Abstract
Fibroblast growth factor 2 (FGF-2) is a potent mediator of stem cell differentiation and proliferation. Although FGF-2 has a well-established role in promoting bone tissue formation, flaws in its delivery have limited its clinical utility. Polyelectrolyte multilayer films represent a novel system for FGF-2 delivery that has promise for local, precisely controlled and sustained release of FGF-2 from surfaces of interest including medical implants and tissue engineering scaffolds. In this work, the loading and release of FGF-2 from synthetic hydrolytically degradable multilayer thin films of various architectures is explored; drug loading was tunable using at least three parameters (number of nanolayers, counterpolyanion, and type of degradable polycation) and yielded values of 7-45 ng/cm2 of FGF-2. Release time varied between 24 hours and approximately five days. FGF-2 released from these films retained in vitro activity, promoting the proliferation of MC3T3 pre-osteoblast cells. The use of biologically derived counterpolyanions heparin sulfate and chondroitin sulfate in the multilayer structures enhanced FGF-2 activity. The control over drug loading and release kinetics inform future in vivo bone and tissue regeneration models for the exploration of clinical relevance of LbL growth factor delivery films.
Keywords: drug delivery, Fibroblast growth factor 2 (FGF-2), polyelectrolyte multilayer, heparin, poly(β-aminoester)
1. Introduction
Fibroblast Growth Factor-2 (FGF-2) is a potent biologic capable of helping to direct the stem cell differentiation and proliferation of cells as diverse as hepatocytes1, 2, neurons3, 4, cartilage5, and bone. It therefore represents a tremendous potential opportunity in affecting clinical outcomes across a number of different medical conditions in which exogenous cell transplantation, or the recruitment of native stem cells, is a possible strategy. In particular, there is a clear need for enhancing bone creation and regeneration. A bone defect that is too large to bridge can occur in response to trauma, during treatment of bone non-union, or when resecting bone tumors, leading to a lesion which cannot heal6. In reconstructive surgery, frequently the need for allograft bone tissue outstrips the supply available7, particularly in elderly and pediatric populations, limiting therapeutic intervention. In both of these circumstances, it has been found that the introduction of FGF-2 can greatly enhance the rate of healing and the ability of natural bone to bridge defects8-11 due to its ability to promote proliferation both in pre-osteoblast cell populations to increase bone production12, and also in endothelial cell populations which enhance blood supply to the growing tissue13. FGF-2 has further bone forming roles in increasing inorganic phosphate transport14 and in stimulating vascular endothelial growth factor (VEGF) secretion15. However, bolus injections of growth factor are rapidly cleared, leaving little time to exhibit therapeutic effects16, 17. Furthermore, supraphysiologic doses of growth factor are needed for injections due to growth factor dilution in a larger body volume (opening the possibility of off-target side effects), loss of growth factor activity on exposure to blood, and clearance from the blood stream. There is therefore a need for a biomaterial that will locally release sustained low levels of active growth factors for long periods of time.
One means of accomplishing this is to create polymer coatings that can safely contain and release even sensitive biologic drugs in an active form within nanolayered film architectures for controlled release directly from implanted surfaces. Such an implant coating could be beneficial for an orthopedic hip implant, a stent, an implantable tissue engineering scaffold, or a range of surgical fixtures such as screws or sutures. Such release from implant surfaces calls for new, surface based methodologies that allow coating of a current mechanical device rather than traditional bulk polymer encapsulation methods. This can be achieved using Layer-by-Layer (LbL) assembly18; in this technique, positively and negatively charged polymers are adsorbed sequentially onto a charged surface to build a film. LbL assembly is advantageous in that the films are created through a gentle, aqueous process that preserves fragile drug activity7, 19, 20, while the resulting films can be made to be biodegradable, thin, and completely conformal to the device of interest, with easily tunable drug incorporation and film architecture20-22. Furthermore, LbL opens the possibility of sequestering multiple drugs in different layers of the film and creating the opportunity to sequentially release several growth factors or other therapeutic agents such as antibiotics or anti-inflammatory agents23, 24 through surface erosion of the film. Finally, because conformal LbL deposition is possible on a wide variety of biomedically relevant materials, including titanium, ceramic, polymer, and glass, there are many potential medical applications of LbL.
Early drug release experiments with LbL films using hydrogen bonded films showed that these films fall apart rapidly at near-neutral pH, allowing a rather instantaneous method of drug release25. However, such release is impossible to tune, and on a shorter time span than most controlled drug delivery applications require. Another approach is to pre-construct LbL films out of inert polymers and then load drug into the permeable network for diffusive or pH induced release26-28. While such porous LbL systems work well for the delivery of small molecules, the delivery of substantially larger protein therapeutics is difficult, as the therapeutic gets trapped in the polymer mesh and cannot diffuse out. Therapeutic drugs can either be encapsulated in the core, or in the LbL film surrounding the core itself29. Drugs in the core can either be crystalline drug which therefore is ready for solubilization and release after LbL coating30, 31, encased in a polymer which is subsequently leached out32, 33, or absorbed into the capsule once the template core is dissolved34. However, as non-degradable polymers are typically used, release is diffusive in nature and thus not easily tuned, and such microcapsules do not take advantage of the conformal nature of LbL in the implant setting, in addition to typically employing high ionic strength or high pH as a means of release (conditions rarely encountered in drug delivery applications in vivo). These approaches are thus not suitable for proteins.
