Abstract
Objective
Infection and bone resorption are major complications of cranioplasty and have been well recognized. However, there are few clinical series describing the epidural fluid collection (EFC) as complication of cranioplasty. This study was planned to identify the predictive factors and fate of EFC after cranioplasty.
Methods
We reviewed retrospectively the demographic, clinical, and radiographic data in 59 patients who underwent a first cranioplsty following decompressive craniectomy during a period of 6 years, from January 2004 to December 2009. We compared demographic, clinical, and radiographic factors between EFC group and no EFC group. The predictive factors associated with the development of EFC were assessed by logistic regression analysis.
Results
Overall, 22 of 59 patients (37.3%) suffered from EFC following cranioplasty. EFC had disappeared (n=6, 31.8%) or regressed (n=6, 31.8%) over time on follow up brain computed tomographic (CT) scans. However, 5 patients (22.7%) required reoperation due to symptomatic and persistent EFC. Predictive factors for EFC were male [odds ratio (OR), 5.48; 95% CI, 1.26-23.79], air bubbles in the epidural space (OR, 12.52; 95% CI, 2.26-69.28), and dural calcification on postoperative brain CT scan (OR, 4.21; 95% CI, 1.12-15.84).
Conclusion
The most of EFCs could be treated by conservative therapy. Air bubble in the epidural space and dural calcification are proposed to be the predictive factors in the formation of EFC after cranioplasty.
Keywords: Cranioplasty, Epidural fluid collection
INTRODUCTION
Decompressive craniectomy is widely performed in patients suffering from medically refractory elevation of intracranial pressure and is known to improve clinical outcome1,9,15). The patients who survive after decompressive craniectomy need to undergo cranioplasty.
The complications, including infection, hematoma, and bone graft resorption following cranioplasty have been well studied and considered as a significant cause of postoperative morbidity6,8,11,12). However, reports of epidural fluid collection (EFC) after cranioplasty are uncommon and limited to isolated case examples and small series6,10).
In our neurosurgical practice, we treated patients who developed EFC following cranioplasty. We thus investigated the incidence of EFC and predictive factors associated with its development. Also, this study was focused on the presumptive mechanism of EFC development and its fate.
MATERIALS AND METHODS
We conducted retrospective collection of demographic, clinical, and radiographic data in 59 patients who underwent a first cranioplasty following decompressive craniectomy during a period of 6 years, between January 2004 and December 2009. Patients underwent decompressive craniectomy for the control of increased intracranial pressure.
The decision to undertake cranioplasty depended on the judgment of each individual surgeon. Although operative techniques of cranioplasty were different among neurosurgeons, bone flaps removed during the initial craniectomy were frozen and stored in bone bank. The previous scars were incised, and scalp flap was carefully detached from the underlying dura and brain. Any dural tears and defects were repaired. After achieving meticulous hemostasis in the epidural spaces with bipolar coagulation and hemostatic agents, circumferential and bone flap dural tack-up sutures were placed to reduce epidural dead space. The bone flap was replaced and fixed in place with microscrews and plates. In all patients, a closed epidural and subgaleal drainage system was left in place for 2 to 4 days.
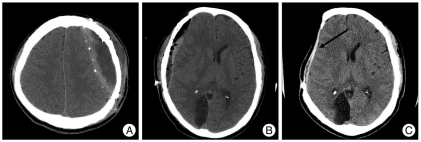
A brain computed tomographic (CT) scan was taken in order to determine EFC, postoperative air bubble in the epidural space, and preoperative dural calcification before cranioplasty within 7 days and after cranioplasty within 3 days (Fig. 1). We defined EFC as low density fluid in the epidural space on brain CT scan within 3 days after cranioplasty.
Fig. 1.
Brain axial computed tomographic scans demonstrating epidural fluid collection (A), postoperative air bubble in the epidural space (B), and preoperative dural calcification (arrow) (C).
The following factors were taken into consideration during analysis : sex, age at time of cranioplasty, initial diagnosis (trauma, subarachnoid hemorrhage, cerebral infarction, and brain tumor), time between craniectomy and cranioplasty, operative time, size of bone flap (3.14 X long axis X short axis), graft material, need to revise cranioplasty, presence of complication, presence of postoperative air bubble in the epidural space, and presence of dural calcification. We excluded the patients who underwent ventriculoperitoneal shunt before cranioplasty.
Next, patients were classified as having EFC and no EFC. Statistical comparisons were made to determine the difference between EFC group and no EFC group. Comparisons between two groups were analyzed using the independent t-test and chi-square test. All data were presented in mean±standard deviation. The result less than p=0.05 was considered statistically significant. We performed multivariate logistic regression analysis with forward selection of variables with probability values less than 0.05 in the univariate analysis. Data were reported with 95% CIs.
