Abstract
Ionotropic glutamate receptors, especially the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor subtype, undergo dynamic trafficking between the surface membrane and intracellular organelles. This trafficking activity determines the efficacy and strength of excitatory synapses and is subject to modulation by changing synaptic inputs. Given the possibility that glutamate receptors in the central nervous system might be a sensitive target of anesthetic agents, this study investigated the possible impact of anesthesia on trafficking and subcellular expression of AMPA receptors in adult mouse brain neurons in vivo. We found that anesthesia induced by a systemic injection of pentobarbital did not alter total protein levels of three AMPA receptor subunits (GluR1–3) in cortical neurons. However, an anesthetic dose of pentobarbital reduced GluR1 and GluR3 proteins in the surface pool and elevated these proteins in the intracellular pool of cortical neurons. The similar redistribution of GluR1/3 was observed in mouse striatal neurons. Pentobarbital did not significantly alter GluR2 expression in the two pools. Chloral hydrate at an anesthetic dose also reduced surface GluR1/3 expression and increased intracellular levels of these proteins. The effect of pentobarbital on subcellular distribution of AMPA receptors was reversible. Altered subcellular distribution of GluR1/3 returned to normal levels after the anesthesia subsided. These data indicate that anesthesia induced by pentobarbital and chloral hydrate can alter AMPA receptor trafficking in both cortical and striatal neurons. This alteration is characterized by the concurrent loss and addition of GluR1/3 subunits in the respective surface and intracellular pools.
Keywords: pentobarbital, chloral hydrate, glutamate, GluR1, GluR3, trafficking
INTRODUCTION
Glutamatergic transmission plays a central role in regulating neuronal and synaptic activity in the central nervous system. Glutamate released from glutamatergic nerve terminals interacts with postsynaptic glutamate receptors, mainly ionotropic and metabotropic glutamate receptors, to modulate postsynaptic neurons (Dingledine et al., 1999). Ionotropic glutamate receptors are broadly distributed in the brain and are divided into different subtypes based on their pharmacological properties. The α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor is the key subtype that mediates fast synaptic transmission. Like other subtypes of ionotropic glutamate receptors, the AMPA receptor is a ligand-gated ion channel. Activation of this channel allows small cation ion influxes (Na+), resulting in excitatory postsynaptic currents. The AMPA receptor becomes functional when they are assembled into a homo- or heteromeric complex with various compositions of different subunits (Mansour et al., 2001; Greger et al., 2003). Variable AMPA receptor subunit stoichiometry renders a biochemical basis for the functional distinction of AMPA receptors and for multiple models of AMPA receptor-mediated synaptic transmission and plasticity (Dingledine et al., 1999).
AMPA receptor function is also modulated by their trafficking and subcellular localization. As an ion channel exclusively expressed in a membrane-bound form, AMPA receptors are normally bound to either the surface membrane or the membrane of intracellular organelles, including endoplasmic reticulum, Golgi apparatus, and vesicles (Kennedy and Ehlers, 2006). The numbers of receptors in surface and intracellular membrane compartments are balanced via reciprocal trafficking (Groc and Choquet, 2006; Kennedy and Ehlers, 2006). Under basal conditions, the receptor is synthesized and transported from the intracellular organelles to the surface membrane (externalization trafficking). Meanwhile, the surface receptor is removed through endocytosis to the intracellular sites (internalization trafficking). These trafficking activities regulate the number of receptors at synaptic sites and determine the efficacy and strength of excitatory synapses (Groc and Choquet, 2006; Kennedy and Ehlers, 2006). Of note, trafficking of AMPA receptors is highly sensitive to changing synaptic inputs and represents a key process subjected to the modulation by a variety of extracellular signals (Lissin et al., 1998; Elias and Nicoll, 2007; Newpher and Ehlers, 2008).
Anesthetic agents affect specific molecular targets in the central nervous system to achieve their biological actions. Increasing evidence indicates that the AMPA receptor is among the targets sensitive to anesthetics (Harris et al., 1995; Yamakura et al., 1995a; Krampfl et al., 2005; Haines et al., 2008). However, at present, little is known about the effect of anesthetics on AMPA receptor trafficking in neuronal cells in vivo. In this study, we initiated an effort to investigate the potential impact of general anesthesia on AMPA receptor trafficking in vivo. We selected pentobarbital and chloral hydrate to induce general anesthesia because they are commonly used in biochemical and physiological experiments in laboratory animal studies. We found that anesthesia induced by both drugs led to a significant reduction of AMPA receptor GluR1 and GluR3 subunits in the surface compartment and a proportional increase in these subunits in the intracellular compartment. These data demonstrate a selective loss of GluR1/3 AMPA receptors in the surface membrane following anesthesia in vivo.
MATERIALS AND METHODS
Animals
C57BL/6J male mice (2–3 month old) were used (Charles River, New York, NY) and were individually housed in a controlled environment at a constant temperature of 23°C and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12/12 h light/dark cycle. Mice were allowed 6–7 days of habituation to the animal colony. All procedures performed were approved by the Institutional Animal Care and Use Committee (Kansas City, MO, USA) and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Anesthesia
General anesthesia was induced by intraperitoneal (i.p.) injections of pentobarbital sodium (Nembutal, Abbott Labratories, North Chicago, IL) at a dose of 50 mg/kg. A vehicle solution (50% dH2O, 40% propylene glycol, and 10% ethanol) served as a control. Anesthesia was assessed by loss of righting reflex (LORR), and time to induce LORR was usually between 2–5 min (tested every 20 sec). In some mice, anesthesia was induced by an i.p. injection of chloral hydrate (Sigma, St. Louis, MO) at a dose of 400–500 mg/kg in phosphate-buffered saline (PBS). The animals injected with PBS alone served as a control.
