Abstract
Objective
The authors investigated the changes of cortical sensorimotor activity in functional MRI (fMRI) and functional recovery in spinal cord injury (SCI) patients who had been treated by bone marrow cell transplantation.
Methods
Nineteen patients with SCI were included in this study; ten patients with clinical improvement and nine without. The cortical sensorimotor activations were studied using the proprioceptive stimulation during the fMRI.
Results
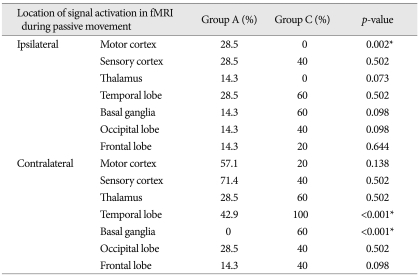
Diagnostic accuracy of fMRI with neurological improvement was 70.0% and 44.4% for sensitivity and specificity, respectively. Signal activation in the ipsilateral motor cortex in fMRI was commonly observed in the clinically neurological improved group (p-value=0.002). Signal activation in the contralateral temporal lobe and basal ganglia was more commonly found in the neurological unimproved group (p-value<0.001). Signal activation in other locations was not statistically different.
Conclusion
In patients with SCI, activation patterns of fMRI between patients with neurologic recovery and those without varied. Such plasticity should be considered in evaluating SCI interventions based on behavioral and neurological measurements.
Keywords: Sensorimotor, Functional MRI, Spinal cord injury, Bone marrow cell, Proprioceptive
INTRODUCTION
Patients with spinal cord injury (SCI) usually have permanent and often devastating neurologic deficits and disability. The cure for SCI has remained unsuccessful in spite of the development of new treatment tools including cell transplantation technology20). This technology has demonstrated that bone marrow cells (BMCs) differentiate into mature neurons or glial cells under specific experimental conditions and that the transplantation of BMCs could promote functional improvements after SCI2). In our previous study, it was suggested that BMC transplantation and administration of granulocyte macrophage colony stimulating factor (GM-CSF) could be one strategy for repairing SCI11,22).
Generally, neurologic status using the American Spinal Injury Association scale (AIS) and electrophysiological methods have been used to assess the therapeutic effect of SCI. The AIS is a proven and effective means of determining the function retained by a patient after trauma to the spinal cord. However, this assessment has been limited to information that could be revealed by external signs and could not reveal the full extent of the condition of the cord, or how the condition changed over time, that is unless a change in the cord's condition was revealed by external signs. Recent experiments using somatosensory-evoked potential (SSEP) or motor evoked potential (MEP) mapping revealed the recovery of corticospinal neural integrity after SCI4,24). However, the use of these electrophysiological techniques in predicting neurological outcomes is still under debate and the standard neurological examination has remained as the first choice for assessing status and probable outcome following SCI13,14). To visualize the corticospinal neuronal integrities in SCI patients, functional brain mapping studies with functional magnetic resolution images (fMRI) and positron emission tomography (PET) have been widely investigated. Functional MRI has been used to study certain clinical problems such as pre-surgical mapping, imaging of the epileptic foci, monitoring recovery after stroke or head trauma, and following treatment using neuropharmaceutical agents16,23). In addition to being a tool used to study the anatomy of the brain, it has also become a powerful tool in the understanding and detection of significant changes in neuronal activity in functional disorders of the brain10).
Previous results obtained from fMRI studies have shown that cortical reorganization appears to be linked to damage in neural pathways. Adaptive reorganization by activation of parallel pathways, local synaptic reorganization or expansion of the functional cortex may additionally compensate for neural injury. It has been indicated that the evidence is unclear for such phenomena in patients with SCI in whom measures of motor cortex reorganization for movement control correlate with disease burden or axon injury17). These cortical functional changes evolve dynamically which is consistent with the notion that they may provide a mechanism to facilitate clinical recovery with an additional synergistic effect of repairing reversible axonal injury.
In the present study, we have described the reorganization of brain activation in patients after SCI and the relationship between their neurologic status and fMRI findings.
