Abstract
Background
The safety and clinical utility of magnetic resonance imaging at 1.5T in patients with cardiac implantable devices such as pacemakers (PM) and implantable cardioverter defibrillators (ICD) have been reported. This study aims to evaluate the extent of artifacts on cardiac magnetic resonance (CMR) in patients with PM and ICD (PM/ICD).
Methods and Results
A total of 71 CMR studies were performed with an established safety protocol in patients with pre-pectoral PM/ICD. The artifact area around the PM/ICD generator was measured in all short axis (SA), horizontal (HLA) and vertical long axis (VLA) SSFP cine planes. The location and extent of artifacts were also assessed in all SA (20 sectors/plane), HLA, and VLA (6 sectors/plane) late gadolinium enhanced CMR (LGE-CMR) planes. The artifact area on cine CMR was significantly larger with ICD versus PM generators in each plane (P<0.001, respectively). In patients with left-sided ICD or biventricular ICD systems, the percentages of sectors with any artifacts on LGE-CMR were 53.7%, 48.0% and 49.2% in SA, HLA and VLA planes, respectively. Meanwhile, patients with left-sided PM or right-sided PM/ICD had fewer artifacts. Anterior and apical regions were severely affected by artifact due to left-sided PM/ICD generators.
Conclusions
In contrast to patients with right-sided PM/ICD and left-sided PM, the anterior and apical left ventricle can be affected by susceptibility artifacts in patients with left-sided ICD. Artifact reduction methodologies will be necessary to improve the performance of CMR in patients with left sided ICD systems.
Keywords: magnetic resonance imaging, artifacts, pacemakers, implantable cardioverter defibrillator
The clinical utility and safety of non-cardiac and cardiac magnetic resonance imaging (MRI) at 1.5T in patients with cardiac implantable devices such as pacemaker (PM) and implantable cardioverter defibrillator (ICD) systems has been investigated in previous reports.1–12 Cardiac magnetic resonance imaging (CMR) can be instrumental for the diagnosis of underlying cardiomyopathies, assessment of cardiac function and myocardial viability, assessment of disease progression, and identification of arrhythmogenic substrates.12–23 However, many patients with cardiac pathology who would otherwise derive benefit from CMR will have received a cardiac device prior to referral for imaging. We previously found that metallic PM and ICD (PM/ICD) can produce susceptibility artifacts due to distortion of MRI magnetic field resulting in bright and dark artifacts surrounding the generator and leads.7 Consequently, the risk to benefit ratio of performing CMR in the setting of PM/ICD may be significantly altered compared to patients with PM/ICD who require non-cardiac MRI.1–8,24 We sought to quantitatively assess susceptibility artifacts on 1.5T CMR utilizing our previously reported safety protocol for patients with PM/ICD. 1–3
Methods
The study protocol was reviewed and approved by the Johns Hopkins Institutional Review Board. Written informed consent was obtained from all patients after potential risks of PM/ICD exposure to MRI scanning were explained.