Western blot analysis
Western blots were performed as described previously (Yang et al., 1004; Mao et al., 2005; Liu et al., 2006). Briefly, mice were sacrificed by cervical dislocation and brains were immediately removed. The cerebral cortex and the striatum were quickly dissected on ice. Brain tissues were sonicated in a sample buffer (RIPA) containing 50 mM Tris-HCl, pH 7.5, 1% Nonidet P-40, 4% ionic detergent sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethanesulfonyl fluoride, 5 μg/ml each of aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, and 1 mM NaF. Protein concentrations were determined with a Pierce BCA assay kit (Rockford, IL). The equal amount of proteins was separated on NuPAGE Novex 4–12% gels (Invitrogen, Carsbad, CA). Proteins were transferred to polyvinylidene fluoride membrane (Millipore, Bedford, MA) and blocked in blocking buffer (5% nonfat dry milk and 0.1% Tween 20) for 1 h. The blots were incubated in primary rabbit polyclonal antibodies against GluR1 (Millipore) or actin (Santa Cruz Biotechnology, Santa Cruz, CA), or mouse antibodies against GluR2 (Millipore), GluR3 (Millipore), or α-actinin (millipore) usually at 1:1,000 overnight at 4°C. This was followed by 1 h incubation in goat anti-rabbit or anti-mouse horseradish peroxidase-linked secondary antibodies (Jackson Immunoresearch Laboratory, West Grove, PA) at 1:5000. Immunoblots were developed with the enhanced chemiluminescence reagents (ECL; Amersham Pharmacia Biotech, Piscataway, NJ), and captured into Kodak Image Station 2000R. Kaleidoscope-prestained standards (Bio-Rad, Hercules, CA) were used for protein size determination. The density of immunoblots was measured using the Kodak 1D Image Analysis software, and all bands were normalized to total actin amounts and then to basal values. Values are expressed as percentages of basal values.
In vivo surface receptor crosslinking assays
Assays were performed as described previously (Boudreau and Wolf, 2005; Sun et al., 2008; Mao et al., 2009; LacKamp et al., 2009). Briefly, surface receptor expression was detected using a membrane-impermeable crosslinking agent bis(sulfosuccinimidyl)suberate (BS3), which only crosslinks proteins on the surface of cells. Brains were removed following cervical dislocation. Coronal sections (400 µm) were cut using a vibratome. The cerebral cortex and the striatum including the caudate putamen and nucleus accumbens were dissected from the slices and added into eppendorf tubes containing ice-cold oxygenated artificial cerebrospinal fluid (ACSF) containing (mM) 119 NaCl, 3.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26.2 NaHCO3, and 11 glucose, bubbled continuously with 95% O2/5% CO2 (pH 7.4). BS3 (Pierce, Rockford, IL) was added to 2 mM and incubated with gentle agitation for 30 min at 4°C. The crosslinking reaction was terminated by quenching with 20 mM of glycine (10 min, 4°C). The tissues were then washed four times in cold ACSF (10 min each), homogenized to obtain total protein homogenate, and analyzed directly by SDS-PAGE (4–12% Tris-glycine gels, Invitrogen).
Behavioral assessment
Righting reflex was assessed after administration of pentobarbital or chloral hydrate. Righting reflex scores were evaluated according to the rating scale: a score of 0 indicated a normal right reflex; +1 indicated that the mouse righted itself within 2 s on all three trials (slightly impaired righting reflex); +2 indicated that the latency to righting was > 2 s, but < 10 s at the best response in three trials (moderately or severely impaired righting reflex); +3 corresponded to absence of righting reflex (no righting within 10 s on all three trials) (Irifune et al., 2003).
Statistics
The results are presented as means ± SEM, and were evaluated using a one- or two-way analysis of variance, as appropriate, followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means. Probability levels of < 0.05 were considered statistically significant.
RESULTS
Effects of pentobarbital-induced anesthesia on total AMPA receptor expression
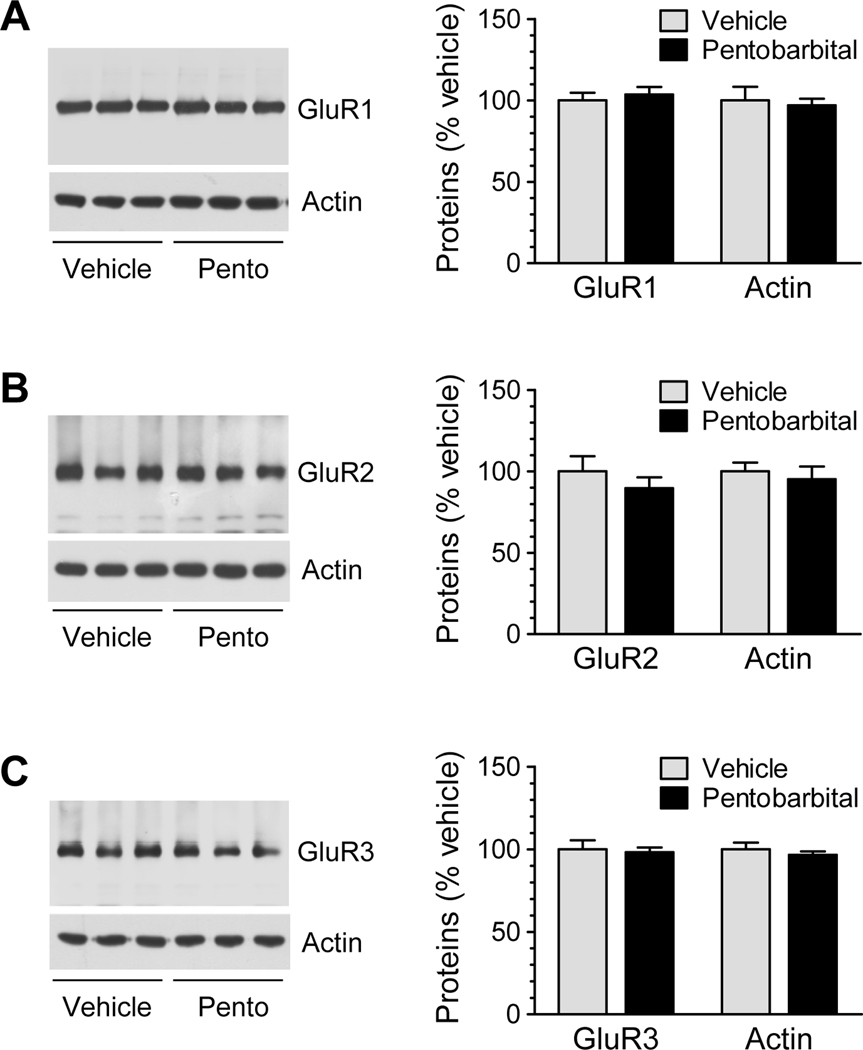
We first examined whether anesthesia has any impact on total cellular levels of AMPA receptors in cortical neurons. We subjected mice to a single injection of pentobarbital at an anesthetic dose (50 mg/kg, i.p.). We confirmed a state of deep anesthesia by observing the loss of righting reflex (LORR) at a few minutes after pentobarbital injection. Anesthetized mice and control animals which received a vehicle injection were sacrificed 15 min after drug injection for analyzing AMPA receptor subunit proteins in cortical neurons. We found that total GluR1 levels in anesthetized animals were not different from that in control animals (Fig. 1A). Similar results were also observed for the other two subunits (Fig. 1B for GluR2, and Fig. 1C for GluR3). Thus, anesthesia induced by pentobarbital appears to have no significant impact on the total cellular AMPA receptor expression in cortical neurons in vivo.
