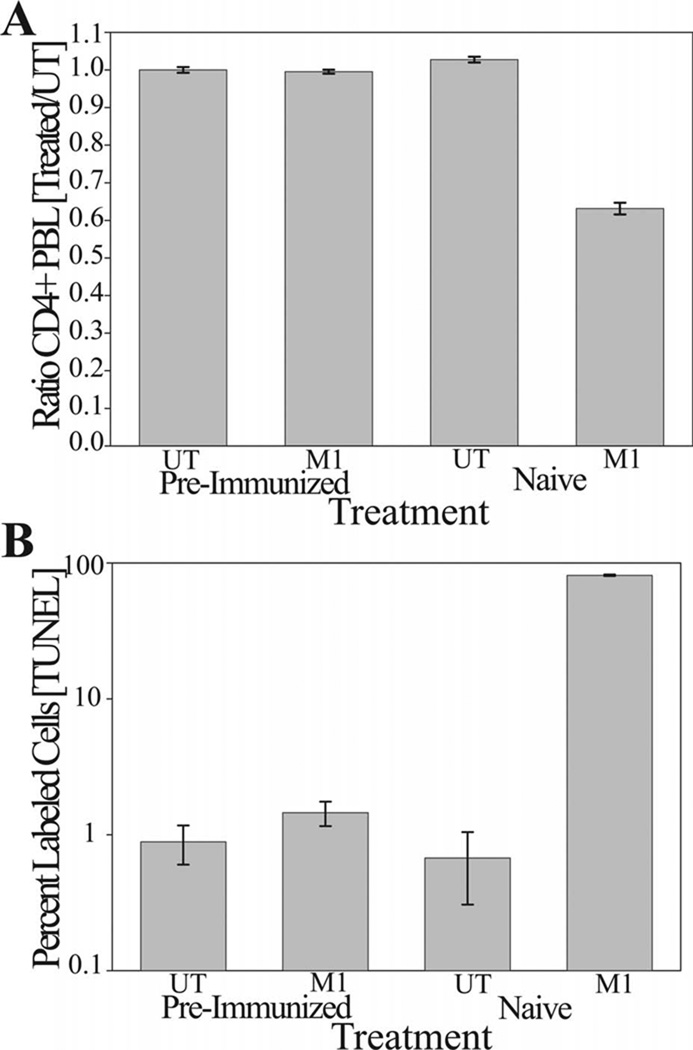
Immunization of Mice with Nef Peptide M1 Can Protect against Lymphocyte Depletion
Mice were either naïve or preimmunized with KLH-NefM1 and subsequently analyzed to show that the preimmunized mice were producing a humoral response to NefM1 (data not shown). Both the preimmunized and the naive mouse set were either untreated or challenged as above with a three-week NefM1 treatment, sacrificed, and PBLs harvested and analyzed for lymphocyte depletion (Figure 4A) and apoptosis induction in CD4+ PBLs (Figure 4B). The immune response induction protected those mice from induction of lymphocyte depletion by the subsequent challenge with NefM1 (Figure 4A, preimmunized-M1 vs naive-M1). This correlated with a concurrent protection from NefM1 apoptosis induction in CD4+ PBLs (Figure 4B, compare preimmunized-M1 to naive-M1). Alternatively, the lymphocytes from naive (unimmunized) mice displayed all the hallmarks of NefM1 challenge, CD4+ PBL depletion (Figure 4A, naive-M1), and increased levels of apoptosis in CD4+ PBLs (Figure 4B, naive-M1).
Fig 4.
The protective effect of induction of a humoral response against NefM1. A cohort of nine mice was immunized with KLH-NefM1, and a humoral immune response was induced and demonstrated in each mouse. Another cohort of six unimmunized mice was used as a control group. The challenge group (M1) consisted of seven preimmunized mice (pre-immunized panel A-bar M1/PreImmunized and panel B-bar M1/PreImmunized), and five unimmunized mice (Naive; panel A-bar M1/Naive and panel B-bar M1/Naive). they were challenged with three weeks of IP injection treatments every 48 hours with NefM1 (1 µg/mouse). The unchallenged, or negative control group (UT) consisted of two pre-immunized mice (panel A-bar UT/pre-immunized, and panel B-bar UT/pre-immunized), and one unimmunized mouse (panel A-bar UT/Naive and panel B-bar UT/Naïve). All mice were then sacrificed, blood collected, and peripheral blood lymphocytes harvested. (A) Cell numbers from each treatment group that were analyzed for CD4+ phenotype, and displayed as a ratio of the number of cells in the treated population to the total number of cells in the untreated population (UT). (B) The percent of CD4+ cells in each treatment group that were TUNEL labeled
Discussion
In previous studies, soluble Nef bound to CXCR4 on the surface of a number of cell types, including lymphocytes, induced apoptosis.1,2 The CXCR4 receptor is highly conserved between humans and mice.8–10 This information led us to believe that Nef could induce apoptosis in primary mouse cells (eg, lymphocytes, thymocytes). If so, the mouse would make a reasonable model to examine the effects of Nef-induced lymphocyte depletion. Introduction of Nef into the bloodstream of the mouse by periodic injection simulates hypothesized secretion of Nef into the bloodstream during HIV-1 infection in humans. Thus, a non-transgenic mouse model would be useful in testing the effects of Nef on lymphocyte depletion. Further, from previous work we concluded that NefM1 and NefsM1 could make useful surrogate probes to study Nef-induced lymphocyte depletion in our animal model.