Device Safety Protocol for CMR in Patients with PM/ICD
Patients were enrolled if they had a clinical necessity for CMR, no acceptable imaging alternative, and PM/ICD found to be safe by previous in vivo or in vitro testing.1–3 Patients with device implantation <6 weeks before CMR and those with epicardial and abandoned leads were excluded. Specific device models included Medtronic EnTrust (T154ATG), GEMIII (7231), Insync (7272), Marquis (7274), Maximo (7232, 7278), Virtuso (D154AWG, D154VRC); Boston Scientific Confient (E030), Contak Renewal (H119, H170, H175, H210, H217, H219), Ventak Prizm (1852, 1860, 1861), Vitality (T125, T135, T165, T167, T175, T177); and St. Jude Medical Atlas (V343, V366), Current (1207–36) Promote (3207–36) ICD and BiV-ICD devices. Additionally, the following PM models were included: Medtronic EnPulse (E2DR01, E2DR21), Kappa (KDR401, KDR701, KDR901); Boston Scientific Insignia (1290); St. Jude Medical; Identity (5376, 5386), Integrity (5142, 5342, 5346), and Trilogy (2360). Device interrogation to assess parameters including battery voltage, lead impedance, lead capture thresholds and sensing were performed immediately prior to, immediately after, and at routine clinic follow-up. Pacing mode was programmed to asynchronous in PM-dependent patients without a hemodynamically stable escape rhythm, whereas the patients without PM dependence were programmed to the ventricular or dual-chamber-inhibited pacing mode. Magnet response, noise response, ventricular sense response, conducted atrial fibrillation response and tachyarrhythmia functions (monitoring, antitachycaradia pacing, and defibrillation) were turned off before CMR. Devices were reprogrammed to original settings after the completion of CMR. Blood pressure, electrocardiographic telemetry, pulse oximetry, and symptoms were monitored. In addition, a registered nurse trained in advanced cardiac life support and familiar with device programming and trouble shooting was present at all CMR scans. The specific absorption rate (SAR) of MRI sequences was limited to less than 2.0 W/kg during our initial experience (27 of 71 CMR studies).1 After the initial period, given the lack of association between SAR and device parameter changes10,11 and the unreliability of using SAR to guide MRI safety recommendations12, no restrictions beyond standard manufacturer SAR limits were applied in subsequent patients.
Cardiac Magnetic Resonance Imaging
CMR scans were performed with a 1.5T scanner (Avanto, Siemens Medical Systems, PA) with maximum gradient field 45 mT/m and slew rate 200 T/m/s. ECG telemetry, pulse oximetry, blood pressure and symptoms were monitored during the scan. Cine steady-state free precession (SSFP) gradient-echo images were obtained in multiple short axis (SA), horizontal long axis (HLA) and vertical long axis (VLA) planes (echo time 1.1–1.6 ms, repetition time 2.5–3.8 ms, average in-plane resolution 1.4×1.4 mm2; flip angle 45–60°; temporal resolution 40–45 ms). Fifteen minutes after bolus injection of 0.2 mmol/kg intravenous gadolinium contrast, late gadolinium enhanced CMR (LGE-CMR) was obtained in 10–13 SA planes with an inversion-recovery fast-gradient-echo pulse sequence (echo time 1.3–3.9 ms, repetition time 5.4–8.3 ms, average in-plane resolution 1.5×2.0 mm2; 8 mm slice thickness, flip angle 30 degrees). Inversion times (range 175–300 ms) were optimized for each patient to maximize conspicuity of myocardial delayed enhanced area. Single planes of LGE-CMR were also acquired in VLA and HLA planes. In a subgroup of patients, three T2-weighted SA planes acquired by a T2-weighted turbo spin echo sequence before contrast administration (echo time 76 ms, repetition time 1800–2100 ms, average in-plane resolution 1.4×1.4 mm2, 10 mm slice thickness, flip angle 180 degrees), and first pass myocardial perfusion imaging using hybrid fast gradient echo/echo planar technique in 4 SA planes (echo time 1.0–1.1ms, repetition time 164–256ms, average in-plane sequence 1.9×1.9 mm2, 8 mm slice thickness, flip angle 12 degrees) were also performed. Contrast-enhanced MR angiography was additionally performed in a subgroup of patients before and immediately after contrast agent administration (echo time 1.0–1.1ms, repetition time 2.7–2.9 ms, average in-plane sequence 1.0×1.0 mm2, 1 mm slice thickness, flip angle 25 degrees).