Figure 1.
Effects of pentobarbital-induced anesthesia on total cellular levels of AMPA receptor subunits in mouse cortical neurons. (A–C) Effects of anesthesia on total GluR1 (A), GluR2 (B), and GluR3 (C) expression in cortical neurons. Representative immunoblots from three different animals receiving either vehicle or pentobarbital (Pento) are shown to the left of the quantified data. Anesthesia was induced by pentobarbital at 50 mg/kg (i.p.). Mice were sacrificed 15 min after drug injection. Data are presented as means ± SEM (n = 3–6 per group).
Normal subcellular distribution of AMPA receptors
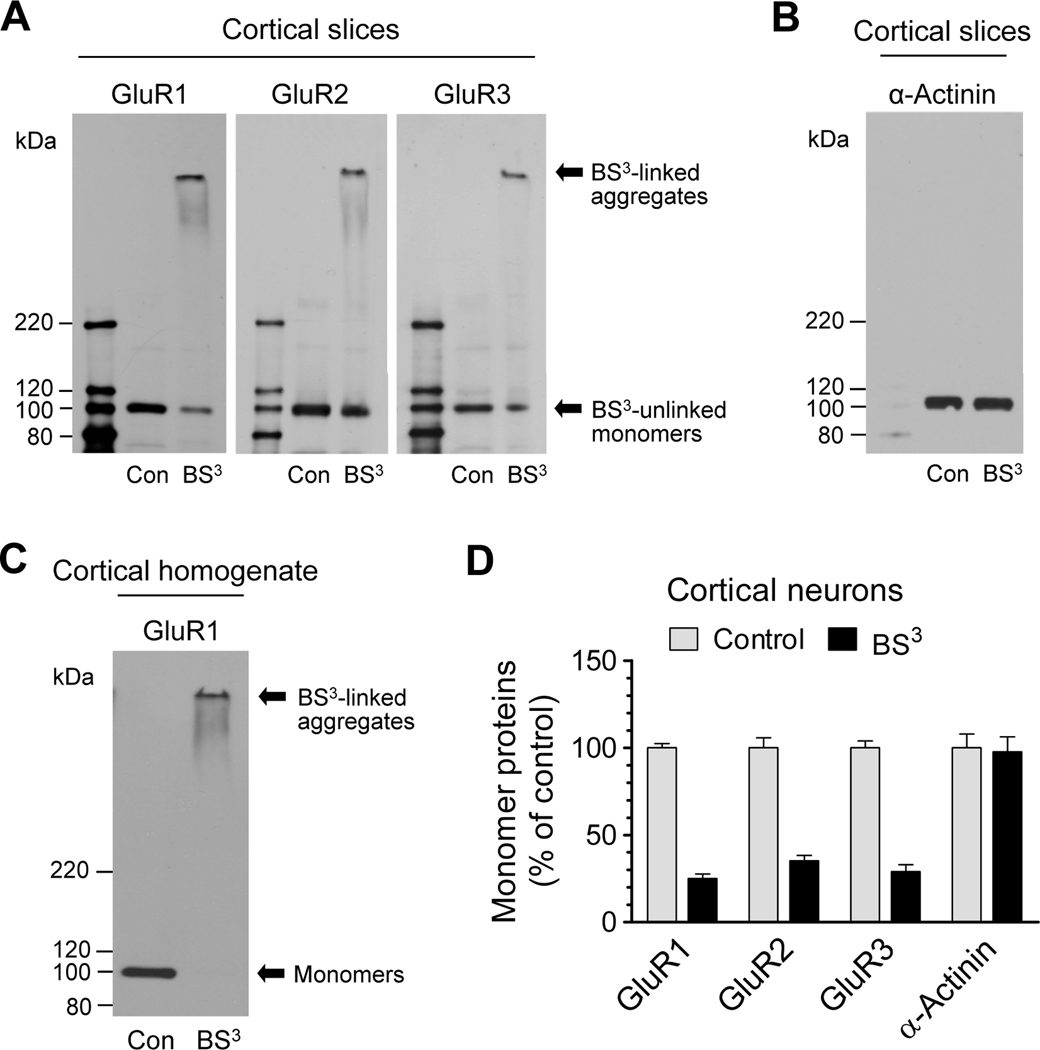
We next investigated whether anesthesia affects AMPA receptor expression in different subcellular compartments. To this end, we first examined normal subcellular distribution of AMPA receptors in mouse cortical neurons. We used a recently developed biochemical crosslinking method to isolate native receptors from different cellular pools in intact cortical neurons in vivo (Boudreau and Wolf, 2005; Sun et al., 2008; Mao et al., 2009; LacKamp et al., 2009). Viable cortical slices were prepared and treated with BS3, a membrane-impermeable reagent selectively crosslinking surface membrane-bound receptors to form high-molecular weight aggregates. These aggregates, on gel electrophoresis, are readily separated from unlinked intracellular receptors bound on light membranes from the intracellular endoplasmic reticulum, Golgi, and vesicular structures. As shown in Fig. 2A, a high molecular weight band (BS3-linked surface receptors) was observed for each subunit in BS3-treated tissue. In addition, a low monomeric molecular weight band (BS3-unlinked intracellular receptors) was shown at a density level less than that in non-BS3-treated control slices (Fig. 2A). The selectivity of BS3 crosslinking was confirmed by the lack of crosslinking effect of BS3 on α-actinin, an intracellular protein (Fig. 2B). Adding BS3 into cortical homogenates completely eliminated the monomeric band of GluR1 (Fig. 2C). Quantification of three subunits in the form of monomers in the intracellular pool revealed a similar distribution pattern (Fig. 2D). Evidently, a smaller portion of these subunits is expressed in the intracellular pool (approximately 20–40%).
Figure 2.
Surface and intracellular expression of AMPA receptors in mouse cortical neurons. (A) Representative GluR1–3 immunoblots from mouse viable cortical slices treated with BS3 or vehicle control (Con). (B) A representative α-actinin immunoblot from mouse viable cortical slices treated with BS3 or vehicle control. (C) An immunoblot of GluR1 from cortical homogenates treated with BS3 or vehicle control. (D) Quantification of monomer subunit protein expression in normal cortical neurons (BS3-unlinked intracellular monomers in BS3-treated samples versus monomers in vehicle control samples). Data are presented as means ± SEM (n = 3–6 per group).