NefM1-treated mice displayed significant decreases in circulating CD4+ lymphocytes with no concomitant decrease in CD8+ lymphocytes, which suggests that NefM1 was also affecting the host’s ability to replenish the CD4 lymphocyte population.11 Hematopoietic progenitor cells (bone marrow) emigrate to the thymus, are entrained, and the resultant mature thymocytes emigrate into the peripheral blood, becoming mature PBLs, replenishing this population.12 Immature thymocytes have been shown to express high levels of CXCR4, making them a potential target for Nef-induced apoptosis.12 Additionally, this process of entrainment involves interactions between the hematopoietic cells and a special thymic epithelium population, thymic nurse cells.13 Some epithelial cell types express CXCR4,14–17 while others do not,18 making it possible that thymic epithelium might also be susceptible to Nef-induced effects. We found that NefM1 induced significant levels of apoptosis in CD4+ cells in the thymus of treated mice. NefM1 was also found to induce apoptosis in CD8+ PBLs, although at that dosage, their numbers were found not to decrease significantly in the peripheral blood. As both CD4+ and CD8+ lymphocytes originate in the thymus, this finding suggests differences in the reactivity of the two populations to NefM1 or in their ability to be replenished.
The validity of soluble Nef inducing bystander effects hinges on 1) a soluble Nef that induces cell killing in the extracellular environment and 2) a weak immune response to the Nef apoptotic epitopes that does not block Nef-induced apoptosis. Our previous experiments1,2 and other studies3,19,20 show that Nef can induce apoptosis in lymphocytes both in cell culture and on in vivo treatment, support the existence of soluble Nef in the extracellular environment, and support the contention of a weak or nonexistent immune response to the Nef apoptotic motifs. We have suggested that this combination of events can contribute to the ability of Nef to induce CD4+ PBL depletion. We tested this hypothesis in our mouse model, and indeed, we found that immunization with KLH-NefM1 induced a humoral response in mice to NefM1. In contrast, no humoral response to NefM1 was observed in mice treated with non-immunogen-linked NefM1, even after several months of treatment (data not shown). Vaccination with KLH-conjugated NefM1 blocked/neutralized subsequent NefM1-induced CD4+ PBL depletion. Consequently, the host organism, which does not naturally raise a neutralizing response to this epitope, can be forced by vaccination to raise a neutralizing response to this epitope. This leads to a key consideration in this study, which is that HIV infection/replication is not being modeled. Consequently, the lack of ability of the mouse to be infected by HIV and the lack of the viruses’ ability to replicate in mouse are not relevant. Rather, through the use of this animal model, we are examining the effects of host exposure (ie, hematopoietic system) to secreted HIV-1 Nef (apoptotic peptides).
Based on our findings, we hypothesize that this lack of response to this apoptotic epitope also occurs in infected humans and contributes to the Nef apoptotic epitope’s induction of CD4+ PBL depletion. We further posit that a detailed analysis of this phenomenon can be made in a mouse model. Finally, we believe that this line of investigation could lead to development of therapeutics (eg, vaccine) targeting the Nef apoptotic motifs. The identified motifs, attached to an appropriate immunogen, could, when injected into a subject, induce a strong immune response against this specific epitope, blocking its contribution towards CD4+ PBL depletion and modulating the onset of Nef-induced pathology that leads to AIDS.
Acknowledgments
We thank Mrs. Dorothea Parker for her help with the immunization studies. This work was supported by NIH/NIGMS/MBRS (grant 58268) and NIH/NCRR/RCMI (grant G12-RR03034). The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HEK 293 cells, anti-Nef polyclonal antibody. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant #C06 RR18386 from NIH/NCRR.