Measurements of Artifact due to PM/ICD
The maximum area of image susceptibility artifact was measured in SA, HLA and VLA planes on cine CMR, and the percentage of cine CMR scans with any artifacts was also assessed in the three different planes (Figure 1A). The artifact size and the percentage of cine CMR scans with any artifacts in each plane were compared between patients with ICD/BiV-ICD and those with PMs. The association of artifact size with generator dimensions (area defined as height×width, thickness, weight, and volume) was evaluated. The feasibility of cardiac function calculation based on cine CMR (ie, clear visualization of myocardial borders free from artifact) was also evaluated in the four groups divided by the type of cardiac devices (ICD/BiV-ICD, PM) and the implanted side of device generator (left, right). The extent of artifacts on LGE-CMR in SA, HLA and VLA planes was recorded. To ascertain regional differences in artifact, the left ventricular myocardium was divided into 20 sectors in each SA plane and 6 sectors in each single HLA and VLA planes (Figure 1B). The percentage of sectors with/without artifact in the three different planes was assessed in the four groups described above. The percentage of sectors with artifact was summarized using the 17-segment model. The extent of artifacts in SA planes of cine CMR was assessed in the same way as the analysis of LGE-CMR. The distance from the generator to the cardiac silhouette on antero-posterior (AP) chest X-ray was measured (if the generator border overlapped the cardiac silhouette the distance was reported as 0), and the association between that distance and the percentage of sectors with artifact in SA, HLA and VLA planes was assessed. In patients with left-sided ICD/BiV-ICD, the artifact effects on cine, T2-weighted, perfusion and LGE-CMR images were compared. Finally, artifact effects due to PM/ICD leads were assessed by measuring the area of artifact surrounding the lead tip in SA cine CMR images. The artifact area surrounding PM/ICD leads was also measured in SA planes of cine CMR at the tip of the lead. In a subgroup of patients who had previously undergone cardiac computed tomography (CT), artifacts characteristics were qualitatively compared between MRI and CT. Cardiac CT images were acquired using a 64-slice CT scanner (Aquillon, Toshiba Medical Systems Corporation, Tochigi, Japan). Image acquisition was performed during one breath-hold at the end-expiratory phase. The duration of scanning was approximately 10 seconds and scanning was retrospectively gated to the cardiac cycle. CT images were reconstructed every 10% of the cardiac cycle with a slice thickness of 1 mm.
Figure 1. Methodology for Measurement of Artifacts on Cine CMR and LGE-CMR.
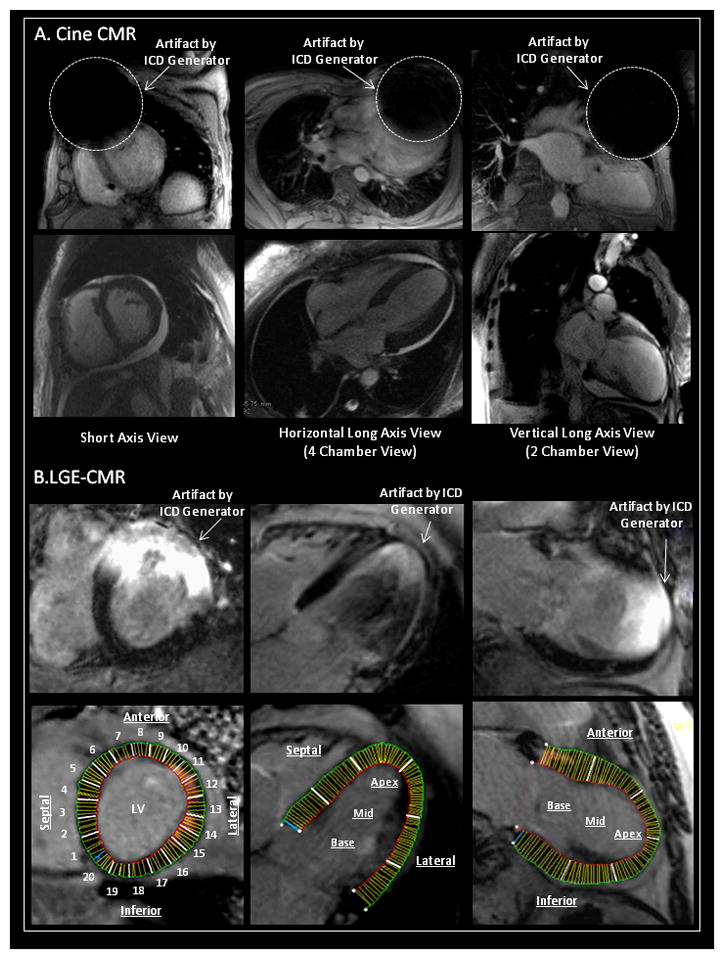
Artifact size on SSFP cine and late gadolinium enhanced cardiac magnetic resonance imaging (LGE-CMR) was measured in SA, HLA and VLA planes. (A) The artifact size on cine CMR due to the PM/ICD was measured. Percent sectors with any artifacts on cine CMR were also assessed in each plane. (B) The regional artifact effects on LGE-CMR due to the generator were quantitatively estimated in each plane (divided into 20 sectors in SA, 6 sectors in HLA, and 6 sectors in VLA planes, respectively).