Although proteins, and in particular growth factors, have been incorporated in LbL films19, 22, 35, it is not typical for these LbL films to incorporate synthetic polymers that are designed to allow biodegradability and growth factor release, which can sometimes hamper efforts to tune drug release due to the constraints of existing biopolymers36. The approach taken in this work is to incorporate a cationic polymer from the class of poly(β-aminoester)s (PBAEs)37. These polymers are created through the simple Michael addition of two commercially available classes of starting materials. Work in our group and others has shown that PBAEs readily incorporate in LbL films38, and that the drug delivery characteristics of PBAEs from LbL can be varied through varying the polymer architecture36, 39, allowing strong control over release through polymer design. It was found that increasing the hydrophobicity in LbL films led to extended release by decreasing water cleavage of the ester bond up until a critical point, at which the films would quickly fall apart and release all of the film contents due to film instability39. Taken together, the ready incorporation of PBAEs in LbL films combined with our deep understanding of the tunability of these polymers represent an excellent opportunity to tune drug release profiles from LbL films via polymer design38. The poly(beta-aminoester)s used in this paper, denoted Poly1 and Poly2, have the same structural characteristics (Figure 2), differing by the addition of two methylene units in the backbone of Poly2, increasing the hydrophobicity in the region next to the ester bond which is hydrolytically cleaved and therefore decreasing degradation.
Figure 2.

The poly(beta-aminoester)s used in this paper, denoted Poly1 and Poly2, have the same structural characteristics, save for the addition of two methylene units in the backbone of Poly2, increasing the hydrophobicity in the region next to the ester bond which is hydrolytically cleaved and therefore decreasing degradation.
A negatively charged polyanion is also required for film construction. Here, heparin sulfate and chondroitin sulfate were chosen to exploit advantageous interactions each of these molecules has with FGF-2. Heparin is well known to highly increase the mitogenic potential of FGF-2 by assisting its receptor binding40, 41 while preserving FGF-2 from heat, pH changes, and proteolysis42. Specific binding sequences43 have been discovered which allow FGF-2 to bind and be sequestered in heparin 44, leading to a developing body of work on the use of heparin for controlled delivery45-48. However, because it is difficult to modify and optimize biopolymers such as heparin as a release material, there are challenges in addressing their inherent materials limitations, such as undesirable release profiles or unintended side effects. Chondroitin, a natural extracellular matrix biopolymer found in cartilage with anti-inflammatory properties has also been shown to enhance FGF-2 mitogenic activity49. In addition, in a previous study the ability to tune the release profile36 from LbL films has been demonstrated, and chondroitin has potential synergism in creating bone and cartilage50-52.
The aim of the present work is to investigate the characteristics of this novel FGF-2 carrier film that combines the advantages of biopolymer enhancement of FGF-2 mitogenic activity with the fine control over release afforded by synthetic polymers, with a focus on creating a conformal thin film that can be coated on implanted medical devices to enhance tissue growth once implanted in vivo.
2. Materials and Methods
2.1 Materials
Linear poly(ethylenimine) (LPEI, Mn = 25000) was bought from Polysciences, Inc (Warrington, PA). Poly (sodium 4-styrenesulfonate) (PSS, Mn = 1000000) and chondroitin were purchased from Sigma-Aldrich (St. Louis, MO). Heparin sodium salt was obtained from Celsus Laboratories (Cincinnati, OH). Poly(β-amino ester)s (PBAEs) Poly 1 and Poly 2 (Figure 2) are cleavable through the ester bond, and differ only in an additional 2 methlyene units in the backbone. These PBAEs were synthesized as previously described37. Fibroblast Growth Factor-2 (17.2 kDa, pI = 9.6) and FGF-2 ELISA kits were obtained from Peprotech (Rocky Hill, NJ). Glass slides (for substrates) were obtained from VWR Scientific (Edison NJ).
2.2 Preparation of polyelectrolyte solutions
LPEI and PSS dipping solutions contained 10mM polymer with respect to repeat unit, adjusted to pH 4.25 and 4.75 respectively. Dipping solutions were prepared in sodium acetate buffer (pH 5.1, 100mM) in the following concentrations: heparin, chondroitin, Poly 1 and Poly2 prepared at 2mg mL−1, and FGF-2 prepared at 1.65 g mL−1.
2.3 Film construction and characterization
Glass substrates (1″ × ¼″) were plasma etched with room air using a Harrick PDC-32G plasma cleaner on high RF power for 5 minutes. Ten base layers of (LPEI/PSS) were deposited upon plasma etched substrates to create a surface area 3/4″× 1/4″ with a Carl Ziess HSM series programmable slide stainer with 5 minutes of soaking per polymer followed by 3 rinses in deionized water. Slides with ten base layers were sequentially dipped in baths of polymers and growth factors to create the following tetralayer architecture: (PolyX/heparin/FGF-2/heparin)n where the PolyX is either Poly1 or Poly2 and n refers to the number of tetralayers deposited on the substrate. A typical dipping protocol is 10 minutes in Poly 1 solution, 3 washes, 7.5 minutes in heparin solution, 3 washes, 10 minutes in FGF-2 solution, 2 washes and 7.5 minutes in heparin solution with 3 washes (all washes were performed with non-pH adjusted deionized water). The thickness of films was measured by scoring the samples with a razor blade and measuring the step height using a P10 Surface Profiler with a 2 μm tip radius stylus.