Effects of pentobarbital-induced anesthesia on subcellular expression of AMPA receptors
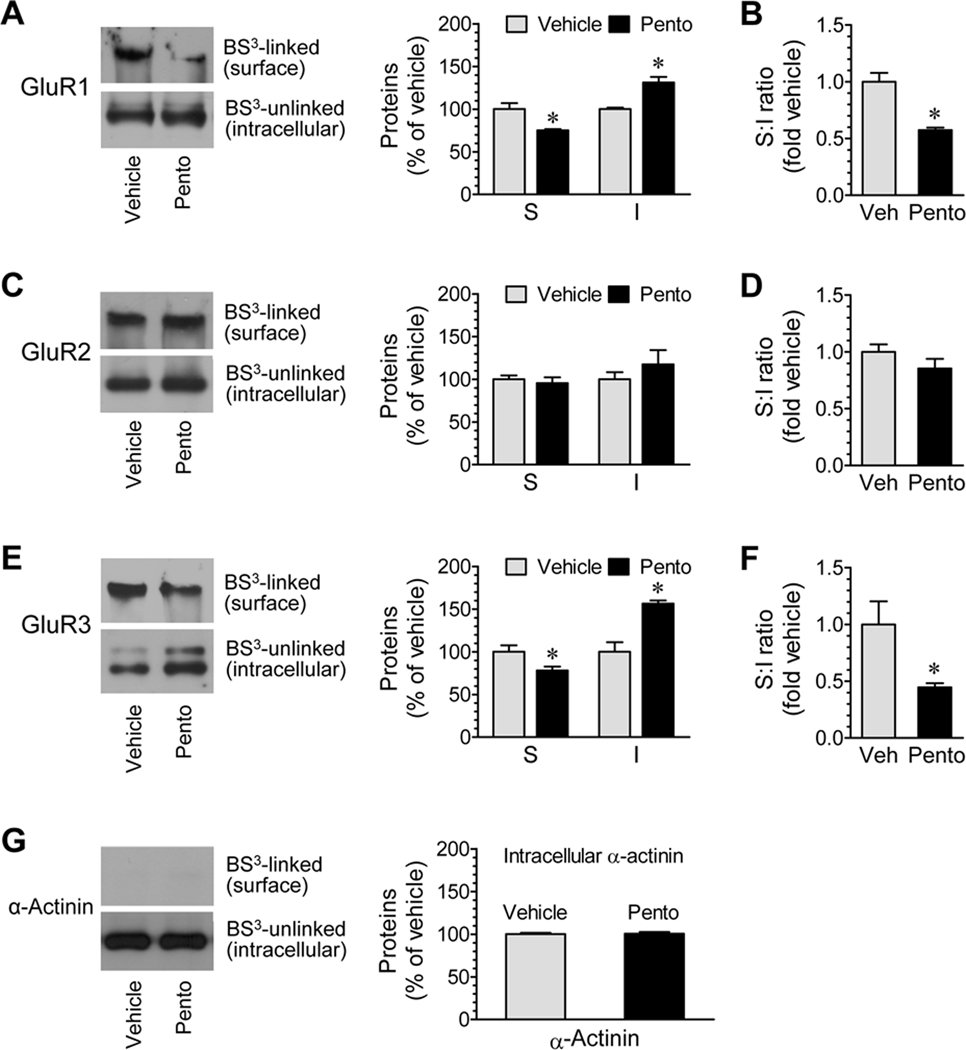
Given that AMPA receptors are normally distributed in both surface and intracellular pools (Boudreau and Wolf, 2005; Van Dolah et al., 2011), we then examined whether anesthesia alters the receptor expression in these defined subcellular pools. Mice were injected with pentobarbital (50 mg/kg, i.p.) and were sacrificed 15 min after drug injection for BS3 crosslinking assays. We found that the amount of BS3-linked GluR1, i.e., surface expressed GluR1, was reduced in anesthetized animals relative to control animals (75.0 ± 1.5% of vehicle, P < 0.05; Fig. 3A). In contrast, BS3-unlinked GluR1 (intracellular GluR1) in anesthetized animals was elevated to 131.2 ± 6.6% of vehicle (P < 0.05; Fig. 3A). The ratio of surface to intracellular GluR1 was significantly lower in anesthetized animals than in control animals due to a decrease and increase in GluR1 in the surface and intracellular pools, respectively (Fig. 3B). Unlike GluR1, GluR2 was less sensitive to anesthesia. Its expression levels in the surface and intracellular pools were not significantly altered following anesthesia (Fig. 3C). Neither was the ratio of surface to intracellular GluR2 altered in anesthetized animals (Fig. 3D). With regard to GluR3, changes in the two pools in opposite directions as seen in GluR1 were observed. A significant decrease in the surface pool (78.2 ± 4.5% of vehicle, P < 0.05; Fig. 3E) accompanied by a marked increase in the intracellular pool (156.5 ± 3.6% of vehicle, P < 0.05; Fig. 3E) was seen after anesthesia. As a result, the ratio of surface to intracellular GluR3 was reduced (Fig. 3F). The intracellular protein α-actinin remained stable in its abundance following anesthesia (Fig. 3G). These data demonstrate an induced redistribution of GluR1/3 AMPA receptors in cortical neurons following anesthesia.
Figure 3.
Effects of pentobarbital-induced anesthesia on AMPA receptor subunit expression in surface (S) and intracellular (I) pools of mouse cortical neurons. (A, C, and E) Effects of anesthesia on GluR1 (A), GluR2 (C), and GluR3 (E) expression in the two pools. (B, D, and F) Effects of anesthesia on the ratio of surface to intracellular expression of GluR1 (B), GluR2 (D), and GluR3 (F). (G) Effects of anesthesia on α-actinin expression. Note that anesthesia reduced GluR1 and GluR3 abundance in the surface pool while increased them in the intracellular pool. Anesthesia was induced by pentobarbital (Pento) at 50 mg/kg (i.p.). Mice were sacrificed 15 min after drug injection. Control mice received a vehicle (Veh) injection. Representative immunoblots are shown left to the quantified data (A, C, and E). The data are expressed in terms of means ± SEM (n = 6 per group). *: P < 0.05 versus vehicle.
