Abstract
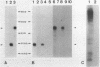
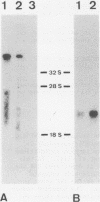
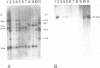
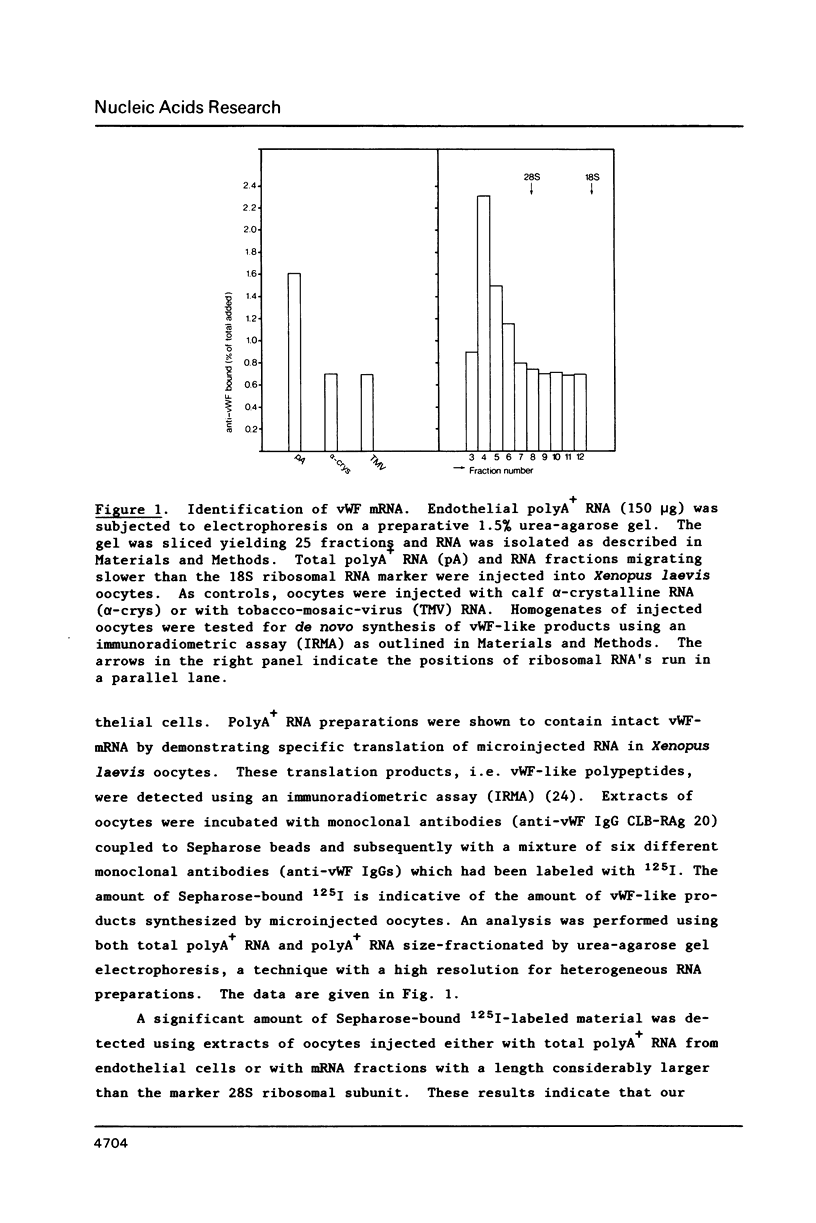
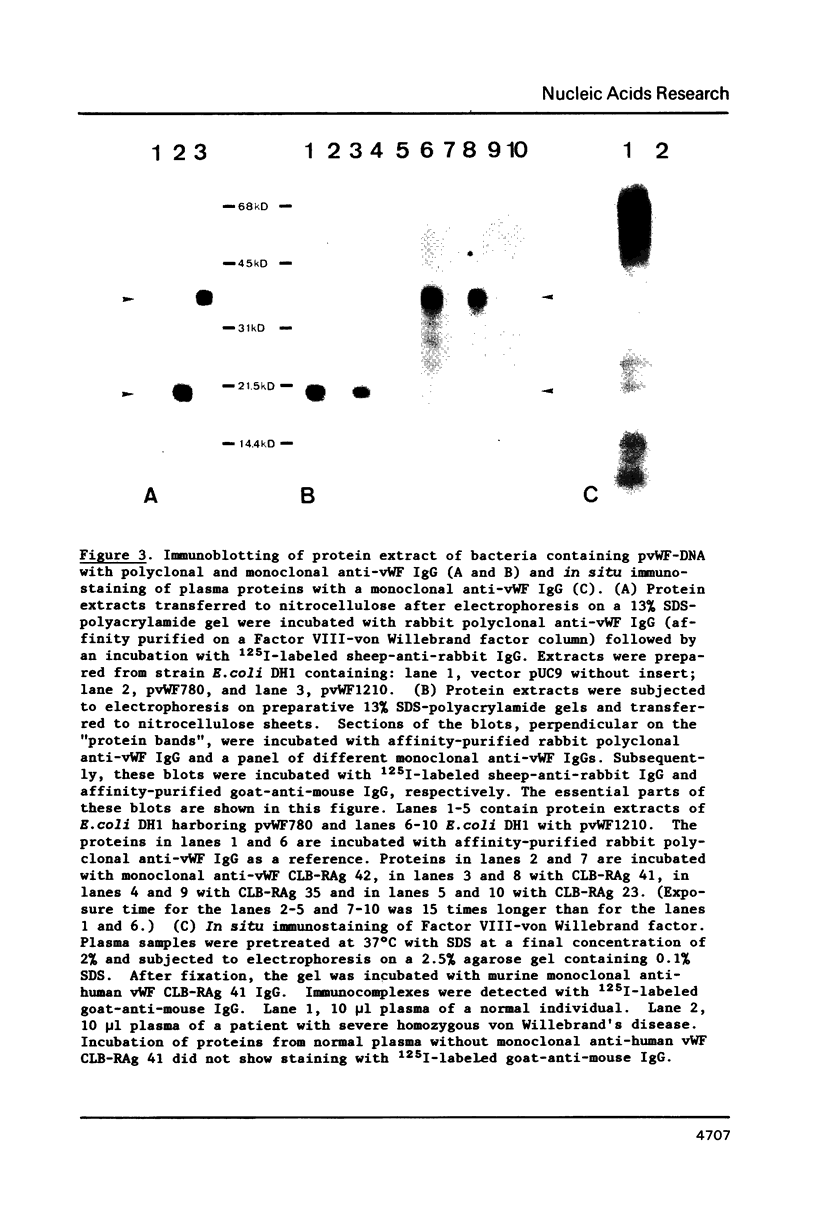
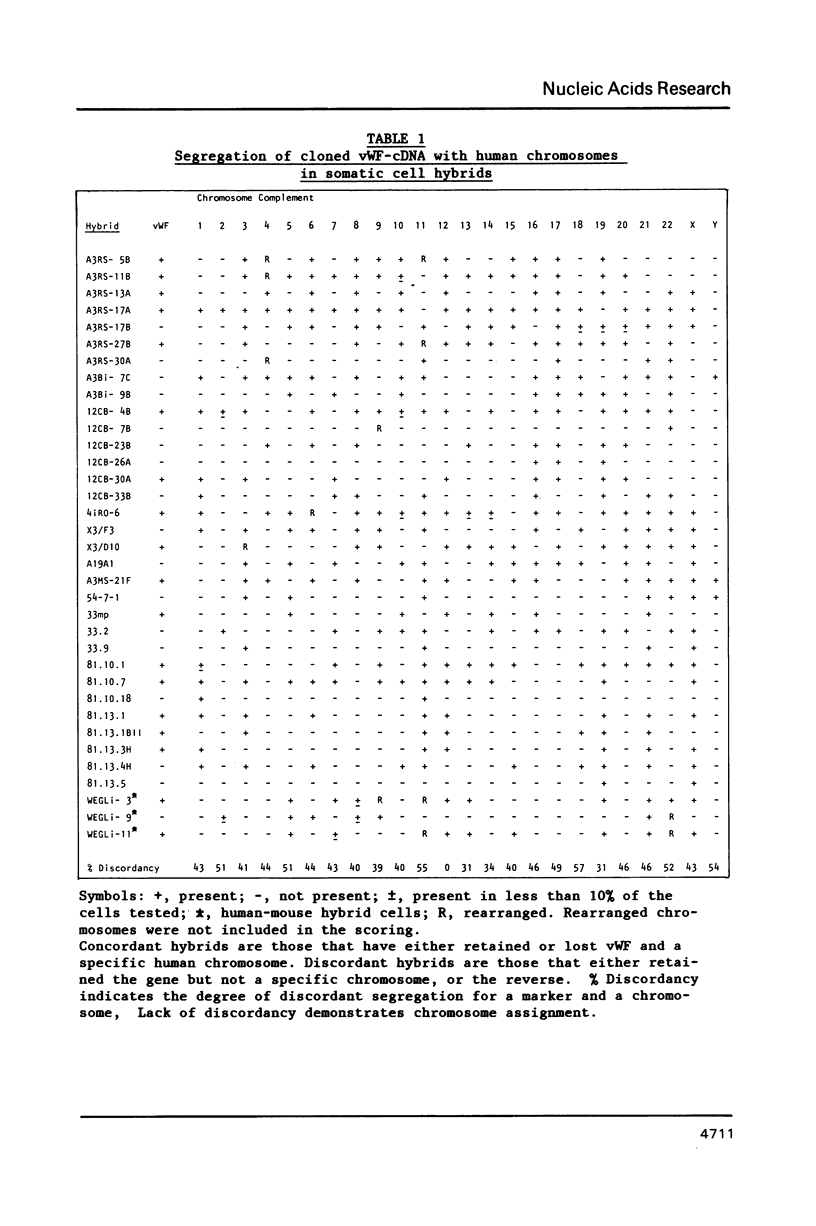
Von Willebrand Factor (vWF) mRNA was identified in fractionated polyA+ RNA preparations isolated from cultured human endothelial cells. Micro-injection of specific polyA+ RNA fractions in Xenopus laevis oocytes provoked the synthesis of a vWF-like product which could be detected with an immunoradiometric assay relying on Sepharose-linked monoclonal anti-vWF IgG and different radiolabeled monoclonal anti-vWF IgGs. A vWF-mRNA-containing polyA+ RNA preparation served as substrate for a size-selected cDNA-expression library of 60 000 colonies which was screened for the synthesis of antigens related to vWF, using polyclonal anti-vWF IgG and a second antibody conjugated with peroxidase. Eight positive colonies were detected of which two reacted strongly in the enzyme-linked assay. Immunoblotting of bacterial extracts of "expression clones" with a monoclonal anti-vWF IgG revealed polypeptides which size fits within the length of the cDNA insertions. Northern blotting of human endothelial RNA, employing fragments of vWF cDNA as probes, showed specific hybridization with a mRNA of about 9000 nucleotides. DNA-sequence analysis of a vWF-cDNA insertion revealed an open reading frame followed by a translation stopcodon. It is argued that the cDNA insertions encode the carboxy-terminal part of the vWF protein. vWF-cDNA probes were employed to map the von Willebrand factor gene on chromosome 12 using a panel of 35 human-rodent somatic cell hybrids.