2.4 Release characterization
FGF-2 films were released into 1 mL of 1% serum medium, consisting of alpha Minimum Essential Medium (αMEM) supplemented with 1% fetal bovine serum and 1% penicillin streptomycin (Invitrogen, Carlsbad CA) at 37°C. At a series of different time points, 0.5 mL of medium and eluted material was removed and 0.5 mL of fresh release medium was replaced. Samples were analyzed using Enzyme Linked ImmunoSorbent Assay (ELISA) development kits and cell proliferation assays (see below) according to manufacturer instructions. ELISA analysis of the release medium (1% serum in αMEM) yielded no FGF-2 above the lower detection limit of the test (62.5 pg/mL). At the conclusion of the experiment, residual film was scraped from the surface with a razor blade and analyzed with ELISA to determine the total FGF-2 load and fractional release.
2.5 Cell culture
MC3T3 E1S4 (ATCC, Manassas, VA) were maintained in growth medium consisting of α-MEM supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were split when subconfluent and used until passage 15.
2.6 FGF-2 activity assay
750 MC3T3 cells were seeded in 96 well plates in 100 uL of growth medium and allowed to attach overnight. The medium was changed to 1% serum and after 24 hours 10 uL of eluted material from each sample or control was mixed with 90 uL of 1% serum and applied in triplicate to the wells. After 72 hours, bromodeoxyuridine (BRDU) was placed in the release medium (BRDU kit, Roche Applied Science, Indianapolis, IN) for 4 hours. Similar to [3H] thymidine assays, BRDU is incorporated into the DNA of proliferating cells, which is later analyzed using ELISA. Plates were analyzed according to manufacturer instructions.
3. Results and Discussion
3.1 Film assembly characterization
Multilayer films with a tetralayer architecture were created by alternately dipping FGF-2, which exhibits a net positive charge under physiological conditions (isoelectric point = 9.6), with a non-cytotoxic polyanion and a hydrolytically degradable polycation from a series of poly(β-amino-esters). Figure 1 depicts a schematic of the LbL dipping process and resulting tetralayer architecture of the films, which is denoted through this paper as [PolyX/Polyanion/FGF-2/Polyanion]n, where the term in brackets represents one repeat unit (tetralayer) of film and n represents the number of repeat units deposited on the substrate. Two poly(β-amino-esters), Poly1 and Poly2, were utilized in this work; they are identical structurally save for two additional methylene units in the backbone of Poly2. Figure 2 shows the structure of both polymers. Previous work 36, 39 has shown that in drug delivery applications, use of Poly2 typically results in longer release periods than Poly1. This has been attributed to increased hydrophobicity from the methylene units adjacent to the hydrolytically cleavable ester bond, which decreases water access to the bond and results in longer degradation times than Poly136, 39.
Figure 1.

Schematic of the Layer-by-Layer tetralayer architecture employed in this paper and mechanism of release, in which surface erosion through polymer hydrolysis allows for sustained, local delivery of growth factor.
LbL films were successfully constructed varying both the polyanion and polycation, resulting in the three following architectures: [P2/chondroitin/FGF-2/chondroitin], [P2/heparin/FGF-2/heparin], and [P1/heparin/FGF-2/heparin]. The correlation between thickness of the films and number of tetralayers deposited was tracked with profilometry for the three film formulations in Figure 3. All film architectures tested exhibited a two-regime buildup behavior with a brief period for which thin films with nanometer scale thicknesses are formed, followed by a linear building regime. This characteristic growth pattern has been observed in many LbL systems reported in the literature36, 53, 54. When the film is composed of a few tetralayers (10-20 tetralayers or less), an exponential or superlinear growth behavior is observed in which each additional tetralayer incorporates more material than the previous layer due to interdiffusion of the polymers into the film during deposition. This regime gives way to the second, diffusion-limited linear growth regime, which grows by approximately 200 nm per tetralayer; it is thought that this linear thickness increase is the result of diffusion limitations over the timeframe of the adsorption/absorption step from interdiffusion behavior.
Figure 3.

Film growth is monitored by profilometry. A typical two regime building process is observed in all film formulations, indicating superlinear building and therefore good drug loading potential. In the figure above P1 and P2 = Poly 1 and 2 respectively, H = heparin, and C = chondroitin.
This film growth behavior is typically observed in LbL systems for which one or more of the film components can diffuse into the film during the dipping process, and has been attributed to film rearrangement due to this diffusive process during dipping36, 53-56. This is typically true of biological systems in which lower polymer backbone charge density prevails, or in cases when small, rapidly diffused molecules are entrapped in a film57. Such exponential buildup can be advantageous for drug delivery applications, due to the rapid buildup and incorporation of drug compared to linearly building LbL films. The thickness per tetralayer of film, 100 to 200 nm, is an order of magnitude larger than typical values for electrostatic multilayer systems.
3.2 FGF-2 loading and release
After ensuring that FGF-2 containing films would assemble properly, the release characteristics and drug loading of the films were tested. Figure 4 shows the cumulative release of FGF-2 release samples incubated at 37°C in 1% serum cell medium from 50 tetralayer films of each film formulation of interest. [Poly1/heparin/FGF-2/heparin] films exhibit a high level of release over the first 24 hour period with low-level release thereafter, while Poly2 films show a sustained 3- or 5-day release. These results are interesting from several perspectives.
Figure 4.