MATERIALS AND METHODS
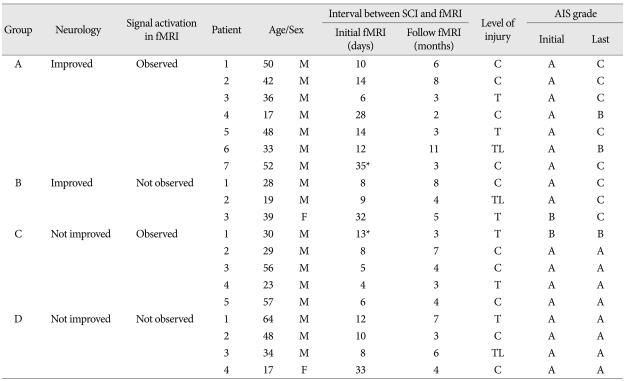
A total of 19 patients, median age 38 years (range 17-64 years), with SCI were included and their demographic data and AIS grade have been provided in Table 1. Seventeen patients had a complete SCI (AIS A) while two had an incomplete (AIS B) SCI. A full neurological examination was performed in order to exclude accompanying neurological disorders of the peripheral and central nervous systems. No patient suffered from a psychiatric disorder or had a history of brain trauma.
Table 1.
Demographic data, clinical data, and AIS grade of 19 patients with SCI
*Activity was observed in the initial fMRI. fMRI : functional magnetic resolution image, SCI : spinal cord injury, AIS : association scale, level of injury C : cervical, level of injury T : thoracic, level of injury TL : thoracolumbar
The patients were grouped into four groups as shown in Table 1. Group A consisted of patients showing neurologic improvement with signal activation on fMRI; Group B included patients showing neurologic improvement without detection of signal activation on fMRI; Group C included patients without neurologic improvement but detection of signal activation on fMRI; and Group D was comprised of patients without neurologic improvement and no detection of signal activation on fMRI. Each group received the same treatment as indicated below.
The BMC transplantation and GM-CSF administration trials were done according to previously reported articles11,22). Briefly, patients first underwent successful spinal cord decompression and spinal fusion and demonstrated persistent complete paralysis below the level of injury. Transplantation was done within 14 days after admission. A laminectomy was performed to provide sufficient access to the transplantation site. After exposure of sufficient surface at the contusion site, 300 µL aliquots of cell paste (total volume of 1.8 mL with 2×108 cells) were injected into six separate points surrounding the margin of the contusion site. After surgery, a total of five cycles (daily for the first 5 days of each month over 5 months) of GM-CSF (Leucogen; LG Life Sciences, Seoul, Korea) was injected subcutaneously (250 µg/m2 of body surface area).
Neurological assessment and electrophysiological testing both were carried out on a number of occasions post-injury in each patient. Functional MRI studies were performed after confirming the complete SCI (median=10 days, range 4-35 days). Patients then underwent successful spinal cord decompression and treatment with BMC transplantation and GM-CSF administration. Follow-up fMRI studies were performed at least 2 months (median=4 months, range 3-11 months) after injury.
The fMRI examination was performed using a 1.5 T GE Signa Horizon scanner. The patients were lying supine with their eyes closed in the scanner and instructed to keep their eyes closed and to maintain relaxation and avoid even minimal movement. The head and the proximal limb were securely fixed to minimize involuntary movements and functional images were acquired using a single-shot echoplanar imaging sequence (TR 3,980 ms, interphase delay 20 ms, TE 60 ms, bandwidth 62.5 kHz, FOV 26×26 cm, matrix size 64×64, slice thickness 5 mm). Each functional image consisted of a time course series of 17 slices and 94 phases (Total 1,598 phases), alternating 4 periods of rest and 4 periods of stimulation (proprio-somesthesic stimulation of the great toe). In each period, 10 scans were performed, excluding 14 initial resting scans. Following the acquisition of the functional data, high-resolution anatomical T1-weighted images were acquired in the same center and field of view. The images were transferred to a personal computer, and fMRI analysis programs were used, statistical parametric mapping (SPM 96, MRC Cyclotron Unit, London, UK) to correct any misalignment from patient movement, and gaussian filtering and high pass filtering to increase the signal : noise ratio and to remove noise. Functional maps were obtained through a pixel-by-pixel analysis, using a t-test with a confidence level of p<0.001, and superimposed over high-resolution anatomical images to display the areas of brain activation. The anatomical location where signal activation on fMRI during passive toe movement was categorized to motor cortex, sensory cortex, basal ganglia, thalamus, frontal lobe, temporal lobe, and occipital lobe according to ipsilateral or contralateral on the passive toe movement side. Stable respiratory and circulatory function by continuous monitoring during at least 1 week prior to fMRI was a prerequisite for inclusion in the study.