Effects of anesthesia on subcellular expression of AMPA receptors in striatal neurons
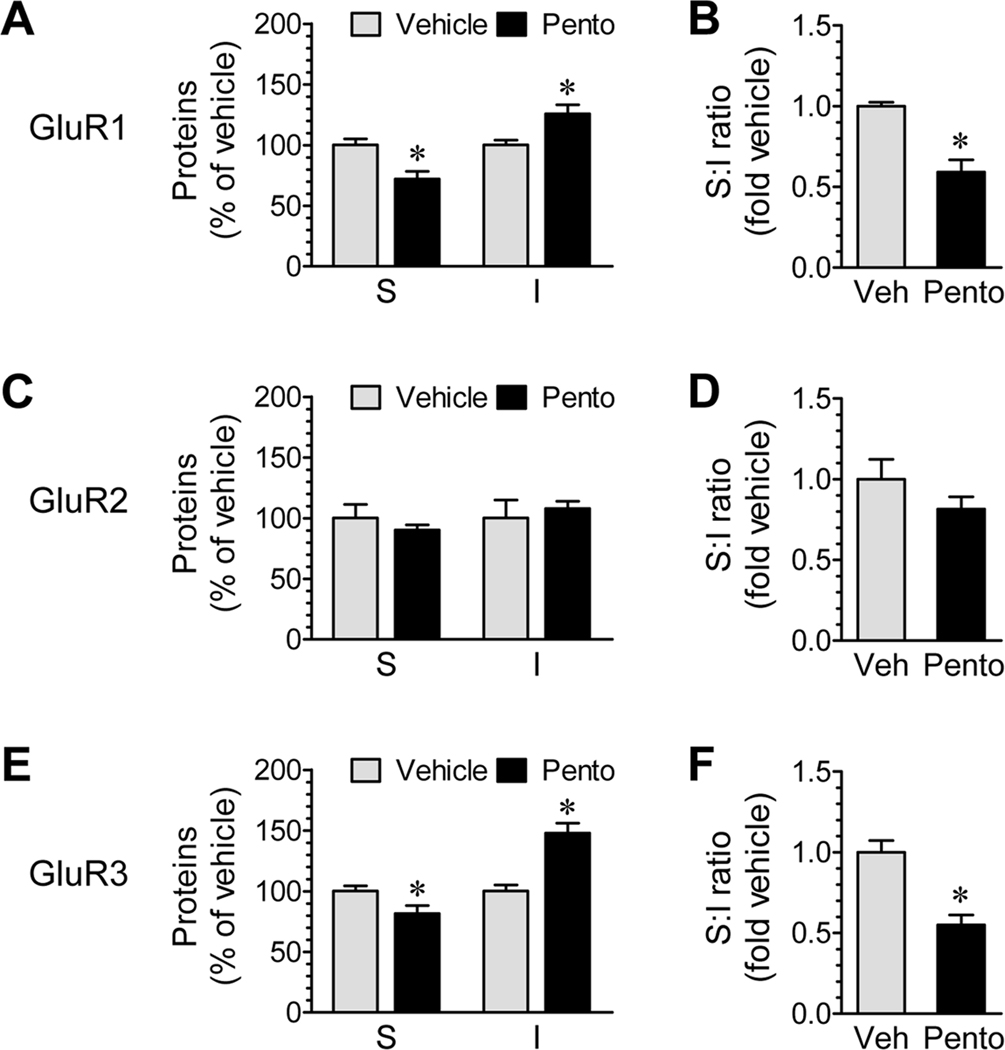
To determine whether the effect of pentobarbital is region-specific, we examined changes in AMPA expression in a major forebrain structure under the cortex: the striatum (caudate putamen and nucleus accumbens). We found that GluR1–3 subunits in striatal neurons responded similarly as those in cortical neurons to pentobarbital-induced anesthesia. For GluR1 and GluR3, their abundance in the surface pool was reduced whereas their levels in the intracellular pool were elevated in anesthetized animals (Fig. 4A for GluR1 and 4E for GluR3). The surface versus intracellular ratios were reduced (Fig. 4B and 4F). GluR2, however, remained insensitive to anesthesia (Fig. 4C and 4D). These data support that anesthesia can induce the GluR1/3 redistribution in other brain regions.
Figure 4.
Effects of pentobarbital-induced anesthesia on AMPA receptor subunit expression in surface (S) and intracellular (I) pools of mouse striatal neurons. (A, C, and E) Effects of anesthesia on GluR1 (A), GluR2 (C), and GluR3 (E) expression in the two pools. (B, D, and F) Effects of anesthesia on the ratio of surface to intracellular expression of GluR1 (B), GluR2 (D), and GluR3 (F). Anesthesia was induced by pentobarbital (Pento) at 50 mg/kg (i.p.). Mice were sacrificed 15 min after drug injection. Control mice received a vehicle (Veh) injection. The data are expressed in terms of means ± SEM (n = 6 per group). *: P < 0.05 versus vehicle.
Effects of chloral hydrate-induced anesthesia on subcellular expression of AMPA receptors
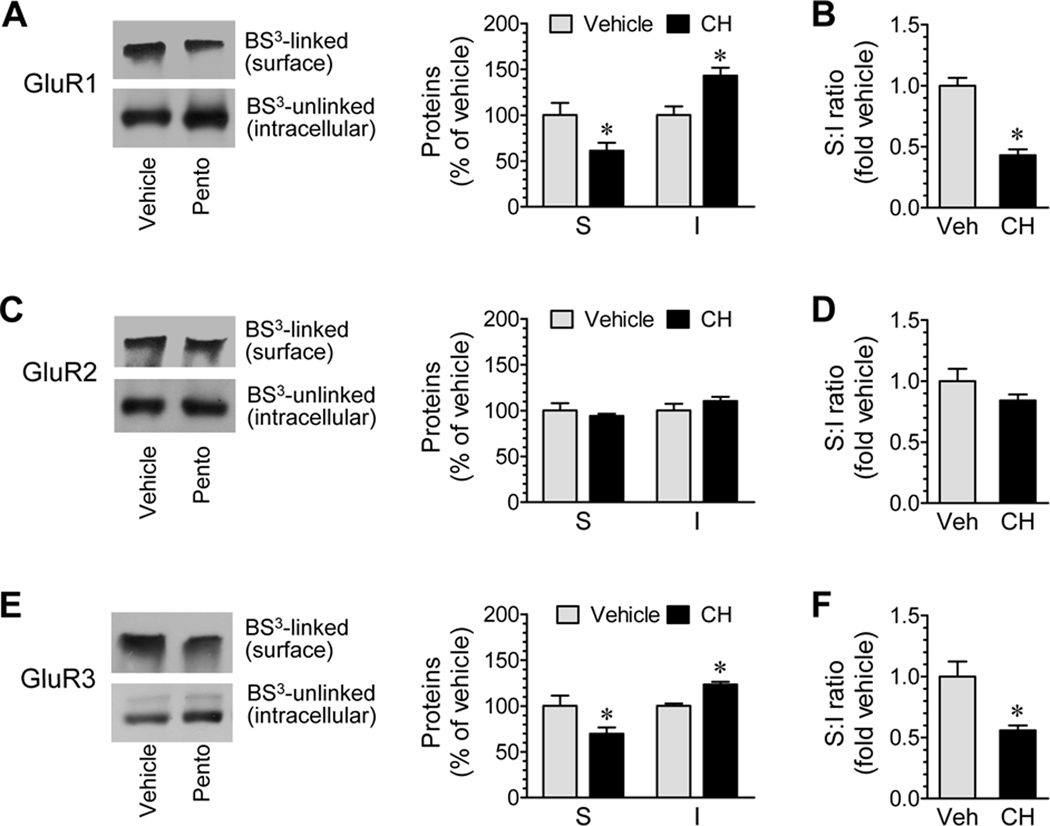
Chloral hydrate is another widely-used general anesthetic. To define whether chloral hydrate has the same effect as pentobarbital, we tested the effect of anesthesia induced by chloral hydrate on subcellular expression of AMPA receptors. To this end, we subjected mice to an i.p. injection of chloral hydrate (400–500 mg/kg) and then sacrificed mice 15 min after drug injection for BS3 assays of AMPA receptor expression in the cerebral cortex. A decrease in surface GluR1 levels and an increase in intracellular GluR1 proteins were seen in anesthetized mice compared to control mice (Fig. 5A). The ratio of surface to intracellular GluR1 expression was reduced (Fig. 5B). Similar results were observed in probing responses of GluR3 to anesthesia (Fig. 5E and 5F). Unlike GluR1/3, GluR2 levels in both surface and intracellular pools were unchanged after anesthesia (Fig. 5C and 5D). There was no significant difference in α-actinin expression between anesthetized and conscious control mice (data not shown). These results indicate that chloral hydrate like pentobarbital can significantly modulate subcellular expression of GluR1/3 AMPA receptors.