Release profiles for different 50 tetralayer LbL film architectures
The release curve has a classic power law shape. Previous experiments with LbL PBAE films show that surface erosion contributes a dominant mechanism of release in these LbL films36, 58, which would be anticipated to yield a linear release profile. In this release mechanism, it is hypothesized that degradation of the polymer backbone yields shorter polymer fragments which can more easily break the ionic crosslinks with the film surface than can the full length polymer, allowing the polymer to elute off of the surface and therefore release drug entrapped below it. The power law curve suggests that in addition to surface erosion, a second release mechanism, likely diffusion, is also at work, accounting for the additional curvature to the release profile. ,.
The drug loading is affected by both the polycation and polyanion used, with maximum loading observed with P2/H (42 ng/cm2), and minimum loading with P1/H (8 ng/ cm2). Comparing first the effect of the polycation, it is seen that P2/H contains five times as much FGF-2 as did P1/H. This may be due in part to the increased hydrophobicity along the P2 backbone, combined with a slightly lower charge density, resulting in a lower effective ionic crosslink density within the film, and hydrophobic pockets within the film that may increase secondary interactions with FGF-2. It has been posited that a lower ionic crosslink density can lead to looser, loopier polymer chain conformations within the film that may also more readily accommodate protein sequestration within the multilayer, and, indeed, model protein Poly2 films are thicker and load more protein than their Poly1 equivalents36. As hypothesized, Poly2 films do release for longer periods of time than Poly1 films. To compare release profiles, the released amounts are plotted as percent of total release in Figure 5; here it is seen that [P2/heparin/FGF-2/heparin] films release over a span of 5 days with 85% of release occurring around day 4 while similar films made with Poly1 ([P1/heparin/FGF-2/heparin] release 85% of their contents in 1 day with slow release of the rest over an additional 2 days. . This supports the notion that, because Poly2 is more hydrophobic and thus less degradable by ester hydrolysis, it takes longer for the film to erode and release its contents.
Figure 5.

Release from different 50 tetralayer LbL architecture films plotted as a fraction of total release show release profiles for P2 films which have a slower release rate than the release from a P1 film.
The polyanion also affects film loading and release. Comparing P2/H and P2/C films, total drug loading capacity of the films is affected, with P2/C films loading approximately 30% of the FGF-2 loaded into the P2/H film. One contributing factor is likely the specific interaction between FGF-2 and heparin, which allows more FGF-2 to be incorporated. However, studies with the model protein lysozyme, which has similar isoelectric point and molecular weight to FGF-2, also showed that P1/H films loaded more lysozyme than P1/C films, indicating that specific binding interactions are not the only contributing factor. In that work, it was posited that specific interactions allow better film packing and increased drug density36. The release of [Poly2/chondroitin/FGF-2/chondroitin] films takes place over about three days while the release of [Poly2/heparin/FGF-2/heparin] films takes place over 5 days; these results are consistent with the work done with lysozyme in which heparin films release over a longer period of time.
In Figure 6, [P2/heparin/FGF-2/heparin] films ranging from 10 to 50 tetralayers thick were released to generate a family of curves, showing that the delivered dose and the time span of release of FGF-2 from these LbL films can be easily tuned simply by changing the number of tetralayers used. In comparing the release times of each number of tetralayers, it is interesting to note that the release curves overlap during the early phase of release, until the film is exhausted (so, for example, the forty and fifty tetralayer release curves overlap until the forty tetralayer film releases its entire load). This is consistent with a surface erosion mechanism of release, in which each layer releases its contents until the entire film thickness is eroded, yielding release profiles that release for the same time span and release rate within the family of curves.
Figure 6.
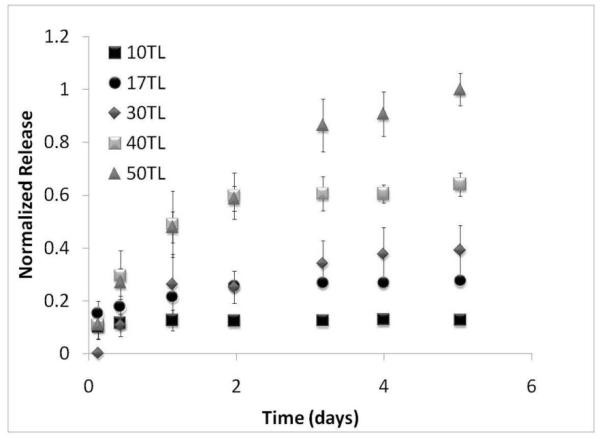
Release is tunable with number of tetralayers, using [P2/Heparin/FGF-2/Heparin] film as an illustration
Finally, it was of interest to pursue the fractional release from the film. After 10, 20, 30, 40, and 50 tetralayers were done releasing as detected by ELISA, any remaining visible film was scraped from the surface with a razor blade and vortexed in collection medium. The resulting, non released fraction was run on ELISA to detect unreleased FGF-2 in the polymer matrix. In all cases, the fractional release was 95% or higher of the total drug load (Table 1).
Table1.