This study was approved by the Institutional Review Board at our institution and all procedures were performed after obtaining written informed consent. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Evaluation of the statistical significance of differences in signal activation areas was carried out using Chi-square tests. Tests were considered significant at p-values less than 0.05. Statistical comparisons were performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic data and neurologic assessment
Demographic and clinical data from 19 patients with SCI have been summarized in Table 1. The AIS for each patient on admission and last follow-up, and at the time of the first and last fMRI studies also have been included.
Among the patients with SCI, seven patients showed neurologic improvement with significant fMRI signal changes (Group A). Three patients demonstrated neurologic improvement without showing fMRI signal changes (Group B). Five patients showed no improvement in neurologic function; however, fMRI signal changes were found (Group C). Four patients didn't experience neurologic improvement or fMRI signal changes (Group D).
Seventeen patients had AIS grade A and two patients had AIS grade B. The neurologic status of the group had improved in seven patients from AIS grade A to grade C, in three patients from AIS grade A to grade B, and in one patient from AIS grade B to grade C. The levels of SCI were thoracic lesions in 6 patients, cervical in 10 patients, and thoracolumbar in 3 patients.
The median age of the 19 patients with SCI was 38 years with a range 17-64 years, and there were no significant differences in the mean age between the groups (Group A : 39.7 years, Group B : 28.6, Group C : 39, Group D : 40.75). The median time interval between spinal injuries to the first fMRI was 10 days, ranging from 4-35 days, and the median time interval between spinal injuries to the last fMRI was 4 months, with a range of 3-11 months.
Brain activation during passive toe movement in SCI patients
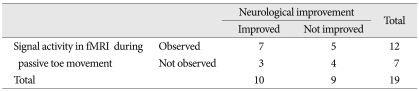
Diagnostic accuracy of fMRI in the assessment of improving SCI patients showed 70.0% and 44.4% for sensitivity and specificity, respectively. Further, 58.3% were positive predictive values and 57.1% were negative predictive values (Table 2).
Table 2.
Diagnostic accuracy of fMRI in the assessment of improving SCI patients
fMRI : functional magnetic resolution image, SCI : spinal cord injury
Preoperative initial fMRI revealed that only two of the 19 patients showed signal activities after toe stimulation (Table 1); however, among these, one patient improved neurological status while the other failed to improve. The signal activity on initial fMRI was not statistically different (p=0.644).
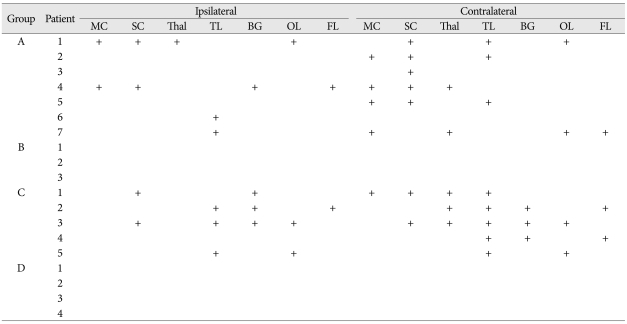
The positive signal activation on follow-up fMRI during passive toe movement was observed in 12 patients and the anatomical location of signal activation in fMRI has been provided in Table 3, 4. The signal activation of the motor cortex in fMRI was observed in total six patients, two signal activations of the ipsilateral motor cortex in group A, and five signal activations of the ipsilateral motor cortex in groups A and C. The signal activation of the ipsilateral motor cortex on fMRI was shown to be statistically significant (p=0.002); however, the activation of contralateral motor cortex was not statistically different. The signal activation of the contralateral temporal lobe and basal ganglia was more commonly found in group C (p<0.001). The signal activation of other locations (sensory cortex, thalamus, frontal lobe and occipital lobe) on fMRI was observed in patients with SCI, but the authors could not find any statistical differences between groups A and C (Table 3, 4).
Table 3.
Cerebral parenchymal locations with positive signal activation on fMRI during passive toe movement
MC : motor cortex, SC : sensory cortex, Thal : thalamus, TL : temporal lobe, BG : basal ganglia, OL : occipital lobe, FL : frontal lobe, + : positive signal activation, fMRI : functional magnetic resolution image
Table 4.