SSFP=steady state free precession; SA=short axis; HLA=horizontal long axis; VLA=vertical long axis; PM/ICD=pacemaker and implantable cardioverter defibrillator.
Interpretability of CMR Images
The percentage of image series qualitatively defined as “successfully interpretable,” “partially interpretable,” and “impossible to interpret” for each of 4 pulse sequences (LGE-CMR, cine CMR, Perfusion CMR, and MR Angiography) were calculated and stratified by location of device and underlying heart disease. All images were reviewed by 2 independent observers and discrepancies (<5 cases) were resolved by the senior observer.
Statistical Analysis
All values are expressed as mean ± SD. Comparisons of continuous variables were made using Student’s t-test, and categorical variables were compared with chi-squared testing or Fisher’s exact test where appropriate. Spearman’s rank correlation test was used to assess the association between artifact size and parameters related to generator dimensions. Linear regression analysis was used to assess the relationship between the minimum distance from PM/ICD generator to heart on frontal chest X-ray and percent sectors with artifact due to the PM/ICD generator on LGE-CMR. All tests were two tailed and analyses were performed using STATA 10 statistical software (StataCorp, College Station, Texas).
Results
A total of 71 CMR examinations were performed in 66 patients with PM/ICD between November 2003 and March 2010. Of 71 scans, 56 (78.9%) were acquired in patients with ICD or biventricular ICD (BiV-ICD) systems and 15 (21.1%) were acquired in patients with PM systems. All ICD/BiV-ICD systems were implanted in the left infraclavicular pre-pectoral area except 1 BiV-ICD, and 4 PMs which were implanted in the right infraclavicular pre-pectoral area. Patient characteristics are summarized in Table 1. Patients with ICD devices were older and more likely to have structural heart disease than those with PMs. Body mass index (BMI) was similar between the two groups. Patient safety issues have been reported separately.1
Table 1.
Baseline Characteristics
| ICD/BIV-ICD N=56 Scans |
PM N=15 Scans |
P-Value | |
|---|---|---|---|
| Age | 59±15 | 43±16 | 0.001 |
| Female/Male | 8/48 | 7/8 | 0.012 |
| ICD/BiV-ICD | 42 ICD/14 BIV-ICD | (−) | |
| Generator Left-sided/Right-sided | 55/1 | 11/4 | 0.006 |
| Structural Heart Disease | 56 | 13 | 0.042 |
| ICM/NICM/HCM/Congenital/Others | 38/16/1/0/1 | 0/7/1/6/1 | <0.0001 |
| Body Weight [kg] | 81.6±16.5 | 70.3±15.3 | 0.034 |
| Body Height [cm] | 176.8±8.8 | 166.8±16.0 | 0.056 |
| Body Mass Index [kg/m2] | 26.0±4.0 | 25.4±5.3 | 0.688 |
| Ejection Fraction [%] | 34.0±15.9 | 51.5±16.5 | 0.002 |
| LVEDD [mm] | 56.8±10.8 | 45.4±5.6 | 0.006 |
| Possible Cardiac Function Evaluation by Cine CMR | 47/55 (85.5%) | 15/15 (100%) | 0.266 |
| Patients with CT Data | 22 (39.3%) | 4 (26.7%) | 0.548 |
| Generator Size | |||
| Height [mm] | 68.8±6.6 | 46.7±3.8 | <0.0001 |
| Width [mm] | 56.2±5.7 | 46.7±4.4 | <0.0001 |
| Area (Height*Width) [cm2] | 38.7±7.1 | 21.7±2.0 | <0.0001 |
| Thickness [mm] | 13.1±1.8 | 6.9±0.9 | <0.0001 |
| Weight [g] | 81.5±8.9 | 24.3±1.8 | <0.0001 |
| Volume [cc] | 36.4±6.0 | 11.3±0.9 | <0.0001 |
Values are shown as mean ±SD.