Fractional release of [P2/heparin/FGF-2/heparin] films of varying numbers of tetralayers. Greater than or equal to about 95% release is seen at all numbers of tetralayers, suggesting that all FGF-2 incorporated is released.
| # Tetralayers | Percent released |
|---|---|
| 50 | 98.8 |
| 40 | 100.0 |
| 30 | 94.8 |
| 17 | 95.7 |
| 10 | 99.5 |
3.3 In vitro assessment of activity
FGF-2 is known to be a potent mitogen for the pre-osteoblastic MC3T3 cell line59, 60, as well as for human osteoblasts59, which increases the population of pre-osteoblast cells available to become bone cells in vivo. The FGF-2 released from the film was tested in a BRDU proliferation assay (Figure 7). In this assay, only proliferating cells are labeled with BRDU which is later detected by ELISA. FGF-2 released from all three film formulations of interest (P2/C, P2/H, P1/H, Fig 7A, B, C, respectively) shows robust (three or more fold) increases in proliferation over control cells which received no FGF-2. This released FGF-2 maintains proliferative activity far past the period of release from the film, as proliferation rates are elevated for up to 12 days in culture, indicating that FGF-2 retains high levels of activity while in the film. Concentrations of free FGF-2 (100, 50, 5, 0,5 and 0 ng/cm2) introduced into the medium show a dose dependence on FGF-2 (Supplemental figure S1). Interestingly, FGF-2 released from LbL films demonstrate increased ability of up to eight fold negative control values to enhance proliferation compared to the free FGF-2 (about 2 fold), which may be due to the co-release of heparin or chondroitin sulfate. Both heparin and chondroitin are known to increase the mitogenic activity of FGF-249, and thus represent a synergistic addition to the film.
Figure 7.
Released FGF-2 from LbL films retains proliferative effect on MC3T3 cells. Compared with control cells which were not exposed to FGF-2 from the films (“zero” in (A), (B), (C)), cells which were exposed to FGF-2 released from films of different noted architectures showed a marked increase in proliferation rate as measured by BRDU incorporation. (D) All film components tested other than FGF-2 show no positive or negative effect on proliferation compared with control cells not exposed to LbL films (y axis variables are arbitrary units).
To rule out the possibility that heparin, or another film component released from the film, could increase proliferation alone by helping native FGF-2 present in the cell medium serum bind to MC3T3, films were created without FGF-2 (only inactive components) and were released and tested on cells. All other film components (Poly1, Poly2, chondroitin, and heparin) alone or in combination, showed no proliferative effect on the MC3T3 cells (Fig 3D). Thus, the mitogenic activity of FGF-2 encapsulated within these LbL films is preserved and enhanced by co-delivery with heparin or chondroitin.
4. Conclusions
Although growth factors have an acknowledged ability to aid in the formation of new bone tissue, which is still critically needed in many clinical applications, its delivery for clinical applications thus far has been flawed or limited due to loss of activity and inability to control release in a sustained fashion. FGF-2 is one of a family of growth factors that, if released in combination or sequence with other growth factors, could lead to an enhanced bone formation response that would be clinically relevant. LbL is an ideal candidate drug delivery system for such co- and sequential release schemes due to the possibility of sequestering different growth factors in different layers of a constructed film24 that can be used to coat a bone implant or an engineered scaffold for wound healing. In this work, we demonstrate the first successful incorporation and sustained controlled release of FGF-2 from a synthetic, biodegradable polymer LbL drug delivery system intended to work locally at the site of new bone formation. FGF-2 release is tunable through the polycation, polyanion, and number of tetralayers used to construct the film; FGF-2 released from the film exhibits enhanced ability to promote proliferation in pre-osteoblast cells compared with FGF-2 medium supplementation due to co-release of heparin or chondroitin sulfate. We show the ability to release 45 ng/cm2 of growth factor with retained bioactivity over 12 days. While other LbL methods that rely on post-manufacture surface adsorption of growth factor onto LbL films 28, 61 can deliver similar amounts of growth factor, because the growth factor is surface-bound rather than sequestered with a degradable synthetic polymer, no control can be exerted over its release profile, nor is sequential release accessible. In other cases 7, 62, the amounts released are modest, rendering autologous in vivo cell response difficult to achieve. Thus here, we show a unique system which retains LbL’s true potential in biomedical surface modification by combining both a high degree of loading of growth factor in multilayer films while preserving the possibility of sequential release of multiple growth factors or other therapeutic agents. These results significantly enhance our understanding of growth factor delivery from LbL films in a way that will enable in vivo bone regeneration models in future work, and represent a necessary step in understanding how LbL can be used to treat clinically relevant problems in growth factor delivery.
Supplementary Material
Acknowledgement
We thank Dr. David Lynn for helpful and synergistic discussions, and Dr. Robert Langer for his support. Financial support for this work is gratefully acknowledged from Deshpande Center grant 009216-1 and the National Institutes of Health grant 1-R01-AG029601-01. M. Macdonald is thankful for a National Science Foundation Graduate Research Fellowship. This work made use of MRSEC Shared Facilities supported by the National Science Foundation under Award Number DMR-0213282.