Comparison of the proportion of signal activation on fMRI during passive toe movement between groups A and B according to the cerebral parenchymal location
*Significant statistical difference. fMRI : functional magnetic resolution image
Case illustration
Group A Case 5
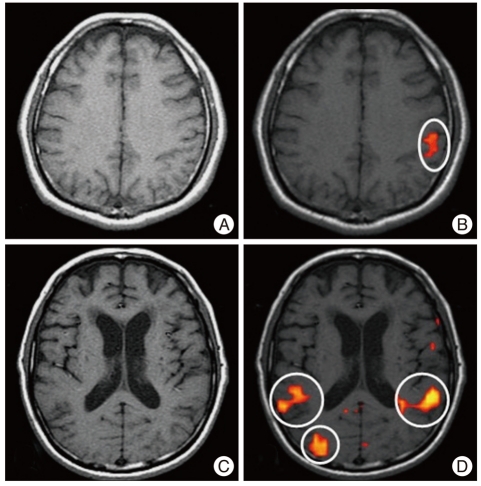
Patient was a 48-year-old man who suffered SCI (AIS grade A) after being injured while repairing a car. The patient could not feel any pin prick or touch sensation below the umbilicus (T10). On initial spinal MRI, T11 and 12 vertebrae were found to be fractured and dislocated. Severe cord contusion was also found. The initial fMRI was done 14 days after injury, and signal activity was not noticed during toe stimulation (Fig. 1A). The patient received BMC transplantation within two weeks of receiving the injury, and GM-CSF was also administered. Sensory improvement was noted 10 days after transplantation. The patient felt dull sensation around the both thigh and knee. Postoperative fMRI was done three months after injury. Following the right great toe controlled repetitive passive toe movement, brain activity was noticed on the contralateral inferior portion of the motor, sensory cortex, and the posterior part of the temporal lobe (Fig. 1B). The last neurologic follow-up at four months showed voluntary flexion of both hips (AIS grade C, muscle power grade III). But, the early sensory recovery of this patient was not confirmed inducing by whether the effect of BMC transplanation or spontaneous recovery.
Fig. 1.
Cortical activation during passive toe movement in two cases of spinal cord injury. A and B : the images of initial and follow-up functional magnetic resolution image (fMRI) for group A case 5. C and D : images of initial and follow-up fMRI for group C case 3.
Group C Case 3
This 56-year-old man was transferred from another institute, and had been operated on with C5-6 anterior and posterior fixation after being injured in a motor vehicle accident. At the initial neurological examination, the patient showed complete quadriplegia (AIS grade A). The patient showed no movement below both wrists and no response of fMRI (Fig. 1C); however, elbow extension movement in both arms was traced. The patient received BMC transplantation approximately two weeks after the injury, and received GM-CSF for five cycles. Significant neurological improvement was not noticed. A postoperative fMRI was done four months after the injury. Following the right great toe proprioceptive stimulation, fMRI activity was noted on the ipsilateral sensory cortex, temporal lobe, occipital lobe, and basal ganglia. Furthermore, the contralateral temporal lobe, occipital lobe, frontal lobe, thalamus, and basal ganglia showed increased signals (Fig. 1D).
DISCUSSION
Transplantation of bone marrow cells and cytokine treatments are emerging therapeutic strategies for SCI in experimental studies1,11,27). Recently, some clinical trials used cell transplantation methods7,12,15). Unfortunately, notwithstanding the remarkable development in therapeutic studies with SCI, our ability to examine the neurologic status of SCI patients has been limited. Some reports using electrophysiological techniques, SSEP or MEP mapping, to investigate corticospinal neural integrity after SCI have been published4,24); however, the use of these to predict neurological outcomes remains under debate and the standard neurological examination remains as the first choice for assessing status and probable outcome following SCI13,14). The current study was performed to verify whether the functional cortical or subcortical changes can occur in patients with SCI after BMC transplantation and GM-CSF treatment. Functional MRI studies have been published using a standardized passive movement to access the neurological status in SCI patients.
