Abstract
Plasma sphingolipids have been shown to predict cognitive impairment and hippocampal volume loss, but there is little research in patients with Alzheimer’s disease dementia (AD). In this study we sought to determine whether plasma ceramides, dihydroceramides (DHCer), sphingomyelins (SM), or dihydrosphingomyelin (DHSM) levels and ratios of SM/ceramide or DHSM/DHCer were predictive of progression in AD. Probable AD patients (n=120) were enrolled in the Alzheimer’s Disease and Memory Disorders Center at Baylor College of Medicine. Plasma sphingolipids were assessed using ESI/MS/MS. Linear mixed effects models were used to examine the relation between baseline plasma sphingolipid levels and cross-sectional and longitudinal performance on the Mini-Mental State Exam (MMSE), Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), and Clinical Dementia Rating-Sum of Boxes (CDR-Sum). Participants were followed a mean of 4.2 visits and 2.3 years. There were no cross-sectional associations. In longitudinal analyses, high levels of DHCer and ceramide were associated with greater progression, but findings did not reach significance (p>0.05). In contrast, higher plasma levels of SM, DHSM, SM/ceramide and DHSM/DHCer ratios were associated with less progression on the MMSE and ADAS-Cog; the ratios were the strongest predictors of clinical progression. Compared to the lowest tertiles, the highest tertiles of DHSM/DHCer and SM/ceramide ratios declined 1.35 points (p=0.001) and 1.19 (p=0.004) less per year on the MMSE and increased 3.18 points (p=0.001) and 2.42 (p=0.016) less per year on the ADAS-Cog. These results suggest that increased SM/ceramide and DHSM/DHCer ratios dose-dependently predict slower progression among AD patients and may be sensitive blood-based biomarkers for clinical progression.
Keywords: Alzheimer’s disease, biomarker, plasma, sphingomyelin, dihydrosphingomyelin, ceramide, dihydroceramide, sphingosine, sphinganine
Introduction
Alzheimer’s disease dementia (AD) is a progressive neurodegenerative disorder. Current available therapies, including cholinesterase inhibitors and memantine, have small symptomatic benefits but are not regarded as disease modifying. While many studies have examined factors associated with incident AD-dementia, fewer studies have focused on factors associated with progression after a diagnosis. Several studies have noted both “fast” and “slow” progressors, demonstrating that the rate of progression after a diagnosis of AD can vary considerably [1–3]. Although much of the variance in progression rate can be explained by cognitive reserve (as estimated by premorbid IQ), intrinsic progression rate, and the relative persistence of anti-dementia drug treatment [4,5], the biological basis for varying rates of progression is not well understood. The identification of a biological marker that could predict rate of progression would be of great benefit to families and caregivers of patients and facilitate the development of more effective therapeutic approaches.
Sphingolipid metabolism is a dynamic process that modulates the formation of a number of bioactive metabolites and second messengers that are critical in cellular signaling. In brain, the proper balance of sphingolipids is essential for normal neuronal function, and subtle changes in sphingolipid balance may be intimately involved in neurodegenerative diseases, including AD ([6, 7] for recent reviews). Sphingomyelins (SM) are major components of cell membranes and are especially enriched in the central nervous system. This class of lipid is a critical component of membrane microdomains known as lipid rafts. These specialized membrane regions are important sites that play roles in signal transduction by regulating protein trafficking, sorting and scaffolding [8]. The processing of APP by beta- and gamma-secretase has specifically been associated with these domains [9]. Ceramides are biologically active sphingolipids that are both precursors to SM synthesis and can be formed by the hydrolysis of SM. A number of ceramide species appear to function as second messengers that regulate a large variety of cellular events including differentiation, proliferation, and apoptosis through temporal and spatial coding that can differentially activate signaling cascades [10]. Clinical, laboratory and animal studies thus far suggest that perturbations in SM and ceramide balance may contribute to the pathophysiology of AD, particularly the formation of amyloid-beta, associated amyloid-plaques, and subsequent neurodegeneration [11–16] Sphingolipid levels in brain tissue of AD patients, compared to cognitively normal controls, have also been found to be altered [12, 16–19].
Previously we reported that blood levels of ceramides and SM were cross-sectionally associated with memory impairment in an epidemiological study [16, 20], and varied by AD severity in a well-defined clinic population of normal controls, amnestic mild cognitive impairment (aMCI) and early probable AD patients [21]. High blood ceramide levels in the latter study were also predictive of one-year cognitive decline and hippocampal volume loss among aMCI patients. However, plasma ceramides and SM have not been assessed as predictors of progression among AD patients. Therefore, in the present study we examined whether blood sphingolipid levels were cross-sectionally associated with, and longitudinally predictive of, cognitive and functional progression in a large, well-characterized group of AD patients. The majority of sphingolipid research conducted to date has focused on SM and ceramides containing a sphingosine backbone. Little is known about the neurobiological functions of dihydroceramides (DHCer) and dihydrosphingomyelins (DHSM), that contain a sphinganine backbone. In particular, the relative distribution of these products has not been examined in autopsy or clinical studies of AD. In this study, we sought to examine plasma ceramides, DHCer, SM, DHSM, and the ratios of SM to Ceramides and DHSM to DHCer as predictors of clinical progression in patients with AD-dementia.
Materials and Methods
Patients
The participants in this study were patients followed in the Alzheimer’s Disease and Memory Disorders Center (ADMDC) at Baylor College of Medicine (BCM) who had agreed to participate in a research database approved by the Institutional Review Board at BCM and met criteria for the diagnosis of probable Alzheimer’s disease (AD) as determined by the NINCDS-ADRDA [22]. All patients underwent an evaluation by a neurologist and completed a standardized dementia workup that has been in continuous use since 1989 [23]. A detailed medical history and interview with the patient and informant, neurological and physical examinations, a neuroimaging scan, neuropsychological testing, and screening laboratory studies were performed at the initial visit. The initial battery of neuropsychological tests is repeated along with neurological and physical exams annually. Patients are given an opportunity to provide blood specimens for storage in a blood bank for future biomarker analyses after additional informed consent. Approximately 5 ml of non-fasting blood is drawn into 3 tubes and frozen at −80 degrees in a freezer whose samples are logged and managed by Freezerworks software. The present study included all AD patients who had stored serum samples and had been seen for a comprehensive assessment in at least two consecutive years.