Footnotes
Supporting Information Available Graph showing proliferative response of MC3T3 preosteoblast cells to varying concentrations of FGF-2 added to 1% serum cocurrently with film sample exposure to the cells. Dose response can be seen to varying concentrations of FGF-2. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Bonora-Centelles A, Jover R, Mirabet V, Lahoz A, Carbonell F, Castell JV, Gomez-Lechon MJ. Sequential Hepatogenic Transdifferentiation of Adipose Tissue-Derived Stem Cells: Relevance of Different Extracellular Signaling Molecules, Transcription Factors Involved, and Expression of New Key Marker Genes. Cell Transplant. 2009;18(12):1319–1340. doi: 10.3727/096368909X12483162197321. [DOI] [PubMed] [Google Scholar]
- 2.Touboul T, Hannan NRF, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, Di Santo J, Weber A, Vallier L. Generation of Functional Hepatocytes from Human Embryonic Stem Cells Under Chemically Defined Conditions that Recapitulate Liver Development. Hepatology. 2010;51(5):1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- 3.Kang K, Song MR. Diverse FGF receptor signaling controls astrocyte specification and proliferation. Biochem Biophys Res Commun. 2010;395(3):324–329. doi: 10.1016/j.bbrc.2010.03.174. [DOI] [PubMed] [Google Scholar]
- 4.Kasai M, J. T, Fukumitsu H, Furukawa S. FGF-2-responsive and spinal cord-resident cells improve locomotor function after spinal cord injury. J Neurotrauma. 2009 doi: 10.1089/neu.2009.1108. doi:10.1089/neu.2009.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takafuji H, Suzuki T, Okubo Y, Fujimura K, Bessho K. Regeneration of articular cartilage defects in the temporomandibular joint of rabbits by fibroblast growth factor-2: a pilot study. Int J Oral Maxillofac Surg. 2007;36(10):934–937. doi: 10.1016/j.ijom.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Betz OB, Betz VM, Nazarian A, Pilapil CG, Vrahas MS, Bouxsein ML, Gerstenfeld LC, Einhorn TA, Evans CH. Direct percutaneous gene delivery to enhance healing of segmental bone defects. Journal of Bone and Joint Surgery-American Volume. 2006;88A(2):355–365. doi: 10.2106/JBJS.E.00464. [DOI] [PubMed] [Google Scholar]
- 7.Dierich A, Le Guen E, Messaddeq N, Stoltz JF, Netter P, Schaaf P, Voegel JC, Benkirane-Jessel N. Bone formation mediated by synergy-acting growth factors embedded in a polyelectrolyte multilayer film. Adv Mater (Weinheim, Ger) 2007;19(5):693–697. [Google Scholar]
- 8.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone - Biology and clinical applications. Journal of Bone and Joint Surgery-American Volume. 2002;84A(6):1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Ma T, Gutnick J, Salazar B, Larsen MD, Suenaga E, Zilber S, Huang Z, Huddleston J, Smith RL, Goodman S. Modulation of allograft incorporation by growth factors over a prolonged continuous infusion of duration in vivo. Bone. 2007;41(3):386–392. doi: 10.1016/j.bone.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Murakami S, Takayama S, Ikezawa K, Shimabukuro Y, Kitamura M, Nozaki T, Terashima A, Asano T, Okada H. Regeneration of periodontal tissues by basic fibroblast growth factor. J Periodontal Res. 1999;34(7):425–430. doi: 10.1111/j.1600-0765.1999.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 11.Power RA, Iwaniec UT, Wronski TJ. Changes in gene expression associated with the bone anabolic effects of basic fibroblast growth factor in aged ovariectomized rats. Bone. 2002;31(1):143–148. doi: 10.1016/s8756-3282(02)00799-8. [DOI] [PubMed] [Google Scholar]
- 12.Hanada K, Dennis JE, Caplan AI. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res. 1997;12(10):1606–1614. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]
- 13.Cote MF, Laroche G, Gagnon E, Chevallier P, Doillon CJ. Denatured collagen as support for a FGF-2 delivery system: physicochemical characterizations and in vitro release kinetics and bioactivity. Biomaterials. 2004;25(17):3761–3772. doi: 10.1016/j.biomaterials.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Palmer G, Bonjour JP, Caverzasio J. Stimulation of sodium-dependent phosphate transport and signaling mechanisms induced by basic fibroblast growth factor in MC3T3-E1 osteoblast-like cells. Journal of Bone and Mineral Research. 2000;15(1):95–102. doi: 10.1359/jbmr.2000.15.1.95. [DOI] [PubMed] [Google Scholar]
- 15.Tokuda H, Kozawa O, Uematsu T. Basic fibroblast growth factor stimulates vascular endothelial growth factor release in osteoblasts: Divergent regulation by p42/p44 mitogen-activated protein kinase and p38 mitogen-activated protein kinase. Journal of Bone and Mineral Research. 2000;15(12):2371–2379. doi: 10.1359/jbmr.2000.15.12.2371. [DOI] [PubMed] [Google Scholar]
- 16.Hsu HP, Zanella JM, Peckham SM, Spector M. Comparing ectopic bone growth induced by rhBMP-2 on an absorbable collagen sponge in rat and rabbit models. J Orthop Res. 2006;24(8):1660–1669. doi: 10.1002/jor.20204. [DOI] [PubMed] [Google Scholar]
- 17.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 18.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277(5330):1232–1237. [Google Scholar]