In this study, two of 19 patients showed signal activities during passive toe stimulation on preoperative initial fMRI. This initial signal activity did not affect neurological improvement. But, after BMC transplantation and GM-CSF treatment, positive signal activation on follow-up fMRI during passive toe movement was observed in 12 patients. Simply, the proportion of positive signal activity on fMRI increased from 10.5% to 63.2%. This suggests that BMC transplantation and administration of GM-CSF could be a strategy for repairing SCI as suggested in other reports11,22). However, the control group (no BMC transplantation or GM-CSF treatment) was not presented in this study, and therefore we can only do so much to assert the effect of BMC transplantation or GM-CSF treatment.
The positive signal activity on fMRI showed relatively high diagnostic accuracy with respect to sensitivity (70.0%), but relatively low specificity (44.4%) as indicated in Table 2. Approximately 30% (3/10) of patients in the clinical improvement group failed to demonstrate significant signal activities on fMRI. Alternatively, 55% (5/9) of patients in AIS grade A group showed signal activities on fMRI during repetitive passive toe movement. These findings may imply that changes in signal activity do not signify a direct correlation with clinical neurologic status. Interestingly, positive signal activation of the ipsilateral motor cortex on fMRI was commonly observed in the clinically neurological improved group (p=0.002), and signal activation of the contralateral temporal lobe and contralateral basal ganglia was more commonly found in the neurological unimproved group (p<0.001).
The functional reorganization induced by neuronal differentiation in SCI patients appears to be reversible and dynamic. Indeed, the partial motor recovery demonstrated by the paraplegic patients has been associated with activation of the corresponding contralateral sensorimotor cortex5). Comparable data on central motor reorganization in patients with cervical root avulsion also has been shown by transcranial magnetic stimulation after surgical anastomosis with intercostal nerves8). These data can be interpreted in the context of a flexible, plastic functional relationship between different components of sensorimotor circuits in adult humans. Relative preservation of sensorimotor maps, as a result, at the cerebral cortex, brainstem, and thalamic levels, might have occurred after transplantation, driven by the new sensory inputs coming from the lower limb. Potential subcortical structures, such as the basal ganglia and thalamus for the extensive reorganization of the sensorimotor cortical map have been described21,29). These high order subcortical stations, receiving functional influences from the restored sensorimotor inputs, might have allowed the full expression of pre-existing pathways, thus unmasking previously inactive subcortical-cortical or spinal-cortical connections.
Subcortical structures may have been the first site of the functional reorganization. As a consequence of the changes in sensorimotor input-output patterns of the lower limb, the mechanisms responsible for long-term cortical changes might have been induced. The thalamus has been known to process afferent input from the spinal cord. In addition, the thalamus also serves as a relay nucleus to the motor cortex with internal loops from the basal ganglia and the cerebellum, thus matching corticospinal output with spino-cerebellar afferent input18). It has been assumed that by reducing afferent input from the spinal cord, a more complex processing of the remaining input leads to stronger activation, or possibly to disinhibition, of the neuronal centers involved, i.e. the thalamus and basal ganglia. It is also possible that the bilateral cortical changes may be secondary effects mediated by thalamic and basal ganglia inputs. These changes are necessary in order to recover sensorimotor functions in SCI patients with respect to the altered spinal functions.
An increased recruitment of the contra- or ipsilateral primary sensorimotor cortex has already been demonstrated in patients with myelitis and has been related to the time elapsed between the onset of clinical symptoms and evaluation, the level of spinal cord injury, and the degree of daily extremity activity25). In this study, it was found that recruitment of the ipsilateral sensorimotor cortex was a predominant finding in patients with improving neurologic functions (p=0.002) (Table 4). These findings imply that the regeneration of neurons or restoration of spinal cord function could explain the regaining of cortical sensorimotor activity.
Neural remodeling within the sensorimotor networks thus appears also to be possible in the mature nervous system. In an attempt to limit the functional consequences of cord damage, SCI patients tend to recruit regions that are usually activated only in healthy individuals when performing complex or novel tasks. This is also supported by findings showing an increased activation of regions of the sensorimotor network in the frontal (medial and inferior frontal gyrus) and parietal lobes (postcentral gyrus, precuneus, and intraparietal lobule) after SCI5). These regions are reciprocally interconnected and have extensive projections to the primary sensorimotor cortex and the spinal cord.