Cognitive and Global Function Measures
Outcomes reflecting progression of AD-dementia included the Mini Mental Status Exam (MMSE; [24]), Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog; [25]) and the Clinical Dementia Rating (CDR) Scale [26]. The MMSE is a widely used test of global cognition and ranges from 0–30 points. Higher scores reflect better performance. The ADAS-Cog is a global cognitive measure and includes memory, language, and praxis. The error score ranges from 0–70 and higher scores reflect more cognitive impairment. The ADAS-Cog is thought to be more sensitive to smaller changes in cognitive function than the MMSE and is a primary endpoint in AD treatment trials, but the test is less widely used in clinical settings. The CDR examines functioning in six domains: memory, orientation, judgment/problem solving, community affairs, home/hobbies, and personal care. The CDR is assessed with a semi-structured interview and has excellent reliability and validity [27]. Scores include a composite score (CDR-composite) and Sum of Boxes (CDR-Sum), which is the sum of ratings in each of six domains with a range of 0 (no impairment) to 18 (maximum impairment in all domains). The CDR-Sum was chosen as the principal outcome here, instead of the composite, because of its greater range and demonstrated sensitivity to change in MCI and AD (e.g. [28]).
Measurement of Plasma Lipids
Non-fasting blood was drawn at baseline and at annual follow-up exams; plasma was isolated and frozen at −80°C until processing. A crude lipid extract was obtained from plasma samples according to a modified Bligh and Dyer procedure [29]. Three volumes of 100% methanol containing 30 mM ammonium acetate were added to each plasma sample and the mixture was vortexed. Four volumes of chloroform were then added, vortexed and centrifuged at 1,000g for 10 minutes. The bottom (chloroform) layer was removed, dried and re-suspended in 100% methanol for LC/MS/MS.
Ceramides were detected and quantified by LC/MS/MS using multiple reaction monitoring (MRM). This procedure was based on high performance liquid chromatography (HPLC) for temporal resolution of compounds with subsequent introduction into the mass spectrometer for detection and quantification by mass/charge. Samples were injected using a PAL autosampler into a PerkElmer HPLC equipped with a phenomenex, luna 100×2 mm, 5 μm, C18 column coupled with guard column containing identical packing material (Phenomenex, Torrance, CA). For a typical run, the LC column was first pre-equilibrated for 0.5 min with the first mobile phase consisting of 85% methanol, 15% H20, and 5 mM ammonium formate. The column was then eluted with the second mobile phase consisting of 99% methanol, 1% formic acid, and 5 mM ammonium formate at the flow rate of 100.0 μl/min. The eluted sample was then injected into the ion source. The detection, and quantitation of each analyte was carried out by ESI/MS/MS in MRM mode monitoring the parent compound, and products by ion scan.
Molecular species of ceramides, DHCer, SM and DHSM were identified and quantified separately by backbone structure (sphinganine or sphingosine), acyl chain length and saturation (ranging from 16:0 to 26:1 carbons). The levels of all carbon chain lengths within each molecular species (ceramide, DHCer, SM, and DHSM) were highly correlated after adjusting for Bonferroni correction (p<0.0001). We therefore summed the levels of all carbon-chain lengths within each species. There were six main predictive variables of interest: 1) total ceramides; 2) total DHCer; 3) total SM; 4) total DHSM; 5) SM/Ceramide ratio; and 6) DHSM/DHCer ratio.
Ratios were examined for those compounds with identical acyl chain lengths and saturation (for example, SM/ceramide 16:0 was the ratio of SM 16:0 to ceramide 16:0) in addition to total, which was calculated by the summation of all detectable species within each class of sphingolipid (ceramide, SM, DHCer, and DHSM).
Statistical Analysis
The plasma total ceramides, DHCer, SM and DHSM were not normally distributed and, therefore, were log-transformed. Data were also analyzed in tertiles to determine whether there was a dose-response or threshold effect of sphingolipid content on cognition, as has been previously described [21]. To examine the effects of the baseline plasma sphingolipids on dementia progression, we examined the average change in MMSE, ADAS-Cog, and CDR-sum from the visit at which the plasma sphingolipids were assayed, using linear mixed effects models and treating subject-specific intercepts and time as random effects. This approach allowed us to assess the effects of key fixed factors, such as baseline plasma lipid levels, on average rate of change for the MMSE, ADAS-Cog, and CDR-Sum, while accounting for the dependence between within-subject repeated measures.
The following variables were examined as potentially confounding factors: age at the initial visit, sex, race/ethnicity, years of education, presence of cardiovascular disease, diabetes, hypercholesterolemia, and hypertriglyceridemia, BMI, the presence of one or more APOE ε4 alleles, duration of dementia, and the pre-progression rate (previously found to be an important predictor in this sample and calculated as the MMSE score [expected 30] – MMSE score [initial])/physician’s estimate of symptom duration [in years]; [1]. Covariates were retained in the models if the comparison between likelihood ratio (LR) test statistics between models with and without the covariates were significant (p<0.05). Thus, final models included sex, race, duration of dementia, and pre-progression rate. Because higher scores on the MMSE reflect better performance, while higher scores on the ADAS-Cog and CDR-Sum reflect worse performance, a positive sign on the MMSE reflects better performance, but a negative sign on the other outcomes reflects better performance. The a priori p-value was set at p<0.05. All analyses were conducted using STATA Version 11.1 (StataCorp, College Station, TX).