Figure 5.
Effects of chloral hydrate-induced anesthesia on AMPA receptor subunit expression in surface (S) and intracellular (I) pools of mouse cortical neurons. (A, C, and E) Effects of anesthesia on GluR1 (A), GluR2 (C), and GluR3 (E) expression in the two pools. (B, D, and F) Effects of anesthesia on the ratio of surface to intracellular expression of GluR1 (B), GluR2 (D), and GluR3 (F). Anesthesia was induced by chloral hydrate (CH) at 400–500 mg/kg (i.p.). Mice were sacrificed 15 min after drug injection. Representative immunoblots are shown left to the quantified data (A, C, and E). The data are expressed in terms of means ± SEM (n = 6 per group). *: P < 0.05 versus vehicle.
Temporal property of anesthesia-induced AMPA receptor redistribution
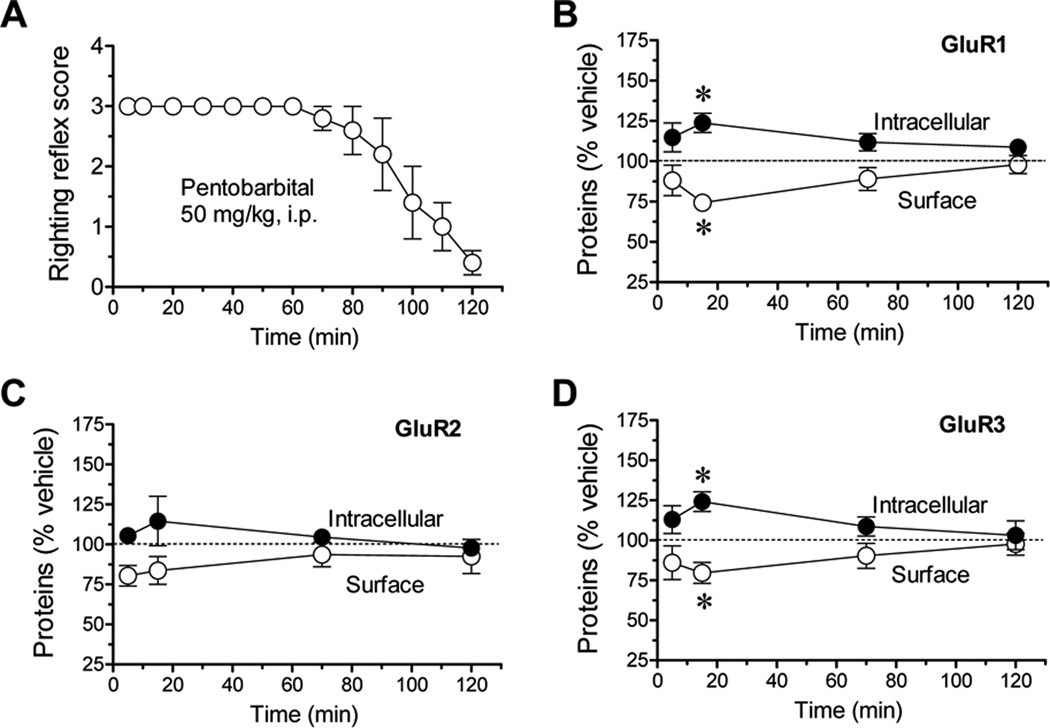
To correlate kinetics of anesthesia and changes in AMPA receptor expression, we carried out a time-course study. As assessed by the behavioral LORR, pentobarbital at a dose of 50 mg/kg produced a relatively short-lived anesthesia (Fig. 6A). At 5 min after drug injection, no righting reflex existed, indicating a rapid induction of anesthesia. Animals started to gain righting reflexes 90–110 min after injection (Fig. 6A). In BS3 assays of AMPA receptors in the cortex, changes in surface and intracellular levels of GluR1 were insignificant at an early time point (5 min) (Fig. 6B). The significant changes as manifested by a decrease in the surface pool and an increase in the intracellular pool were observed 15 min after pentobarbital injection (Fig. 6B). These changes were reduced at 70 min and largely recovered at 2 h (Fig. 6B). GluR2 in both pools showed no significant changes at all time points surveyed (Fig. 6C). GluR3 responded to anesthesia in the same temporal pattern seen in GluR1 (Fig. 6D). These data demonstrate that anesthesia induces a transient and reversible redistribution of GluR1/3 AMPA receptors in mouse cortical neurons.
Figure 6.