ICD=ICD; BiV-ICD=biventricular-ICD; LVEDD = left ventricular end-diastolic diameter; CT=computed tomography.
The estimated whole-body averaged SAR in each image acquisition sequence is reported in Supplemental Figure 1. No clinically significant PM/ICD parameter changes requiring system revision or re-programming were noted after CMR. The clinical indications for CMR studies were as follows; (i) General assessment of myocardial function and viability in patients with underlying heart disease (26 scans; 37%); (ii) Diagnosis of suspected cardiac conditions (arrhythmogenic right ventricular cardiomyopathy, cardiac sarcoidosis, myocarditis, etc) (7 scans; 10%); (iii) Preoperative evaluation of cardiac function, viability and anatomy (prior to coronary artery bypass grafting, left ventricular plasty for ischemic left ventricular aneurysm, valve surgery, heart transplant, radiofrequency catheter ablation, or device upgrade to BiV-ICD) (27 scans; 38%); and (iv) Postoperative evaluation (coronary artery bypass grafting, valve surgery, or congenital heart disease) (11 scans; 15%).
Artifact Size on Cine CMR
Artifact sizes in SA, HLA and VLA planes on cine CMR has been shown in the Supplemental Table. Susceptibility artifacts were present on cardiac cine CMR in 100% of SA planes, 26.9% of HLA planes and 76.1% of VLA planes in patients with left and right-sided ICD/BiV-ICD systems. In contrast, artifacts were observed in 93.3% of SA planes, 23.1% of HLA planes and 33.3% of VLA planes in patients with left and right-sided PM systems. Artifacts were more likely to be present on SA planes compared to HLA planes (P=0.0001). In VLA planes of cine CMR, artifacts were more common in patients with ICD/BiV-ICD than those with PMs (P=0.012). The artifact size on every plane of cine CMR was significantly greater in patients with ICD/BiV-ICD compared with those with PM. Artifacts size in patients with ICD/BiV-ICD systems was significantly smaller in HLA planes than in SA or VLA planes (P<0.0001). Supplemental Figure 2 illustrates the correlation between artifact size in each plane and generator dimensions. The artifact area surrounding the tip of PM/ICD leads on cine CMR averaged 1.05±0.35 cm2 and 1.09±0.38 cm2 for PM and ICD leads, respectively.
Artifact Effects on Cardiac Function Evaluation by Cine CMR
CMR images of patients with left-sided ICD/BiV-ICD systems had more artifact effects on SA plane of cine CMR compared with those in patients with left-sided PM and right-sided PM/ICD (18.9 vs 0%, P<0.0001, respectively) (Supplemental Figure 3A). The anterior region on SA planes of cine CMR were more likely to be affected by artifact than other regions (P<0.0001) (Supplemental Figure 3B). Severe artifacts observed in more than half of the myocardial sectors on cine CMR precluded accurate cardiac function evaluation in 8 scans (14.5%). In contrast, it was possible to evaluate cardiac function by cine CMR in 47 of 55 CMR scans (85.5 %) in the setting of left-sided ICD/BiV-ICD systems and all CMR scans (16 scans) in patients with left-sided PM and right-sided PM/ICD. Patients in whom cardiac function evaluation was possible had higher BMI and greater left ventricular end-diastolic diameter (LVEDD) compared to those in whom cardiac function could not be calculated (P=0.019 for BMI, P=0.045 for LVEDD, Table 2).