Results
Description of Participant Characteristics
The present study included 120 AD participants with available baseline plasma sphingolipids. The baseline population characteristics are described in Table 1. The participants’ mean age was 71.8 (SD=8.8) years and 60.8% were female. On average, patients were mildly-moderately demented, but there was a range of severities. The baseline sample mean MMSE was 19.9 (SD = 6.7; range 2–29), mean ADAS-Cog was 23.9 (SD=15.0; range 4–68), and mean CDR-Sum was 6.8 (SD=4.0; range 0.5–18). All participants had at least one follow-up visit (mean (SD) = 4.2 (3.2), range 2–16) equating to a mean of 2.3 years (SD = 2.1), range 0.8–12.5) of follow-up after the examination with assays of the plasma sphingolipids. Mean total ceramide, DHCer, SM and DHSM (or specific chain lengths of each lipid class) did not significantly (p>0.1) vary by dementia severity at baseline, as defined by the global CDR scale where 0.5 = very mild dementia; 1 = mild dementia, 2 = moderate dementia, and 3 = severe dementia.
Table 1.
Baseline Demographic, Health–Related Characteristics and Cognitive Performance
| Baseline | ||
|---|---|---|
| Characteristics | N | Mean (SD); n(%) |
| Age | 120 | 71.8 (8.8) |
| Female | 120 | 73 (60.8%) |
| Caucasian | 120 | 107 (89.2%) |
| Years of education | 120 | 13.7 (3.4) |
| Presence of CVD | 99 | 9 (9.1%) |
| Presence of Diabetes | 98 | 6 (6.1%) |
| Hypercholesterolemia | 77 | 28 (49.4%) |
| Hypertriglycerides | 77 | 18 (23.4%) |
| BMI | 85 | 24.8 (5.1) |
| Smoking Status | 119 | |
| Current | 9 (8.0%) | |
| Former | 62 (52.1%) | |
| Never Smoked | 48 (40.3%) | |
| APOE E4 allele | 115 | |
| 0 | 46 (40.0%) | |
| 1 | 48 (41.7%) | |
| 2 | 21 (18.3%) | |
| MMSE | 120 | 19.9 (6.7) |
| ADAS-Cog | 112 | 23.9 (15.0) |
| CDR-Sum | 115 | 6.8 (4.0) |
CVD = Cardiovascuar Disease; MMSE = Mini-Mental State Examination; CDR-Sum = Clinical Dementia Rating Scale - Sum of Boxscores; ADAS-COG = Alzheimer’s Disease Assessment Scale-Cognitive Subscale
Cross-sectional and Longitudinal Relationships Between Plasma Sphingolipids and AD Progression
Linear mixed effects models were used to examine the relationship between plasma ceramide, DHCer, SM, DHSM, SM/ceramide ratio and DHSM/DHCer ratio and cognitive tests, controlling for sex, race, duration of dementia, and pre-progression rate (Table 2). There were no cross-sectional associations between any plasma sphingolipid or ratio and cognitive or functional performance at baseline, but baseline sphingolipids did predict rate of decline. Higher baseline levels of DHCer, as a continuous variable, were predictive of greater decline on the MMSE (b = −0.67, 95% CI: −1.34, 0.00), but not other tests. While there was a similar trend for plasma ceramide levels, this association was not significant at p<0.05. In contrast to the ceramide results, higher levels of plasma DHSM and SM, as both a continuous variable and in tertiles, were associated with less progression, indicated by a positive score on the MMSE and a negative score on the ADAS-Cog (Table 2). For example, compared to the lowest tertile, the highest tertile of DHSM and SM declined 0.84 points (95% CI: 0.01, 1.67) and 1.15 points (95% CI: 0.34, 1.97) less points per year on the MMSE. While higher levels of DHSM and SM were also associated with faster progression on the ADAS-Cog and CDR-Sum, these findings were not as strong.
Table 2.
Baseline Plasma Sphingolipid Levels Predict Progression Among AD participants (n=120)*
| MMSE | ADAS-Cog | CDR-Sum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid (Baseline) | Lipid* time | Lipid (Baseline) | Lipid* time | Lipid (Baseline) | Lipid* time | ||||||||
| Lipid Tertile | beta (95% CI) | p | beta (95% CI) | p | beta (95% CI) | p | beta (95% CI) | p | beta (95% CI) | p | beta (95% CI) | p | |
| Dihydroceramide (DHCer) | |||||||||||||
| Cont. Tertiles | 0.69 (−1.01, 2.40) | 0.424 | −0.67 (−1.34, 0.00) | 0.050 | −1.83 (−5.90, 2.23) | 0.377 | 1.36 (−0.25, 2.23) | 0.098 | 0.33 (−0.86, 1.5) | 0.583 | 0.17 (−0.28, 0.62) | 0.454 | |
| 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | |||||||
| 2 | −0.40 (−2.37, 1.56) | 0.686 | −0.36 (−1.15, 0.42) | 0.364 | 1.69 (−2.99, 6.37) | 0.479 | −0.15 (−2.09, 1.79) | 0.879 | 0.71 (−0.65, 2.07) | 0.304 | 0.01 (−0.52, 0.53) | 0.979 | |