Time-dependent effects of anesthesia on the righting reflex behavior and compartment-specific expression of AMPA receptor subunits in mouse cortical neurons. (A) Effects of anesthesia on righting reflex scores. (B, C, and D) Effects of anesthesia on GluR1 (B), GluR2 (C), and GluR3 (D) expression in the two pools at different time points (5, 15, 70, and 120 min). Anesthesia was induced by pentobarbital (50 mg/kg, i.p.). Mice were scored in righting reflect assessments at indicated times (A) or sacrificed at different time points for protein expression assays (B–D). The data are expressed in means ± SEM (n = 4–6 per group). *: P < 0.05 versus vehicle.
DISCUSSION
The neurotransmitter-gated ion channels are sensitive targets of anesthetic agents. The inhibitory GABAA receptor in particular has been thoroughly investigated (Rudolph and Antkowiak, 2004; Hemmings et al., 2005), whereas the excitatory glutamate receptor has received relatively lesser attention (Hudspith, 1997; Zhu et al., 1997; Grasshoff et al., 2006). This study investigated the dynamic influence of anesthesia on subcellular expression of AMPA receptors in in vivo adult mouse brains. All three AMPA receptor subunits surveyed in cortical neurons are normally distributed in both surface and intracellular pools at variable levels. Anesthesia induced by the general anesthetic pentobarbital reduced and elevated the surface and intracellular GluR1/3 expression, respectively, in cortical and striatal neurons. GluR2, however, remained stable in its surface and intracellular levels following anesthesia. Anesthesia induced by another general anesthetic chloral hydrate produced similar results. In a time-course study, pentobarbital caused a transient and reversible anesthesia and redistribution of GluR1/3. These results support a notion that anesthesia possesses the ability to alter the AMPA receptor-mediated glutamatergic transmission via a mechanism involving the redistribution of GluR1/3.
The anesthesia-induced redistribution of AMPA receptors was characterized by the following observations. First, the response of AMPA receptors was time-dependent. The reliable and significant redistribution of GluR1/3 was seen after anesthesia was induced. This change was reversible and returned to normal levels after recovery of animals from anesthesia. Second, anesthesia caused the similar redistribution of GluR1/3 in both cortical and striatal neurons, indicating an existence of the possible global effect of anesthesia on AMPA receptors. Third, the effect of anesthesia may not be drug-selective. The two anesthetic agents (pentobarbital and chloral hydrate) surveyed in this study similarly affected GluR1/3 expression. Finally, the loss of the receptor in the surface pool was accompanied by the accumulation of the receptor in the intracellular pool. Since the total cellular level of GluR1/3 remained stable, changes in externalization and/or internalization trafficking should have accounted for this type of redistribution. Specifically, the delivery process of the receptor from the intracellular organelles to the surface membrane, that is, an externalization trafficking process, might be suppressed. Alternatively, an internalization trafficking process removing surface receptors to intracellular sites might be accelerated. Either suppressed externalization or accelerated internalization or both could lead to a redistribution pattern characterized by the subtraction of the surface receptor and the addition of the intracellular receptor under the condition that the total number of receptors was unchanged. Future studies will need to elucidate precise mechanisms underlying the anesthesia-induced GluR1/3 redistribution.
This study did not explore the direct impact of the loss of surface-expressed GluR1/3 AMPA receptors on the efficacy and strength of excitatory synapses containing AMPA receptors (Kennedy and Ehlers, 2006). However, the loss of surface GluR1/3 usually reduces the strength of excitatory synapses. If the loss occurred at synaptic sites, it is likely to directly weaken synaptic transmission. If the loss, however, occurred at extrasynaptic sites, it may exhibit the different spatiotemporal properties in affecting AMPA receptor function and excitatory synapses. With regard to the role of ligand-gated ion channels in mediating general anesthesia, inhibitory ion channels, i.e., GABAA receptors, seem to be a common substrate mediating anesthesia induced by a variety of general anesthetics (Krasowski and Harrison, 1999; Hemmings et al., 2005). Pentobarbital is a known effective agent augmenting the GABAA-mediated inhibitory transmission (Davies et al., 1997; Pistis et al., 1997). Like pentobarbital, chloral hydrate or trichloroethanol, an active metabolite of chloral hydrate, potentiates the function of GABAA receptors (Lovinger et al., 1993; Peoples and Weight, 1994; Garrett and Gan, 1998; Krasowski and Harrison, 2000). Thus, the GABAA receptor is a well-defined target of anesthetics to induce anesthesia. Compared to this receptor, the direct role of ionotropic glutamate receptors in producing anesthesia is less clear (Hudspith, 1997; Dinse et al., 2005). In electrophysiological experiments, pentobarbital suppressed AMPA receptors, especially GluR2-containing AMPA receptors, in Xenopus oocytes (Taverna et al., 1994; Yamakura et al., 1995b). AMPA receptors were less sensitive to inhibition by pentobarbital in acutely dissociated hippocampal pyramidal neurons from GluR2 null mutant mice (Joo et al., 1999). However, GluR2 null mutant mice were more sensitive to pentobarbital anesthesia in behavioral assays in vivo (Joo et al., 1999). In our neurochemical assays in vivo, GluR1 and GluR3 in cortical and striatal neurons seemed to be more likely to redistribute after pentobarbital. These data underscore the complexity of inhibition of AMPA receptors by pentobarbital. Chloral hydrate reduced glutamate levels in the striatum (Kreuter et al., 2004) and trichloroethanol inhibited glutamatergic transmission in hippocampal slices (Lovinger et al., 1993). However, the potency of chloral hydrate for inhibiting glutamatergic transmission was lower than its potency for enhancing GABAA receptor activity (Lovinger et al., 1993).
Pentobarbital is a choice of drugs for treating status epilepticus, a neurological disorder associated with malfunctional glutamate receptors (Miller and Drislane, 2008). Pentobarbital has also been reported to reduce rat cortical AMPA excitotoxicity (Kimbro et al., 2000). Of note, inhibition of Ca2+-permeable AMPA receptors containing GluR1 and 3 is neuroprotective (Jayakar and Dikshit, 2004). Further studies need to elucidate potential contributions of the pentobarbital-induced loss of surface GluR1/3 AMPA receptors to the epilepticus therapy and the neuroprotection against excitotoxicity.