References
- 1.James CO, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J Virol. 2004;78:3099–3109. doi: 10.1128/JVI.78.6.3099-3109.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang MB, Jin LL, James CO, Khan M, Powell MD, Bond VC. Characterization of Nef-CXCR4 interactions important for apoptosis induction. J Virol. 2004;78:11084–11096. doi: 10.1128/JVI.78.20.11084-11096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujii Y, Otake K, Tashiro M, Adachi A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 1996;393:93–96. doi: 10.1016/0014-5793(96)00859-9. [DOI] [PubMed] [Google Scholar]
- 4.Guy B, Riviere Y, Dott K, Regnault A, Kieny MP. Mutational analysis of the HIV Nef protein. Virology. 1990;176:413–425. doi: 10.1016/0042-6822(90)90011-f. [DOI] [PubMed] [Google Scholar]
- 5.Addo MM, Yu XG, Rathod A, et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol. 2003;77:2081–2092. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novitsky V, Cao H, Rybak N, et al. Magnitude and frequency of cytotoxic T-lymphocyte responses: identification of immunodominant regions of human immunodeficiency virus type 1 subtype C. J Virol. 2002;76:10155–10168. doi: 10.1128/JVI.76.20.10155-10168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choppin J, Cohen W, Bianco A, et al. Characteristics of HIV-1 Nef regions containing multiple CD8+ T cell epitopes: wealth of HLA-binding motifs and sensitivity to proteasome degradation. J Immunol. 2001;166:6164–6169. doi: 10.4049/jimmunol.166.10.6164. [DOI] [PubMed] [Google Scholar]
- 8.Frodl R, Gierschik P, Moepps B. Genomic organization and expression of the CXCR4 gene in mouse and man: absence of a splice variant corresponding to mouse CXCR4-B in human tissues. J Recept Signal Transduct Res. 1998;18:321–344. doi: 10.3109/10799899809047750. [DOI] [PubMed] [Google Scholar]
- 9.Heesen M, Berman MA, Hopken UE, Gerard NP, Dorf ME. Alternate splicing of mouse fusin/CXC chemokine receptor-4: stromal cell-derived factor-1alpha is a ligand for both CXC chemokine receptor-4 isoforms. J Immunol. 1997;158:3561–3564. [PubMed] [Google Scholar]
- 10.Bieniasz PD, Fridell RA, Anthony K, Cullen BR. Murine CXCR-4 is a functional coreceptor for T-cell-tropic and dual-tropic strains of human immunodeficiency virus type 1. J Virol. 1997;71:7097–7100. doi: 10.1128/jvi.71.9.7097-7100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellerstein M, Hanley MB, Cesar D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 12.Berkowitz RD, Beckerman KP, Schall TJ, McCune JM. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–3710. [PubMed] [Google Scholar]
- 13.Su DM, Manley NR. Hoxa3 and pax1 transcription factors regulate the ability of fetal thymic epithelial cells to promote thymocyte development. J Immunol. 2000;164:5753–5760. doi: 10.4049/jimmunol.164.11.5753. [DOI] [PubMed] [Google Scholar]
- 14.Horejsh D, Ruckwardt TJ, David PC. CXCR4-dependent HIV-1 infection of differentiated epithelial cells. Virus Res. 2002;90:275–286. doi: 10.1016/s0168-1702(02)00232-0. [DOI] [PubMed] [Google Scholar]
- 15.Jordan NJ, Kolios G, Abbot SE, et al. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest. 1999;104:1061–1069. doi: 10.1172/JCI6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayali AG, Van Gunst K, Campbell IL, et al. The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. J Cell Biol. 2003;163:859–869. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchou I, Misery L, Sabido O, et al. Functional HIV CXCR4 coreceptor on human epithelial Langerhans cells and infection by HIV strain X4. J Leukoc Biol. 2001;70:313–321. [PubMed] [Google Scholar]
- 18.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 19.Macreadie IG, Castelli LA, Lucantoni A, Azad AA. Stress- and sequence-dependent release into the culture medium of HIV-1 Nef produced in Saccharomyces cerevisiae. Gene. 1995;162:239–243. doi: 10.1016/0378-1119(95)00316-x. [DOI] [PubMed] [Google Scholar]
- 20.Guy B, Riviere Y, Dott K, Regnault A, Kieny MP. Mutational analysis of the HIV nef protein. Virology. 1990;176:413–425. doi: 10.1016/0042-6822(90)90011-f. [DOI] [PubMed] [Google Scholar]