| 3 | 0.38 (−1.59, 2.35) | 0.705 | −0.56 (−1.34, 0.23) | 0.166 | −1.23 (−5.91, 3.46) | 0.608 | 0.79 (−1.14, 2.71) | 0.424 | 0.74 (−0.63, 2.11) | 0.291 | 0.04 (−0.49, 0.57) | 0.882 | |
| Ceramide | |||||||||||||
| Cont. Tertiles | 1.06 (−0.64, 2.76) | 0.223 | −0.41 (−1.10, 0.27) | 0.234 | −3.42 (−7.46, 0.62) | 0.097 | 0.75 (−0.90, 2.40) | 0.374 | −0.12 (−1.32, 1.07) | 0.842 | 0.11 (−0.35, 0.56) | 0.644 | |
| 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | |||||||
| 2 | 1.10 (−0.85, 3.06) | 0.268 | −0.32 (−1.10, 0.46) | 0.424 | −0.24 (−4.88, 4.40) | 0.920 | −0.55 (−2.48, 1.39) | 0.580 | −0.33 (−1.71, 1.05) | 0.639 | 0.08 (−0.44, 0.59) | 0.768 | |
| 3 | 1.46 (−0.51, 3.42) | 0.146 | −0.63 (−1.41, 0.16) | 0.116 | −4.17 (−8.83, 0.49) | 0.238 | 1.26 (−0.67, 3.20) | 0.199 | −0.29 (−1.68, 1.10) | 0.683 | 0.30 (−0.23, 0.82) | 0.272 | |
| Dihydrosphingomyelin (DHSM) | |||||||||||||
| Cont. Tertiles | 0.35 (−0.96, 1.66) | 0.599 | 0.81 (0.27, 1.36) | 0.003 | −1.19 (−4.32, 1.94) | 0.458 | −1.70 (−3.03, −0.37) | 0.012 | −0.57 (−1.48, 0.34) | 0.220 | −0.27 (−0.63, 0.09) | 0.140 | |
| 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | |||||||
| 2 | 0.56 (−1.33, 2.44) | 0.565 | −0.13 (−0.95, 0.69) | 0.758 | −0.80 (−5.34, 3.75) | 0.731 | −0.49 (−2.51, 1.53) | 0.635 | −0.93 (−2.24, 0.38) | 0.163 | 0.05 (−0.49, 0.60) | 0.846 | |
| 3 | 0.81 (−1.08, 2.70) | 0.402 | 0.84 (0.01, 1.67) | 0.046 | −1.49 (−6.03, 3.05) | 0.520 | −1.63 (−3.69, 0.44) | 0.123 | −1.01 (−2.32, 0.29) | 0.127 | −0.29 (−0.84, 0.25) | 0.293 | |
| Sphingomyelin (SM) | |||||||||||||
| Cont. Tertiles | −0.15 (−1.36, 1.05) | 0.804 | 0.86 (0.36, 1.36) | 0.001 | −0.25 (−3.13, 2.63) | 0.863 | −1.58 (−2.80, −0.35) | 0.012 | −0.15 (−0.99, 0.69) | 0.726 | −0.25 (−0.68, −0.03) | 0.035 | |
| 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | |||||||
| 2 | 0.26 (−1.67, 2.19) | 0.792 | 0.16 (−0.64, 0.97) | 0.695 | −1.81 (−6.44, 2.82) | 0.444 | −0.03 (−2.01, 1.96) | 0.979 | 0.19 (−1.15, 1.53) | 0.781 | 0.02 (−0.52, 0.55) | 0.956 | |
| 3 | −0.14 (−2.03, 1.75) | 0.885 | 1.15 (0.34, 1.97) | 0.006 | −0.17 (4.67, 4.32) | 0.940 | −2.05 (−4.05, −0.05) | 0.044 | −0.40 (−1.71, 0.92) | 0.555 | −0.42 (−0.95, 0.12) | 0.127 | |
| DHSM/DHCer Ratio | |||||||||||||
| Cont. Tertiles | −2.17 (−18.37, 14.04) | 0.793 | 12.35 (5.93, 18.78) | <0.001 | 4.49 (−34.67, 43.65) | 0.822 | −25.46 (−40.97, −9.94) | 0.001 | −6.01 (−17.28, 5.26) | 0.296 | −3.58 (−8.07, 0.91) | 0.118 | |
| 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | |||||||
| 2 | 0.23 (−1.69, 2.15) | 0.813 | 1.08 (0.30, 1.87) | 0.007 | 0.02 (−4.64, 4.68) | 0.993 | −2.77 (−4.66, −0.88) | 0.004 | −1.03 (−2.36, 0.30) | 0.130 | −0.31 (−0.86, 0.24) | 0.271 | |
| 3 | −0.77 (−2.71, 1.16) | 0.434 | 1.35 (0.56, 2.13) | 0.001 | 2.12 (−2.58, 6.82) | 0.377 | −3.18 (−5.06, −1.30) | 0.001 | −0.57 (−1.91, 0.78) | 0.409 | −0.39 (−0.94, 0.16) | 0.163 | |
| SM/Cer Ratio | |||||||||||||
| Cont. Tertiles | −7.61 (−20.78, 5.57) | 0.258 | 0.89 (0.40, 1.37) | <0.001 | 20.12 (−11.58, 51.83) | 0.213 | −1.63 (−2.82, −0.45) | 0.007 | −0.05 (−9.25, 9.15) | 0.991 | −0.33 (−0.66, −0.001) | 0.050 | |
| 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | |||||||
| 2 | −0.86 (−2.77, 1.05) | 0.376 | 0.37 (−0.42, 1.17) | 0.358 | 2.96 (−1.65, 7.57) | 0.208 | −1.08 (−3.01, 0.85) | 0.274 | −0.35 (−1.69, 0.99) | 0.609 | −0.11 (−0.66, 0.43) | 0.684 | |
| 3 | −1.42 (−3.38, 0.53) | 0.154 | 1.19 (0.38, 2.00) | 0.004 | 3.85 (−0.86, 8.57) | 0.109 | −2.42 (−4.40, −0.45) | 0.016 | −0.07 (−1.45, 1.31) | 0.917 | −0.42 (−0.98, 0.15) | 0.148 | |
All models control for sex, race, duration of diagnosis and pre-progression rate
Cont = Continuous; Tertile 1 = Low est, Tertile 3 = Highest
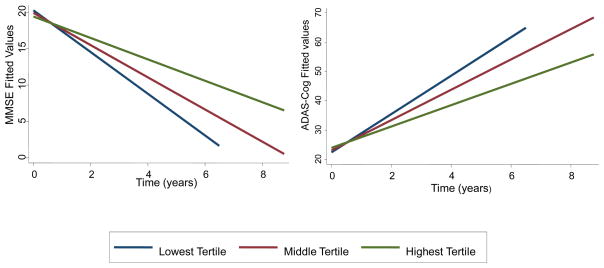
Ceramides are both a precursor to SM (and DHCer to DHSM), and can be formed through the catabolism of SM. We therefore, examined the ratios of these compounds. These ratios were more predictive of progression than any individual lipid species (Table 2 and Figure 1). For example, compared to the lowest tertile, the highest tertile of DHSM/DHCer ratio was associated with 1.35 points more decline per year on the MMSE (95% CI: 0.56, 2.13) and with 3.18 points greater increase per year on the ADAS-Cog (95% CI: 1.30, 5.06).