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom A. L. Acquired immunodeficiency syndrome and other possible immunological disorders in European haemophiliacs. Lancet. 1984 Jun 30;1(8392):1452–1455. doi: 10.1016/s0140-6736(84)91942-1. [DOI] [PubMed] [Google Scholar]
- Bolhuis P. A., Sakariassen K. S., Sander H. J., Bouma B. N., Sixma J. J. Binding of factor VIII-von Willebrand factor to human arterial subendothelium precedes increased platelet adhesion and enhances platelet spreading. J Lab Clin Med. 1981 Apr;97(4):568–576. [PubMed] [Google Scholar]
- Cabilly S., Riggs A. D., Pande H., Shively J. E., Holmes W. E., Rey M., Perry L. J., Wetzel R., Heyneker H. L. Generation of antibody activity from immunoglobulin polypeptide chains produced in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3273–3277. doi: 10.1073/pnas.81.11.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell C. J., Reisner H. M., Graham J. B. Endothelial cell hybrids and the suspension of factor VIII related antigen expression. Br J Haematol. 1980 Dec;46(4):613–620. doi: 10.1111/j.1365-2141.1980.tb06019.x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer L. W. The factor VIII complex: structure and function. Blood. 1981 Jul;58(1):1–13. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lynch D. C., Williams R., Zimmerman T. S., Kirby E. P., Livingston D. M. Biosynthesis of the subunits of factor VIIIR by bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2738–2742. doi: 10.1073/pnas.80.9.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. C., Zimmerman T. S., Collins C. J., Brown M., Morin M. J., Ling E. H., Livingston D. M. Molecular cloning of cDNA for human von Willebrand factor: authentication by a new method. Cell. 1985 May;41(1):49–56. doi: 10.1016/0092-8674(85)90060-1. [DOI] [PubMed] [Google Scholar]