- 19.Benkirane-Jessel N, Lavalle P, Hubsch E, Holl V, Senger B, Haikel Y, Voegel JC, Ogier J, Schaaf P. Short-time timing of the biological activity of functionalized polyelectrolyte multilayers. Adv Funct Mater. 2005;15(4):648–654. [Google Scholar]
- 20.Chuang HF, Smith RC, Hammond PT. Polyelectrolyte multilayers for tunable release of antibiotics. Biomacromolecules. 2008;9(6):1660–1668. doi: 10.1021/bm800185h. [DOI] [PubMed] [Google Scholar]
- 21.Wood KC, Boedicker JQ, Lynn DM, Hammond PT. Tunable drug release from hydrolytically degradable layer-by-layer thin films. Langmuir. 2005;21(4):1603–9. doi: 10.1021/la0476480. [DOI] [PubMed] [Google Scholar]
- 22.Picart C, Schneider A, Etienne O, Mutterer J, Schaaf P, Egles C, Jessel N, Voegel JC. Controlled degradability of polysaccharide multilayer films in vitro and in vivo. Adv Funct Mater. 2005;15(11):1771–1780. [Google Scholar]
- 23.Kim BS, Smith RC, Poon Z, Hammond PT. MAD (Multiagent Delivery) Nanolayer: Delivering Multiple Therapeutics from Hierarchically Assembled Surface Coatings. Langmuir. 2009;25(24):14086–14092. doi: 10.1021/la9017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood KC, Chuang Helen F., Batten Robert D., Lynn David M., Hammond Paula T. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. PNAS. 2006;103(27):10207–10212. doi: 10.1073/pnas.0602884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukhishvili SA, Granick S. Layered, erasable polymer multilayers formed by hydrogen-bonded sequential self-assembly. Macromolecules. 2002;35(1):301–310. [Google Scholar]
- 26.Berg MC, Zhai L, Cohen RE, Rubner MF. Controlled drug release from porous polyelectrolyte multilayers. Biomacromolecules. 2006;7(1):357–364. doi: 10.1021/bm050174e. [DOI] [PubMed] [Google Scholar]
- 27.Chung AJ, Rubner MF. Methods of loading and releasing low molecular weight cationic molecules in weak polyelectrolyte multilayer films. Langmuir. 2002;18(4):1176–1183. [Google Scholar]
- 28.Crouzier T, Ren K, Nicolas C, Roy C, Picart C. Layer-By-Layer Films as a Biomimetic Reservoir for rhBMP-2 Delivery: Controlled Differentiation of Myoblasts to Osteoblasts. Small. 2009;5(5):598–608. doi: 10.1002/smll.200800804. [DOI] [PubMed] [Google Scholar]
- 29.Khopade AJ, Arulsudar N, Khopade SA, Hartmann J. Ultrathin antibiotic walled microcapsules. Biomacromolecules. 2005;6(1):229–234. doi: 10.1021/bm049554a. [DOI] [PubMed] [Google Scholar]
- 30.Caruso F, Trau D, Mohwald H, Renneberg R. Enzyme encapsulation in layer-by-layer engineered polymer multilayer capsules. Langmuir. 2000;16(4):1485–1488. [Google Scholar]
- 31.Pargaonkar N, Lvov YM, Li N, Steenekamp JH, de Villiers MM. Controlled release of dexamethasone from microcapsules produced by polyelectrolyte layer-by-layer nanoassembly. Pharm Res. 2005;22(5):826–835. doi: 10.1007/s11095-005-2600-0. [DOI] [PubMed] [Google Scholar]
- 32.Bhadra D, Gupta G, Bhadra S, Umamaheshwari RB, Jain NK. Multicomposite ultrathin capsules for sustained ocular delivery of ciprofloxacin hydrochloride. J Pharm Pharm Sci. 2004;7(2):241–251. [PubMed] [Google Scholar]
- 33.Caruso F, Yang WJ, Trau D, Renneberg R. Microencapsulation of uncharged low molecular weight organic materials by polyelectrolyte multilayer self-assembly. Langmuir. 2000;16(23):8932–8936. [Google Scholar]
- 34.Zhu HG, Srivastava R, McShane MJ. Spontaneous loading of positively charged macromolecules into alginate-templated polyelectrolyte multilayer microcapsules. Biomacromolecules. 2005;6(4):2221–2228. doi: 10.1021/bm0501656. [DOI] [PubMed] [Google Scholar]
- 35.Onda M, Lvov Y, Ariga K, Kunitake T. Sequential reaction and product separation on molecular films of glucoamylase and glucose oxidase assembled on an ultrafilter. J Ferment Bioeng. 1996;82(5):502–506. [Google Scholar]
- 36.Macdonald M, Rodriguez NM, Smith R, Hammond PT. Release of a model protein from biodegradable self assembled films for surface delivery applications. J Control Release. 2008;131(3):228–234. doi: 10.1016/j.jconrel.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynn DM, Langer R. Degradable poly(beta-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122:10761–10768. [Google Scholar]
- 38.Vazquez E, Dewitt DM, Hammond PT, Lynn DM. Construction of hydrolytically-degradable thin films via layer-by-layer deposition of degradable polyelectrolytes. J Am Chem Soc. 2002;124(47):13992–13993. doi: 10.1021/ja026405w. [DOI] [PubMed] [Google Scholar]
- 39.Smith RC, Leung A, Kim BS, Hammond PT. Hydrophobic Effects in the Critical Destabilization and Release Dynamics of Degradable Multilayer Films. Chemistry of Materials. 2009;21(6):1108–1115. doi: 10.1021/cm802972d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapraeger AC, Krufka A, Olwin BB. Requirement of Heparan-Sulfate for Bfgf-Mediated Fibroblast Growth and Myoblast Differentiation. Science. 1991;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 41.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell-Surface, Heparin-Like Molecules Are Required for Binding of Basic Fibroblast Growth-Factor to Its High-Affinity Receptor. Cell. 1991;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 42.Masuoka K, Ishihara M, Asazuma T, Hattori H, Matsui T, Takase B, Kanatami Y, Fujita M, Saito Y, Yura H, Fujikawa K, Nemoto K. The interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials. 2005;26(16):3277–3284. doi: 10.1016/j.biomaterials.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 43.Maccarana M, Casu B, Lindahl U. Minimal Sequence in Heparin Heparan-Sulfate Required for Binding of Basic Fibroblast Growth-Factor. J Biol Chem. 1993;268(32):23898–23905. [PubMed] [Google Scholar]