Figure 1. A higher ratio of DHSM to DHCer is predictive of slower decline on the MMSE and ADAS-Cog in AD patients.
DHSM = dihydrosphingomyelin; DHCer = dihydroceramide; MMSE = Mini-Mental State Examination; ADAS-Cog = Alzheimer’s Disease Assessment Scale-Cognitive Subscale
Sensitivity Analysis
In order to determine whether the above-described results were due to a global lipid change, we further assessed the specificity of these lipids by also examining total cholesterol and triglyceride levels. Among the 120 AD patients with assayed sphingolipids, there were 74 individuals (62%) with clinical information on cholesterol and triglyceride levels, recorded according to whether the blood levels were abnormal (defined as >200 mg/dl for cholesterol and >150 mg/dl for triglycerides) or normal. There was no relationship between baseline total cholesterol or triglyceride levels and progression (Table 3). Despite the lower sample size and power in this subset of samples, baseline plasma sphingolipids still significantly and strongly predicted progression on the MMSE, ADAS-Cog and CDR-Sum.
Table 3.
Baseline Plasma Sphingolipid Levels, But Not Cholesterol or Triglycerides, Predict Progression Among AD participants (n=74)
| MMSE | ADAS −Cog | CDR−Sum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid (Baseline) | Lipid* time | Lipid (Baseline) | Lipid* time | Lipid (Baseline) | Lipid* time | ||||||||
| beta (95% CI) | p | beta (95% CI) | p | beta (95% CI) | p | beta (95% CI) | p | beta (95% CI) | p | beta (95% CI) | p | ||
| Dihydroceramide (DHCer) | |||||||||||||
| Tertiles | 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||||
| 2 | −0.55 (−2.85, 1.75) | 0.640 | −1.10 (−2.04, −0.15) | 0.023 | 2.97 (−2.66, 8.61) | 0.301 | 1.16 (−1.11, 3.42) | 0.316 | 0.91 (−0.70, 2.52) | 0.270 | 0.48 (−0.16, 1.13) | 0.139 | |
| 3 | 0.46 (−1.91, 2.82) | 0.707 | −0.65 (−1.65, 0.36) | 0.207 | −1.55 (−7.32, 4.22) | 0.598 | 0.75 (−1.67, 3.17) | 0.544 | 0.39 (−1.28, 2.06) | 0.648 | 0.13 (−0.55, 0.82) | 0.707 | |
| Ceramide | |||||||||||||
| Tertiles | 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||||
| 2 | 0.34 (−2.00, 2.69) | 0.776 | −0.56 (−1.55, 0.43) | 0.265 | 1.67 (−3.93, 7.26) | 0.559 | 0.78 (−1.55, 3.12) | 0.510 | 0.12 (−1.55, 1.79) | 0.887 | 0.23 (−0.45, 0.90) | 0.506 | |
| 3 | 1.81 (−0.43, 4.05) | 0.113 | −0.62 (−1.57, 0.34) | 0.208 | −5.22 (−10.57, 0.12) | 0.056 | 1.70 (−0.57, 3.96) | 0.142 | −0.63 (−2.24, 0.97) | 0.439 | 0.25 (−0.41, 0.91) | 0.462 | |
| Dihydrosphingomyelin (DHSM) | |||||||||||||
| Tertiles | 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||||
| 2 | 0.54 (−1.73, 2.81) | 0.639 | 0.89 (−0.13, 1.92) | 0.087 | −0.67 (−6.39, 5.04) | 0.818 | −2.77 (−5.18, −0.37) | 0.024 | −1.33 (−2.92, 0.27) | 0.104 | −0.36 (−1.10, 0.38) | 0.335 | |
| 3 | −0.26 (−2.33, 1.82) | 0.810 | 1.56 (0.61, 2.50) | 0.001 | 0.46 (−4.76, 5.69) | 0.862 | −3.00 (−5.28, −0.73) | 0.010 | −0.43 (−1.90, 1.05) | 0.573 | −0.55 (−1.25, 0.15) | 0.122 | |
| Sphingomyelin (SM) | |||||||||||||
| Tertiles | 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||||
| 2 | 0.11 (−2.18, 2.41) | 0.922 | 0.92 (−0.08, 1.92) | 0.073 | −2.96 (−8.65, 2.73) | 0.307 | −1.06 (−3.51, 1.40) | 0.400 | −0.15 (−1.80, 1.51) | 0.861 | −0.35 (−1.08, 0.38) | 0.345 | |
| 3 | −1.31 (−3.36, 0.74) | 0.211 | 1.69 (0.78, 2.61) | <0.001 | 1.82 (−3.24, 6.89) | 0.481 | −2.90 (−5.11, −0.69) | 0.010 | 0.21 (−1.29, 1.71) | 0.781 | −0.52 (−1.20, 0.16) | 0.135 | |
| DHSM/DHCer Ratio | |||||||||||||
| Tertiles | 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||||
| 2 | 0.88 (−1.39, 3.14) | 0.447 | 1.49 (0.50, 2.49) | 0.003 | 1.11 (−6.81, 4.60) | 0.704 | −3.59 (−5.97, −1.22) | 0.003 | −1.03 (−2.36, 0.30) | 0.130 | −0.31 (−0.86, 0.24) | 0.271 | |
| 3 | −0.83 (−2.95, 1.30) | 0.444 | 1.85 (0.93, 2.78) | <0.001 | 1.80 (−3.56, 7.17) | 0.510 | −3.76 (−5.97, −1.55) | 0.001 | −0.57 (−1.91, 0.78) | 0.409 | −0.39 (−0.94, 0.16) | 0.163 | |
| SM/Cer Ratio | |||||||||||||
| Tertiles | 1 | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||||||
| 2 | 0.16 (−2.11, 2.43) | 0.892 | 0.08 (−0.93, 1.09) | 0.877 | 0.28 (−5.34, 5.91) | 0.922 | −0.13 (−2.52, 2.27) | 0.918 | −0.25 (−1.90, 1.40) | 0.768 | −0.02 (−0.75, 0.71) | 0.954 | |
| 3 | −1.69 (−3.79, 0.40) | 0.113 | 1.42 (0.47, 2.36) | 0.003 | 4.53 (−0.65, 9.71) | 0.087 | −2.74 (−5.00, −0.48) | 0.018 | 0.50 (−1.04, 2.04) | 0.526 | −0.52 (−1.22, 0.18) | 0.148 | |
| Abnormal chol | |||||||||||||
| Cont. | 0.001 (−1.84, 1.85) | 0.999 | −0.24 (−1.04, 0.56) | 0.556 | 1.19 (−3.35, 5.73) | 0.607 | −0.44 (−2.33, 1.44) | 0.644 | 0.05 (−1.26, 1.35) | 0.943 | 0.33 (−0.21, 0.88) | 0.232 | |
| Abnormal TG | |||||||||||||
| Cont. | −1.02 (−3.11, 1.06) | 0.337 | 0.47 (−0.45, 1.38) | 0.317 | 2.44 (−2.74, 7.61) | 0.357 | −0.80 (−3.05, 1.44) | 0.482 | −0.51 (−1.99, 0.96) | 0.495 | −0.25 (−0.88, 0.38) | 0.443 | |
All models control for sex, race, duration of diagnosis and pre-progression rate
Discussion