- Mikaelsson M. E., Forsman N., Oswaldsson U. M. Human factor VIII: a calcium-linked protein complex. Blood. 1983 Nov;62(5):1006–1015. [PubMed] [Google Scholar]
- Nachman R., Levine R., Jaffe E. A. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977 Oct;60(4):914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnoff O. D. Some complications of the therapy of classic hemophilia. J Lab Clin Med. 1984 May;103(5):653–659. [PubMed] [Google Scholar]
- Reinders J. H., De Groot P. G., Gonsalves M. D., Zandbergen J., Loesberg C., Van Mourik J. A. Isolation of a storage and secretory organelle containing Von Willebrand protein from cultured human endothelial cells. Biochim Biophys Acta. 1984 Jul 20;804(3):361–369. doi: 10.1016/0167-4889(84)90140-x. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Sakariassen K. S., Bolhuis P. A., Sixma J. J. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 1979 Jun 14;279(5714):636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodetz J. M., Paulson J. C., McKee P. A. Carbohydrate composition and identification of blood group A, B, and H oligosaccharide structures on human Factor VIII/von Willebrand factor. J Biol Chem. 1979 Nov 10;254(21):10754–10760. [PubMed] [Google Scholar]
- Stel H. V., Sakariassen K. S., Scholte B. J., Veerman E. C., van der Kwast T. H., de Groot P. G., Sixma J. J., van Mourik J. A. Characterization of 25 monoclonal antibodies to factor VIII-von Willebrand factor: relationship between ristocetin-induced platelet aggregation and platelet adherence to subendothelium. Blood. 1984 Jun;63(6):1408–1415. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp T. B., Weiss H. J., Baumgartner H. R. Decreased adhesion of platelets to subendothelium in von Willebrand's disease. J Lab Clin Med. 1974 Feb;83(2):296–300. [PubMed] [Google Scholar]