RESULTS
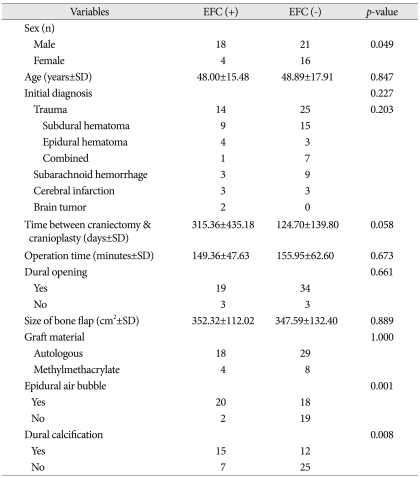
During this study period, 59 patients were examined. Mean age of the study population was 58.5±16.9 years, 39 were men (66.1%) and 20 were women (33.9%). The baseline characteristics of the examined patients are shown in Table 1.
Table 1.
Demographic and clinical data in 59 patients underwent cranioplasty
SD : standard deviation
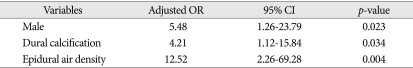
Overall, 22 of 59 patients (37.3%) suffered from EFC following cranioplasty. In the univariate analysis, sex (p=0.049), the presence of postoperative air bubble in the epidural space (p=0.001), and preoperative dural calcification (p=0.008) were statistically significant in EFC group (Table 2). In the multivariate logistic regression analysis, predictive factors for the occurrence of EFC were male [odds ratio (OR), 5.48; 95% CI, 1.26-23.79], air bubbles in the epidural space (OR, 12.52; 95% CI, 2.26-69.28), and dural calcification (OR, 4.21; 95% CI, 1.12-15.84) (Table 3).
Table 2.
Clinical and radiographic data between epidural fluid collection group and no epidural fluid collection group
EFC : epidural fluid collection, SD : standard deviation
Table 3.
Multivariate logistic regression analysis of predictive factors related to epidural fluid collection after cranioplasty
OR : odds ratio, CI : confidence interval
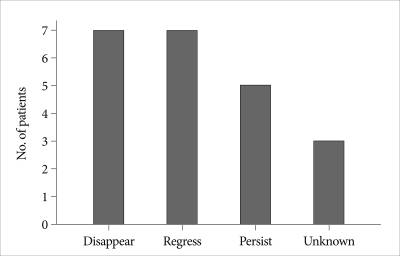
The mean brain CT follow-up periods in EFC group were 11.5±17.9 months (range 0.2-63.7 months). The most of EFC had disappeared (n=6, 31.8%) or regressed (n=6, 31.8%) over time on follow up brain CT scans. However, 5 patients (22.7%) did require reoperation due to symptomatic and persistent EFC (Fig. 2).
Fig. 2.
Bar graph showing the fate of epidural fluid collection over time.
DISCUSSION
The current study demonstrated an overall rate of EFC following cranioplasty of 37.3%. This is notably higher than reported previously6). In a series of 213 patients, Chang et al.6) reported that 13 patients (6.1%) experienced fluid collection complications. However, in this study, there was no explanation for either the definition of EFC or the fate of EFC. There are several factors that may explain why this study revealed that EFC following cranioplasty was far more common than previously suspected. First, we included all patients demonstrating EFC on follow up brain CT within 72 hours, regardless of the amount of EFC. Second, enough EFC to produce a detectable change in the neurological status was a relatively rare event and a substantial number of EFC was disappeared or regressed over time. Accordingly, the true incidence of EFC might have been underestimated.
Data from recent studies have indicated that cranioplasty following decompressive craniectomy was associated with a high complication rates, ranged from 16.4% to 34%4,6,8,12). However, these studies mainly analyzed on major complications including infection, hematoma formation, and bone flap resorption. Therefore, complication rates including minor complication such as EFC were higher in our series.
Although the mechanism of the occurrence of EFC is unknown, we supposed that EFC is not a single entity but rather complex results of several different factors. We postulated that 3 factors might account for this phenomenon. First, cerebrospinal fluid (CSF) leaked through a dural defect which was created by tack up suture or previously damaged dura. Second, dural stiffness due to dural calcification prevented the expansion of brain, resulting in creation of epidural dead space. The mechanism of the calcification is still unclear. However, it is believed to be caused by poor circulation and absorption in the subdural space, cell necrosis, and hyalinization of connective tissue caused by vascular thrombosis2). The time interval between the initial bleeding and the development of calcification varies from 1 month to over 3 years2,5,7). In our practice, although the time interval between craniectomy and cranioplasty was based largely on individual patient recovery, and not standardized, it was longer in patients who developed EFC compared with patients who did not and showed a trend toward significance (p=0.058). Lastly, air bubble in the epidural space might have acted as a precursor of inflammatory process, resulting in the formation of exudate. Aoki3) stressed the significance of air bubble in acute extradural hematoma; the risk of infection and an increase in the possibility of delayed mass effect. Diffusion-weighted magnetic resonance imaging may help distinguish sterile fluid collections from empyema by revealing restricted diffusion in infected collections13,14). Although we did not find the evidence of infection in 5 patients who underwent reoperation due to symptomatic EFC, further study will be needed to elucidate the relationship between the occurrence of EFC and air bubble in the epidural space.