In the present study, we sought to determine if plasma ceramides, SM, DHCer, DHSM, and the ratios of SM to ceramides and DHSM to DHCer were associated with cognitive and functional performance of AD subjects in cross-sectional and longitudinal analyses. Our four primary findings are: 1) there were no cross-sectional relationships between any of the plasma lipids or ratios and performance on the MMSE, ADAS-Cog or CDR-Sum, 2) in longitudinal analyses, low levels of DHCer, and high levels of SM and DHSM predict slower decline on cognitive measures including the MMSE and ADAS-Cog, but not on the CDR-Sum, 3) the ratios of DHSM/DHCer and SM/ceramide were strongly related to cognitive decline and were the most sensitive predictors of progression compared with any individual molecular species of SM or ceramide, and the specific backbone (sphingosine vs. sphingonine) did not appear to overtly affect decline, and 4) plasma cholesterol and triglycerides were not associated with progression on any outcome, suggesting that sphingolipid levels are specific to clinical progression in persons with AD-dementia.
The formation of Aβ-containing plaques, a hallmark of AD pathology, is thought to initiate or contribute to subsequent neuropathology that leads to the symptoms of Alzheimer’s dementia. Pathological modifications in the composition and metabolism of brain lipids is thought to contribute to neuropathological processes in AD by disrupting signaling events and enhancing the formation of Aβ (see [6] for a recent review). A number of sphingolipids, including many ceramides and SMs, are particularly enriched in the central nervous system where in addition to important structural roles, sphingolipid metabolites function as second messengers to modulate a wide variety of signaling events. Both laboratory and animal studies suggest that perturbations in SM and ceramides contribute to the pathophysiology of AD through both direct and indirect connections with Aβ formation, trafficking and clearance [11–15, 30–32]. SM and ceramide are major components of neuronal membranes that strongly influence membrane dynamics, including the structure and function of membrane microdomains. The processing of APP by both β- and γ-secretase enzymes that mediate the formation of pathogenic forms of Aβ, have specifically been associated with sphingolipid levels [9].
Relatively few studies have examined SM and ceramide composition in AD brain tissues and CSF. The studies reported thus far suggest that molecular species of ceramides are elevated and SM are decreased in AD [12, 16, 29, 33, 34]. Ceramides have been found to be increased at the earliest clinically recognizable stage of AD (CDR of 0.5 at the time of death) relative to normal controls [12, 33]. The identification of a blood-based biomarker for clinical progression in this population is especially important as patients with AD-dementia are less likely to be compliant with neuroimaging measures or lumbar puncture for the collection of CSF to measure amyloid-beta and tau. Moreover, information on the trajectory of AD progression would be useful for both caregivers and patients. We previously examined blood sphingolipids and reported that high ceramide levels were associated with an increased risk of memory impairment among cognitively normal controls [20], and were predictive of cognitive decline and hippocampal volume loss in patients with amnestic MCI [21]. In the present study, we examined whether plasma ceramides, DHCer, SM, DHSM, and the ratios of SM to ceramides and DHSM to DHCer were associated with disease severity or could predict cognitive and functional declines among AD-dementia patients. Similar to our previous findings [21], we did not identify a cross-sectional association between individual sphingolipids or ratios of SM to ceramide and outcome measures in this study. However, we did find that sphingolipid levels, especially the ratios of SM/ceramides and DHSM/DHCer, were predictive of clinical progression. These findings, that high levels of SM, DHSM or the ratios of SM/ceramide and DHSM/DHCer are protective, are consistent with findings of lower brain SM in patients with AD-dementia. While higher levels of SM and DHSM, and higher ratios of SM/ceramides and DHSM/DHCer were predictive of slower cognitive decline on the MMSE and ADAS-Cog, there was not an association between these lipids and functional decline on the CDR-Sum. The reason for this discrepancy is not readily apparent, but may relate to the fact that the CDR includes investigation of several domains in addition to cognition, and progression on this measure may be influenced by factors other than, or in addition to, AD-related pathology.
It is interesting to note that our previous studies of cognitively normal controls and amnestic MCI cases found that high ceramide levels were predictive of cognitive progression. In this cohort of AD-dementia patients SM, DHSM, and the SM/ceramide and DHSM/DHCer ratios were most predictive. Together, these results suggest a shift in the metabolic pathways from ceramide to SM over the course of AD and that SM, DHSM, and the SM/ceramide and DHSM/DHCer ratios may be useful predictors of clinical progression in later stages of AD. Although the mechanisms for this shift in sphingolipid metabolism are not currently clear, it is a matter of investigation in our laboratories and by other groups.