Since ipsilateral pathways contribute to the control of hand movements in normal adults and that their recruitment seems to increase with increased task complexity6,28), it is likely that the reported ipsilateral SMC activation might represent a compensatory mechanism with the potential to facilitate corticospinal neuronal firing19). According to previous PET and fMRI studies, which also reported a shifted location of the primary motor hand area in patients with SCI, the present study showed that the somatotopic location of the toe areas in both hemispheres was shifted to other areas (Fig. 1D). Sprouting has been described in the spinal cord, but not reported in the cortex9). In addition, its spatial extent has been too limited to explain by itself the activation of lower limb sensorimotor cortex by the hand representation area. However, it may be possible that a more limited interaction in cortico-cortical connections could have affected the large scale recovery of the crucial sensorimotor areas.
In this study, SCI patients also showed an increased recruitment of areas in the temporal and occipital lobes and of another region in the basal ganglia. The regions in the occipital and temporal lobes, which were found to be more activated in SCI patients, are all part of the visual system. It has been suggested that the afferent rewiring can significantly influence the internal connectivity or microcircuitry of the sensory cortex. Interestingly, the transfer of somatosensory information to the occipital cortex is thought to be mediated by connections between parietal and visual associative areas, which were significantly activated in the SCI patients participating in this study. In addition, the lateral temporal cortex and the parahippocampal gyrus, as well as the parietal cortex, have been considered sites of heteromodal neurons where modality-specific sensory inputs are bound into a multimodal representation3,26). Therefore, the increased recruitment of these regions in patients with SCI might be the result of their enhanced firing secondary to the severe cord damage of afferent and efferent pathways to and from the primary sensory cortices. Our results suggest that proprioceptive inputs from the lower-limbs can induce long-term changes in subcortical and cortical sensorimotor maps, as well as the development of new sensorimotor strategies. Furthermore detected fMRI changes following the transplantation may reflect an adaptive role in limiting the clinical outcomes of SCI.
CONCLUSION
In patients with SCI, activation patterns on fMRI for the patients with neurologic recovery and those without were variable. Such plasticity should be considered in evaluating SCI interventions based on behavioral and neurological measurements.
Acknowledgements
This study was supported by the Inha University College of Medicine and the Korean Ministry of Health.
References
- 1.Bouhy D, Malgrange B, Multon S, Poirrier AL, Scholtes F, Schoenen J, et al. Delayed GM-CSF treatment stimulates axonal regeneration and functional recovery in paraplegic rats via an increased BDNF expression by endogenous macrophages. FASEB J. 2006;20:1239–1241. doi: 10.1096/fj.05-4382fje. [DOI] [PubMed] [Google Scholar]
- 2.Callera F, do Nascimento RX. Delivery of autologous bone marrow precursor cells into the spinal cord via lumbar puncture technique in patients with spinal cord injury : a preliminary safety study. Exp Hematol. 2006;34:130–131. doi: 10.1016/j.exphem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci. 2005;22:2886–2902. doi: 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- 4.Costa P, Bruno A, Bonzanino M, Massaro F, Caruso L, Vincenzo I, et al. Somatosensory- and motor-evoked potential monitoring during spine and spinal cord surgery. Spinal Cord. 2007;45:86–91. doi: 10.1038/sj.sc.3101934. [DOI] [PubMed] [Google Scholar]
- 5.Curt A, Bruehlmeier M, Leenders KL, Roelcke U, Dietz V. Differential effect of spinal cord injury and functional impairment on human brain activation. J Neurotrauma. 2002;19:43–51. doi: 10.1089/089771502753460222. [DOI] [PubMed] [Google Scholar]
- 6.Davare M, Duque J, Vandermeeren Y, Thonnard JL, Olivier E. Role of the ipsilateral primary motor cortex in controlling the timing of hand muscle recruitment. Cereb Cortex. 2007;17:353–362. doi: 10.1093/cercor/bhj152. [DOI] [PubMed] [Google Scholar]
- 7.Dobkin BH, Curt A, Guest J. Cellular transplants in China : observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair. 2006;20:5–13. doi: 10.1177/1545968305284675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foltys H, Kemeny S, Krings T, Boroojerdi B, Sparing R, Thron A, et al. The representation of the plegic hand in the motor cortex : a combined fMRI and TMS study. Neuroreport. 2000;11:147–150. doi: 10.1097/00001756-200001170-00029. [DOI] [PubMed] [Google Scholar]