- 44.Guimond S, Maccarana M, Olwin BB, Lindahl U, Rapraeger AC. Activating and Inhibitory Heparin Sequences for Fgf-2 (Basic Fgf) - Distinct Requirements for Fgf-1, Fgf-2, and Fgf-4. J Biol Chem. 1993;268(32):23906–23914. [PubMed] [Google Scholar]
- 45.Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2) J Biomed Mater Res. 2002;62(1):128–135. doi: 10.1002/jbm.10238. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura S, Ishihara M, Obara K, Masuoka K, Ishizuka T, Kanatani Y, Takase B, Matsui T, Hattori H, Sato T, Kariya Y, Maehara T. Controlled release of fibroblast growth factor-2 from an injectable 6-O-desulfated heparin hydrogel and subsequent effect on in vivo vascularization. Journal of Biomedical Materials Research Part A. 2006;78A(2):364–371. doi: 10.1002/jbm.a.30688. [DOI] [PubMed] [Google Scholar]
- 47.Nie T, Baldwin A, Yamaguchi N, Kiick KL. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J Control Release. 2007;122(3):287–296. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Furst EM, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide-polysaccharide interactions. J Control Release. 2006;114(2):130–142. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nikitovic D, Assouti M, Sifaki M, Katonis P, Krasagakis K, Karamanos NK, Tzanakakis GN. Chondroitin sulfate and heparan sulfate-containing proteoglycans are both partners and targets of basic fibroblast growth factor-mediated proliferation in human metastatic melanoma cell lines. Int J Biochem Cell Biol. 2008;40(1):72–83. doi: 10.1016/j.biocel.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 50.Dziewiatkowski DD. Isolation of Chondroitin Sulfate-S-35 from Articular Cartilage of Rats. J Biol Chem. 1951;189(1):187–190. [PubMed] [Google Scholar]
- 51.Einbinder J, Schubert M. Separation of Chondroitin Sulfate from Cartilage. J Biol Chem. 1950;185(2):725–730. [PubMed] [Google Scholar]
- 52.Schneiders W, Reinstorf A, Ruhnow M, Rehberg S, Heineck J, Hinterseher I, Biewener A, Zwipp H, Rammelt S. Effect of chondroitin sulphate on material properties and bone remodelling around hydroxyapatite/collagen composites. Journal of Biomedical Materials Research Part A. 2008;85A(3):638–645. doi: 10.1002/jbm.a.31611. [DOI] [PubMed] [Google Scholar]
- 53.Porcel C, Lavalle P, Ball V, Decher G, Senger B, Voegel JC, Schaaf P. From exponential to linear growth in polyelectrolyte multilayers. Langmuir. 2006;22(9):4376–4383. doi: 10.1021/la053218d. [DOI] [PubMed] [Google Scholar]
- 54.Porcel C, Lavalle P, Decher G, Senger B, Voegel JC, Schaaf P. Influence of the polyelectrolyte molecular weight on exponentially growing multilayer films in the linear regime. Langmuir. 2007;23(4):1898–1904. doi: 10.1021/la062728k. [DOI] [PubMed] [Google Scholar]
- 55.Jourdainne L, Arntz Y, Senger B, Debry C, Voegel JC, Schaaf P, Lavalle P. Multiple strata of exponentially growing polyelectrolyte multilayer films. Macromolecules. 2007;40(2):316–321. [Google Scholar]
- 56.Lavalle P, Gergely C, Cuisinier FJG, Decher G, Schaaf P, Voegel JC, Picart C. Comparison of the structure of polyelectrolyte multilayer films exhibiting a linear and an exponential growth regime: An in situ atomic force microscopy study. Macromolecules. 2002;35(11):4458–4465. [Google Scholar]
- 57.Shukla A, Fleming KE, Chuang HF, Chau TM, Loose CR, Stephanopoulos GN, Hammond PT. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials. doi: 10.1016/j.biomaterials.2009.11.082. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 58.Smith RC, Riollano M, Leung A, Hammond PT. Layer-by-Layer Platform Technology for Small-Molecule Delivery. Angewandte Chemie-International Edition. 2009;48(47):8974–8977. doi: 10.1002/anie.200902782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaudhary LR, Avioli LV. Identification and activation of mitogen-activated protein (MAP) kinase in normal human osteoblastic and bone marrow stromal cells: Attenuation of MAP kinase activation by cAMP, parathyroid hormone and forskolin. Molecular and Cellular Biochemistry. 1998;178(1-2):59–68. doi: 10.1023/a:1006807221545. [DOI] [PubMed] [Google Scholar]
- 60.Jackson RA, Murali S, Van Wijnen AJ, Stein GS, Nurcombe V, Cool SM. Heparan sulfate regulates the anabolic activity of MC3T3-E1 preosteoblast cells by induction of Runx2. J Cell Physiol. 2007;210(1):38–50. doi: 10.1002/jcp.20813. [DOI] [PubMed] [Google Scholar]
- 61.Muller S, Koenig G, Charpiot A, Debry C, Voegel JC, Lavalle P, Vautier D. VEGF-functionalized polyelectrolyte multilayers as proangiogenic prosthetic coatings. Adv Funct Mater. 2008;18(12):1767–1775. [Google Scholar]
- 62.Facca S, Cortez C, Mendoza-Palomares C, Messadeq N, Dierich A, Johnston APR, Mainard D, Voegel J-C, Caruso F, Benkirane-Jessel N. Active multilayered capsules for in vivo bone formation. Proceedings of the National Academy of Sciences. 107(8):3406–3411. doi: 10.1073/pnas.0908531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