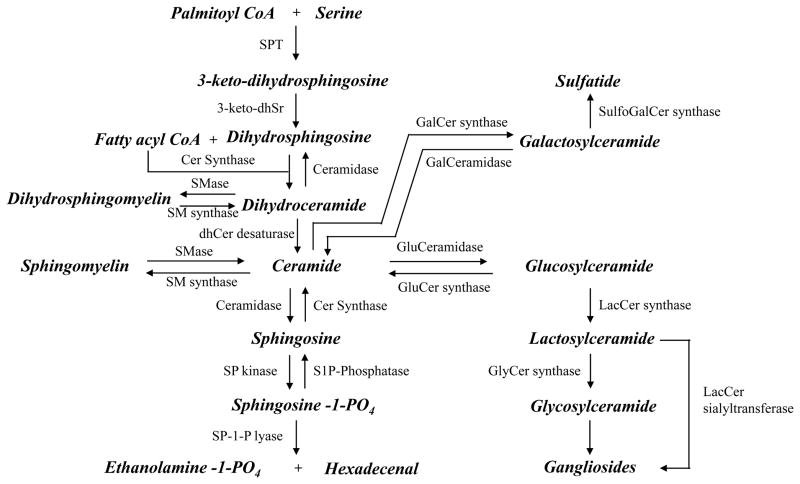
Very few studies in the CNS or of CNS-related disorders have measured DHSM or DHCer, despite the abundance of these sphingolipids in brain [35]. DHSM influences lipid-lipid hydrogen bonding and increases the order in lipid bilayers by increasing the lateral packing density and stability of membrane microdomains [36]. These biophysical effects can modify the location and function of membrane located receptors and signaling proteins with implications for subsequent signaling events (see [6] for a further discussion of sphingolipids and cellular signaling). DHSM is created through dihydroceramide (DHCer), an intermediate in de novo sphingolipid biosynthesis that appears to be efficiently converted to DHSM, dihydroglucosylceramide, and a number of other sphingolipids. However, DHCer is also converted to other complex sphingolipids that contain a desaturated (sphingosine) backbone through desaturase(s) that introduce the 4,5-trans double bond (Figure 2). While the present results suggest that DHCer may be slightly more predictive of progression on the MMSE and ADAS-Cog than ceramides, there was very little difference between these two forms in terms of predictive value. Similarly, while DHSM/DHCer may be a slighter greater predictor of progression, there was little difference when compared to SM/ceramide. Thus, our data would suggest that a particular sphingolipid backbone is not associated with clinical progression, but further research is needed to confirm these conlusions, especially at earlier stages of disease.
Figure 2. Biochemical pathways of sphingolipid metabolism.
Products are indicated in bold and italics. Abbreviations for enzymes are as follows: SPT = Serine palmitoyl transferase, 3-keto-dhSr= 3-keto-dihydrosphingosine reductase, dhCer desaturase = dihydroceramide desaturase, SP-Phosphatase = Sphingosine phosphate phosphatase, SP kinase = Sphingosine kinase, Cer kinase = Ceramide kinase, Cer Synthase = Ceramide synthase, Cer-1-P Phosphatase = Ceramide-1-phosphate phosphatase, SMase = Sphingomyelin synthase, GluCer synthase = glucosylceramide synthase, GluCeramidase = glucosyl ceramidase, GalCer synthase = galactosylceramide synthase, LacCer synthase = Lactosylceramide synthase, GlyCer synthase = Glycosylceramide synthase, LacCer sialytransferase = Lactosylceramide alpha-2,3-sialyltransferase.
There are limitations of this study that do warrant consideration. First, participants were not fasting at the time of the blood draw. A recent paper [37] of ten young, healthy adults has shown that fasting status can affect levels of some blood sphingolipids. While blood glucose levels were not available for all participants in the present study, we have adjusted for blood glucose in previous studies to approximate the time and composition of their last meal and found that this adjustment did not affect the relationship between blood sphingolipids and cognitive progression [20, 21]. Second, information concerning factors that could be associated with both sphingolipids and dementia, including BMI and diagnoses of diabetes and cardiovascular conditions, were not available on all subjects. Nonetheless, controlling for these variables among those for whom this data was available did not alter the relationship between the plasma sphingolipids and AD progression. Lastly, patients with AD-dementia commonly lose weight, which could affect blood lipid levels. As a result, we determined if plasma total cholesterol and triglycerides were associated with AD progression in a subset of subjects for whom these measures were available and we did not find a relationship between total cholesterol and triglycerides and AD progression. We have also shown the specificity of sphingolipids in predicting cognitive impairment and AD in a previous study of cognitively normal controls [20]. However, additional research examining other lipids including phospholipids, glycolipids and fatty acids is warranted.
While the development of a plasma biomarker that is predictive of AD progression would be a significant step forward in the field, the identification of a blood-based marker is complex because many molecules do not cross the blood-brain barrier and the relationship between peripheral molecules and brain metabolism is difficult to establish. We have previously hypothesized that the effects of plasma sphingolipids on cognitive progression among normal controls, amnestic MCI and AD patients could be through direct and/or indirect mechanisms [7]. Using matched CSF and serum samples from ongoing studies with HIV-infected participants we have found a significant correlation between plasma and CSF ceramides and SM [29], suggesting that there may be a direct relationship between blood and brain sphingolipid levels. Additional research examining the blood-CSF relationship of sphingolipids in AD is ongoing in our laboratory. Further, both ceramides and SM have been associated with an increased risk of cardiovascular disease and insulin resistance [38–41], both of which have been associated with an increased risk of AD. Thus, plasma sphingolipids could increase risk of AD indirectly through their increased risk of vascular diseases. Large, longitudinal studies of these lipids with adequate vascular measures are needed to assess whether vascular factors mediate or moderate the effects of plasma sphingolipids on AD development and progression. Nonetheless, while the exact mechanism remains unknown, our present results provide additional evidence that sphingolipid levels in blood may be a sensitive, and easily accessible, biomarker that could be useful in determining and predicting the course and progression of AD.
Acknowledgments
Acknowledgements including sources of support
This study was funded by a grant to both institutions from George P. Mitchell and the late Cynthia W. Mitchell and by the National Institute of Aging (U01 AG037526 and R21 AG28754).
Footnotes
Disclosure: All authors report no conflicts of interest. All authors had full access to all the data in the study and had final responsibility of the decision to submit for publication.