- 9.Fouad K, Pedersen V, Schwab ME, Brösamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol. 2001;11:1766–1770. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- 10.Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, et al. A PET study of the neural systems of stuttering. Nature. 1996;382:158–161. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- 11.Ha Y, Kim YS, Cho JM, Yoon SH, Park SR, Yoon DH, et al. Role of granulocyte-macrophage colony-stimulating factor in preventing apoptosis and improving functional outcome in experimental spinal cord contusion injury. J Neurosurg Spine. 2005;2:55–61. doi: 10.3171/spi.2005.2.1.0055. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Wang H, Chen L, Gu Z, Zhang J, Zhang F, et al. Influence factors for functional improvement after olfactory unsheathing cell transplantation for chronic spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:434–438. [PubMed] [Google Scholar]
- 13.Jacobs SR, Yeaney NK, Herbison GJ, Ditunno JF., Jr Future ambulation prognosis as predicted by somatosensory evoked potentials in motor complete and incomplete quadriplegia. Arch Phys Med Rehabil. 1995;76:635–641. doi: 10.1016/s0003-9993(95)80632-6. [DOI] [PubMed] [Google Scholar]
- 14.Katz RT, Toleikis RJ, Knuth AE. Somatosensory-evoked and dermatomal-evoked potentials are not clinically useful in the prognostication of acute spinal cord injury. Spine (Phila Pa 1976) 1991;16:730–735. doi: 10.1097/00007632-199107000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Knoller N, Auerbach G, Fulga V, Zelig G, Attias J, Bakimer R, et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury : phase I study results. J Neurosurg Spine. 2005;3:173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- 16.Lotze M, Laubis-Herrmann U, Topka H. Combination of TMS and fMRI reveals a specific pattern of reorganization in M1 in patients after complete spinal cord injury. Restor Neurol Neurosci. 2006;24:97–107. [PubMed] [Google Scholar]
- 17.Lotze M, Laubis-Herrmann U, Topka H, Erb M, Grodd W. Reorganization in the primary motor cortex after spinal cord injury - A functional Magnetic Resonance (fMRI) study. Restor Neurol Neurosci. 1999;14:183–187. [PubMed] [Google Scholar]
- 18.Macchi G, Bentivoglio M. Is the "nonspecific" thalamus still "nonspecific"? Arch Ital Biol. 1999;137:201–226. [PubMed] [Google Scholar]
- 19.Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- 20.McDonald JW, Becker D, Holekamp TF, Howard M, Liu S, Lu A, et al. Repair of the injured spinal cord and the potential of embryonic stem cell transplantation. J Neurotrauma. 2004;21:383–393. doi: 10.1089/089771504323004539. [DOI] [PubMed] [Google Scholar]
- 21.Nicolelis MA, Lin RC, Woodward DJ, Chapin JK. Induction of immediate spatiotemporal changes in thalamic networks by peripheral block of ascending cutaneous information. Nature. 1993;361:533–536. doi: 10.1038/361533a0. [DOI] [PubMed] [Google Scholar]
- 22.Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11:913–922. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]
- 23.Roelcke U, Curt A, Otte A, Missimer J, Maguire RP, Dietz V, et al. Influence of spinal cord injury on cerebral sensorimotor systems : a PET study. J Neurol Neurosurg Psychiatry. 1997;62:61–65. doi: 10.1136/jnnp.62.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross IB, Tator CH. Spinal cord blood flow and evoked potential responses after treatment with nimodipine or methylprednisolone in spinal cord-injured rats. Neurosurgery. 1993;33:470–476. doi: 10.1227/00006123-199309000-00017. discussion 476-477. [DOI] [PubMed] [Google Scholar]
- 25.Rocca MA, Agosta F, Martinelli V, Falini A, Comi G, Filippi M. The level of spinal cord involvement influences the pattern of movement-associated cortical recruitment in patients with isolated myelitis. Neuroimage. 2006;30:879–884. doi: 10.1016/j.neuroimage.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey : a retrograde tracer study. J Comp Neurol. 1994;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- 27.Syková E, Jendelová P, Urdzíková L, Lesný P, Hejcl A. Bone marrow stem cells and polymer hydrogels--two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26:1113–1129. doi: 10.1007/s10571-006-9007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- 29.Woods TM, Cusick CG, Pons TP, Taub E, Jones EG. Progressive transneuronal changes in the brainstem and thalamus after long-term dorsal rhizotomies in adult macaque monkeys. J Neurosci. 2000;20:3884–3899. doi: 10.1523/JNEUROSCI.20-10-03884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]