- Tuddenham E. G., Lane R. S., Rotblat F., Johnson A. J., Snape T. J., Middleton S., Kernoff P. B. Response to infusions of polyelectrolyte fractionated human factor VIII concentrate in human haemophilia A and von Willebrand's disease. Br J Haematol. 1982 Oct;52(2):259–267. doi: 10.1111/j.1365-2141.1982.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Veerman E. C., Stel H. V., Huisman J. G., van Mourik J. A. Application of sepharose-linked monoclonal antibodies for the immunoradiometric measurement of factor VIII-procoagulant antigen. Thromb Res. 1984 Jan 1;33(1):89–93. doi: 10.1016/0049-3848(84)90157-9. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells. Identification of a large precursor polypeptide chain. J Biol Chem. 1983 Feb 25;258(4):2065–2067. [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]
- Willems C., Astaldi G. C., De Groot P. G., Janssen M. C., Gonsalvez M. D., Zeijlemaker W. P., Van Mourik J. A., Van Aken W. G. Media conditioned by cultured human vascular endothelial cells inhibit the growth of vascular smooth muscle cells. Exp Cell Res. 1982 May;139(1):191–197. doi: 10.1016/0014-4827(82)90332-9. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Capon D. J., Simonsen C. C., Eaton D. L., Gitschier J., Keyt B., Seeburg P. H., Smith D. H., Hollingshead P., Wion K. L. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984 Nov 22;312(5992):330–337. doi: 10.1038/312330a0. [DOI] [PubMed] [Google Scholar]