References
- 1.Doody RS, Massman P, Dunn JK. A method for estimating progression rates in Alzheimer disease. Arch Neurol. 2001;58:449–454. doi: 10.1001/archneur.58.3.449. [DOI] [PubMed] [Google Scholar]
- 2.Johnsen S, Hughes S, Bullock R, Hindmarch I. Prediction of the rate of decline in cognitive function in Alzheimer’s disease: a model based on simple demographic data and widely used rating scales. Dement Geriatr Cogn Disord. 2003;16:276–282. doi: 10.1159/000072813. [DOI] [PubMed] [Google Scholar]
- 3.Tschanz JT, et al. Progression of Cognitive, Functional and Neuropsychiatric Symptom Domains in a Population Cohort with Alzheimer’s Dementia The Cache County Dementia Progression Study. American Journal of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e3181faec23. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rountree SD, Chan W, Pavlik VN, Darby EJ, Siddiqui S, Doody RS. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimers Res Ther. 2009;1:7. doi: 10.1186/alzrt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doody RS, Pavlik V, Massman P, Rountree S, Darby E, Chan W. Predicting progression of Alzheimer’s disease. Alzheimers Res Ther. 2010;2:2. doi: 10.1186/alzrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haughey NJ, Bandaru VV, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim Biophys Acta. 2010;1801:878–886. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mielke MM, Lyketsos CG. Alterations of the sphingolipid pathway in Alzheimer’s disease: new biomarkers and treatment targets? Neuromolecular Med. 2010;12:331–340. doi: 10.1007/s12017-010-8121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London E, Brown DA. Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim Biophys Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 9.Hur JY, Welander H, Behbahani H, Aoki M, Franberg J, Winblad B, Frykman S, Tjernberg LO. Active gamma-secretase is localized to detergent-resistant membranes in human brain. Febs J. 2008;275:1174–1187. doi: 10.1111/j.1742-4658.2008.06278.x. [DOI] [PubMed] [Google Scholar]
- 10.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–728. doi: 10.1016/s0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 11.Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- 12.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer’s disease. J Biol Chem. 2004;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D, Simons K. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- 15.Mattson MP, Cutler RG, Jo DG. Alzheimer peptides perturb lipid-regulating enzymes. Nat Cell Biol. 2005;7:1045–1047. doi: 10.1038/ncb1105-1045. [DOI] [PubMed] [Google Scholar]
- 16.He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol Aging. 2010;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soderberg M, Edlund C, Alafuzoff I, Kristensson K, Dallner G. Lipid composition in different regions of the brain in Alzheimer’s disease/senile dementia of Alzheimer’s type. J Neurochem. 1992;59:1646–1653. doi: 10.1111/j.1471-4159.1992.tb10994.x. [DOI] [PubMed] [Google Scholar]
- 18.Gottfries CG, Karlsson I, Svennerholm L. Membrane components separate early-onset Alzheimer’s disease from senile dementia of the Alzheimer type. Int Psychogeriatr. 1996;8:365–372. doi: 10.1017/s1041610296002736. [DOI] [PubMed] [Google Scholar]
- 19.Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res. 2001;26:771–782. doi: 10.1023/a:1011603916962. [DOI] [PubMed] [Google Scholar]
- 20.Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010;31:17–24. doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mielke MM, Haughey NJ, Ratnam Bandaru VV, Schech S, Carrick R, Carlson MC, Mori S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimers Dement. 2010;6:378–385. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Doody R, Pavlik V, Massman P, Kenan M, Yeh S, Powell S, Cooke N, Dyer C, Demirovic J, Waring S, Chan W. Changing patient characteristics and survival experience in an Alzheimer’s center patient cohort. Dement Geriatr Cogn Disord. 2005;20:198–208. doi: 10.1159/000087300. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, Sano M, Bieliauskas L, Geldmacher D, Clark C, Thal LJ. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S13–21. [PubMed] [Google Scholar]
- 26.Hughes C, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. The British Journal of Dementia. 1982:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 177–178. [DOI] [PubMed] [Google Scholar]
- 28.Pavlik VN, Doody RS, Massman PJ, Chan W. Influence of premorbid IQ and education on progression of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:367–377. doi: 10.1159/000095640. [DOI] [PubMed] [Google Scholar]
- 29.Bandaru VV, Troncoso J, Wheeler D, Pletnikova O, Wang J, Conant K, Haughey NJ. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer’s but not normal brain. Neurobiol Aging. 2009;30:591–599. doi: 10.1016/j.neurobiolaging.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 31.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 32.Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004;164:123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han X, DMH, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 34.Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, Oka N, Akiguchi I, Furuya S, Hirabayashi Y, Okazaki T. Astroglial expression of ceramide in Alzheimer’s disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130:657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 35.Byrdwell WC, Perry RH. Liquid chromatography with dual parallel mass spectrometry and (31)P nuclear magnetic resonance spectroscopy for analysis of sphingomyelin and dihydrosphingomyelin. I. Bovine brain and chicken egg yolk. J Chromatogr A. 2006;1133:149–171. doi: 10.1016/j.chroma.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Tirri LJ, Ayengar NK, Lipton LC, Chatterjie N, Brockerhoff H. Studies on the hydrogen belts of membranes: III. Glycerol permeability of dihydrosphingomyelin-cholesterol membranes. Lipids. 1978;13:267–269. doi: 10.1007/BF02533668. [DOI] [PubMed] [Google Scholar]
- 37.Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, Klein RL, Hannun YA, Bielawski J, Bielawska A. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res. 2010;51:3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ichi I, Nakahara K, Miyashita Y, Hidaka A, Kutsukake S, Inoue K, Maruyama T, Miwa Y, Harada-Shiba M, Tsushima M, Kojo S. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859–863. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- 39.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 40.Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol. 2010;21:128–135. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- 41.Blachnio-Zabielska AU, Pulka M, Baranowski M, Nikolajuk A, Zabielski P, Gorska M, Gorski J. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. J Cell Physiol. 2011 doi: 10.1002/jcp.22745. Epub ahead of print. [DOI] [PubMed] [Google Scholar]