Acknowledgements
This work was supported by NIH grants (R01-DA10355-15 and R01-MH061469-10) and a gant from the Saint Luke's Foundation (Kansas City, MO, USA)
REFERENCES
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Kirkness EF, Hales TG. Modulation by general anaesthetics of rat GABAA receptors comprised of alpha1 beta 3 and beta 3 subunits expressed in human embryonic kidney 293 cells. Br J Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dinse A, Fohr KJ, Georgieff M, Beyer C, Bulling A, Weight HU. Xenon reduces glutamate-, AMPA-, and kainite-induced membrane currents in cortical neurons. Br J Anaesth. 2005;94:479–485. doi: 10.1093/bja/aei080. [DOI] [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Garrett KM, Gan J. Enhancement of gamma-aminobutyric acidA receptor activity by alpha-chloralose. J Pharmacol Exp Ther. 1998;285:680–686. [PubMed] [Google Scholar]
- Grasshoff C, Drexler B, Rodulph U, Antkowiak B. Anaesthetic drugs: linking molecular actions to clinical effects. Curr Pharm Des. 2006;12:3665–3679. doi: 10.2174/138161206778522038. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326:423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- Haines M, Mao LM, Yang L, Arora A, Fibuch EE, Wang JQ. Modulation of AMPA receptor GluR1 subunit phosphorylation in neurons by the intravenous anesthetic propofol. Br J Anaesth. 2008;100:676–682. doi: 10.1093/bja/aen051. [DOI] [PubMed] [Google Scholar]
- Harris RA, Mihic SJ, Dildy-Mayfield JE, Machu TK. Actions of anesthetics on ligand-gated ion channels: role of receptor subunit composition. FASEB J. 1995;9:1454–1462. doi: 10.1096/fasebj.9.14.7589987. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hudspith MJ. Glutamate: a role in normal brain function, anesthesia, analgesia and CNS injury. Br J Anaesth. 1997;78:731–747. doi: 10.1093/bja/78.6.731. [DOI] [PubMed] [Google Scholar]
- Irifune M, Takarada T, Shimizu Y, Endo C, Katayama S, Dohi T, Kawahara M. Propofol-induced anesthesia in mice is mediated by γ-aminobutyric acid-A and excitatory amino acid receptors. Anesth Analg. 2003;97:424–429. doi: 10.1213/01.ANE.0000059742.62646.40. [DOI] [PubMed] [Google Scholar]
- Jayakar SS, Dikshit M. AMPA receptor regulation mechanisms: future target for safer neuroprotective drugs. Int J Neurosci. 2004;114:695–734. doi: 10.1080/00207450490430453. [DOI] [PubMed] [Google Scholar]
- Joo DT, Xiong Z, MacDonald JF, Jia Z, Roder J, Sonner J, Orser BA. Blockade of glutamate receptors and barbiturate anesthesia: increased sensitivity to pentobarbital-induced anesthesia despite reduced inhibition of AMPA receptors in GluR2 null mutant mice. Anesthesiology. 1999;91:1329–1341. doi: 10.1097/00000542-199911000-00025. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbro JR, Kelly PJ, Drummond JC, Cole DJ, Patel PM. Isoflurane and pentobarbital reduce AMPA toxicity in vivo in the rat cerebral cortex. Anesthesiology. 2000;92:806–812. doi: 10.1097/00000542-200003000-00024. [DOI] [PubMed] [Google Scholar]
- Krampfl K, Cordes A, Schlesinger F, Wolfs H, Bufler J. Effects of propofol on recombinant AMPA receptor channels. Eur J Pharmacol. 2005;511:1–7. doi: 10.1016/j.ejphar.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL. The actions of ether, alcohol and alkane general anesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br J Pharmacol. 2000;129:731–743. doi: 10.1038/sj.bjp.0703087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter JD, Mattson BJ, Wang B, You ZB, Hope BT. Cocaine-induced Fos expression in rat striatum is blocked by chloral hydrate or urethane. Neuroscience. 2004;127:233–242. doi: 10.1016/j.neuroscience.2004.04.047. [DOI] [PubMed] [Google Scholar]
- LacKamp A, Zhang GC, Mao LM, Fibuch EE, Wang JQ. Loss of surface N-methyl-D-aspartate receptor proteins in mouse cortical neurones during anaesthesia induced by chloral hydrate in vivo. Br J Anaesth. 2009;102:515–522. doi: 10.1093/bja/aep009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci USA. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interaction in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Zimmerman SA, Levitin M, Jones MV, Harrison NL. Trichloroethanol potentiates synaptic transmission mediated by gamma-aminobutyric acidA receptors in hippocampal neurons. J Pharmacol Exp Ther. 1993;264:1097–1103. [PubMed] [Google Scholar]
- Mansour M, Nagarajan N, Nehring RB, Clements JD, Rosenmund C. Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron. 2001;32:841–853. doi: 10.1016/s0896-6273(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LC, Drislane FW. Treatment of status epilepticus. Expert Rev Neurother. 2008:1817–1827. doi: 10.1586/14737175.8.12.1817. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples RW, Weight FF. Trichloroethanol potentiation of gamma-aminobutyric acid-activated chloride current in mouse hippocampal neurons. Br J Pharmacol. 1994;113:555–563. doi: 10.1111/j.1476-5381.1994.tb17025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistis M, Belelli D, Peters JA, Lambert JJ. The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in Xenopus laevis ooctyes: a comparative study. Br J Pharmacol. 1997;122:1707–1719. doi: 10.1038/sj.bjp.0701563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna FA, Cameron BR, Hampson DL, Wang LY, MacDonald JF. Sensitivity of AMPA receptors to pentobarbital. Eur J Pharmacol. 1994;267:R3–R5. doi: 10.1016/0922-4106(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Van Dolah DK, Mao LM, Shaffer C, Guo ML, Fibuch EE, Chu XP, Buch S, Wang JQ. Reversible palmitoylation regulates surface stability of AMPA receptors in the nucleus accumbens in response to cocaine in vivo. Biol Psychiatry 2011. 2011 doi: 10.1016/j.biopsych.2010.11.025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Sakimura K, Shimoji K, Mishina M. Effects of propofol on various AMPA-, kainate- and NMDA-selective glutamate receptor channels expressed in Xenopus oocytes. Neurosci Lett. 1995a;188:187–190. doi: 10.1016/0304-3940(95)11431-u. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Sakimura K, Mishina M, Shimoji K. The sensitivity of AMPA-selective glutamate receptor channels to pentobarbital is determined by a single amino acid residue of the alpha 2 subunit. FEBS Lett. 1995b;374:412–414. doi: 10.1016/0014-5793(95)01163-9. [DOI] [PubMed] [Google Scholar]
- Yang L, Mao L, Tang Q, Samdani S, Liu Z, Wang JQ. A novel Ca2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5. J Neurosci. 2004;24:10846–10857. doi: 10.1523/JNEUROSCI.2496-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Cottrell JE, Kass IS. The effect of thiopental and propofol on NMDA and AMPA-mediated glutamate excitotoxicity. Anesthesiology. 1997;87:944–951. doi: 10.1097/00000542-199710000-00030. [DOI] [PubMed] [Google Scholar]