In predicting which patients are most likely to develop EFC after cranioplasty, our data suggest that the most reliable factor is postoperative air bubble in epidural space (OR 12.52, CIs 2.26-69.28). Patients with air bubble demonstrated the greatest predictive factor of EFC when compared with no air bubble (52.6% versus 9.5%, respectively). EFC had disappeared (n=6, 31.8%) or regressed (n=6, 31.8%) over time on follow up brain CT scans. However, 5 patients did require reoperation due to symptomatic and persistent EFC.
This study has some limitations. First, this study is inherently limited by its retrospective analysis including loss of follow-up information, individual discrepancy of follow-up period, and long interval between craniectomy and cranioplasty. As a result, it may not be appropriate to infer direct causal relationships. Second, operative techniques were varied among surgeons. Accordingly, direct comparison in this heterogenous group may have confounded our analysis of predictive factors. However, as far as we know, no authors have analyzed the occurrence of EFC and its predictive factors of development. Thus, despite some limitations, this study may enhance the awareness of EFC as a complication of cranioplasty following craniectomy and may provide additional guidance concerning care of the patient requiring cranioplasty.
CONCLUSION
Air bubble in the epidural space and dural calcification are found to be the predictive factors in the formation of EFC after cranioplasty. The most of EFC could be treated by conservative therapy.
References
- 1.Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469–479. doi: 10.3171/jns.2006.104.4.469. [DOI] [PubMed] [Google Scholar]
- 2.AFRA D. Ossification of subdural hematoma. Reports of two cases. J Neurosurg. 1961;18:393–397. doi: 10.3171/jns.1961.18.3.0393. [DOI] [PubMed] [Google Scholar]
- 3.Aoki N. Air in acute epidural hematoma. Report of two cases. J Neurosurg. 1986;65:555–556. doi: 10.3171/jns.1986.65.4.0555. [DOI] [PubMed] [Google Scholar]
- 4.Beauchamp KM, Kashuk J, Moore EE, Bolles G, Rabb C, Seinfeld J, et al. Cranioplasty after postinjury decompressive craniectomy : is timing of the essence? J Trauma. 2010;69:270–274. doi: 10.1097/TA.0b013e3181e491c2. [DOI] [PubMed] [Google Scholar]
- 5.Chang JH, Choi JY, Chang JW, Park YG, Kim TS, Chung SS. Chronic epidural hematoma with rapid ossification. Childs Nerv Syst. 2002;18:712–716. doi: 10.1007/s00381-002-0664-2. [DOI] [PubMed] [Google Scholar]
- 6.Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010;112:1120–1124. doi: 10.3171/2009.6.JNS09133. [DOI] [PubMed] [Google Scholar]
- 7.Debois V, Lombaert A. Calcified chronic subdural hematoma. Surg Neurol. 1980;14:455–458. [PubMed] [Google Scholar]
- 8.Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy : analysis of 62 cases. Neurosurg Focus. 2009;26:E9. doi: 10.3171/2009.3.FOCUS0962. [DOI] [PubMed] [Google Scholar]
- 9.Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling : indications and results. J Neurosurg. 1999;90:187–196. doi: 10.3171/jns.1999.90.2.0187. [DOI] [PubMed] [Google Scholar]
- 10.Imaizumi S, Owada K, Onuma T, kamii H, Nakajima T. [Epidural fluid collection after cranioplasty using hydroxyapatite ceremics following bone cement] Jpn J Neurosurg (Tokyo) 2000;9:44–47. [Google Scholar]
- 11.Park JS, Lee KS, Shim JJ, Yoon SM, Choi WR, Doh JW. Large defect may cause infectious complications in cranioplasty. J Korean Neurosurg Soc. 2007;42:89–91. [Google Scholar]
- 12.Stephens FL, Mossop CM, Bell RS, Tigno T, Jr, Rosner MK, Kumar A, et al. Cranioplasty complications following wartime decompressive craniectomy. Neurosurg Focus. 2010;28:E3. doi: 10.3171/2010.2.FOCUS1026. [DOI] [PubMed] [Google Scholar]
- 13.Tamaki T, Eguchi T, Sakamoto M, Teramoto A. Use of diffusion-weighted magnetic resonance imaging in empyema after cranioplasty. Br J Neurosurg. 2004;18:40–44. doi: 10.1080/02688690410001660445. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchiya K, Osawa A, Katase S, Fujikawa A, Hachiya J, Aoki S. Diffusion-weighted MRI of subdural and epidural empyemas. Neuroradiology. 2003;45:220–223. doi: 10.1007/s00234-003-0949-5. [DOI] [PubMed] [Google Scholar]
- 15.Whitfield PC, Patel H, Hutchinson PJ, Czosnyka M, Parry D, Melon D, et al. Bifrontal decompressive craniectomy in the management of posttraumatic intracranial hypertension. Br J Neurosurg. 2001;15:500–507. doi: 10.1080/02688690120105110. [DOI] [PubMed] [Google Scholar]