Table 2.
Predictor of Cardiac Function Evaluation by Cine CMR
| Cardiac Function Evaluation by Cine CMR in patients with Left-sided ICD/BiV-ICD
|
P-Value | ||
|---|---|---|---|
| Possible N=47 Scans |
Impossible N=8 Scans |
||
| Age | 58±14 | 64±20 | 0.27 |
| Female/Male | 7/39 | 1/7 | 0.84 |
| Body Mass Index [kg/m2] | 26.9±3.9 | 23.1±2.8 | 0.019* |
| Ejection Fraction [%] | 34.0±16.1 | 34.0±17.1 | 0.996 |
| Left Ventricular End-Diastolic Diameter [mm] | 55.3±10.8 | 65.0±7.7 | 0.045* |
| Artifact Size on Short Axis of Cine MRI [cm2] | 200.3±32.4 | 186.6±19.7 | 0.478 |
| Minimum Distance from Generator to Heart [mm] | |||
| Frontal Chest X-ray | 31.7±20.9 | 15.7±15.0 | 0.08 |
| Lateral Chest X-ray | 32.2±4.0 | 33.2±3.6 | 0.58 |
| Generator | |||
| Height*Width [cm2] | 38.7±6.4 | 39.2±7.5 | 0.82 |
| Thickness [mm] | 13.0±1.8 | 13.0±1.7 | 0.99 |
| Weight [g] | 80.6±8.3 | 81.5±7.2 | 0.78 |
| Volume [cc] | 36.1±5.9 | 35.1±5.0 | 0.65 |
Values are shown as mean ± SD or N.
Significant P-Value defined as P<0.05 are shown by the asterisk (*).
Artifact Effects on LGE-CMR
In patients with left-sided ICD/BiV-ICD systems, artifacts on LGE-CMR were observed in 4501 of 8379 sectors (53.7%) in SA planes, 147 of 306 sectors (48.0%) in HLA planes, and 124 of 252 sectors (49.2%) in VLA planes. On the other hand, no artifact on LGE-CMR was confirmed in HLA and VLA planes in patients with left-sided PM, or any planes in patients with right-sided ICD/BiV-ICD systems. Only 146 of 1493 sectors (9.8%) in SA planes had artifacts in patients with left-sided PM (Figure 2A). The characteristic distribution of artifacts in patients with left-sided ICD/BiV-ICD devices has been summarized in Figure 2B. The anterior regions were more affected by artifact due to the ICD/BiV-ICD generator in SA planes. The apical myocardial regions were also influenced by the artifact compared with basal regions in HLA planes. In VLA planes the anterior apical regions were severely affected by the artifact. In comparison with left-sided ICD/BiV-ICD systems, fewer sectors needed to be excluded due to artifacts of left-sided PM generators (Figure 2C). The percentages of sectors with artifacts on LGE-CMR have been summarized by the 17-segment model (Figure 3). The mean distance from PM/ICD generator to the silhouette of heart on AP chest X-ray was 24.8±16.5 [range; 0–57] mm in patients with left-sided ICD/BiV-ICD and 29.3±20.5 [0–44.6] mm in left-sided PM. The distance from generator to heart was significantly associated with the percentage of the sectors with artifacts on LGE-CMR in each plane (R2=0.474, P<0.0001 in SA planes, R2=0.566, P<0.0001 in HLA planes, R2=0.391, P=0.0001 in VLA planes). The artifacts due to PM/ICD leads were much smaller than those due to the PM/ICD generators. Less artifact effects due to the PM/ICD leads were observed regardless of the image sequence and type of PM/ICD leads such as ICD, PM or coronary sinus leads.
Figure 2. Artifacts Effects on LGE-CMR.
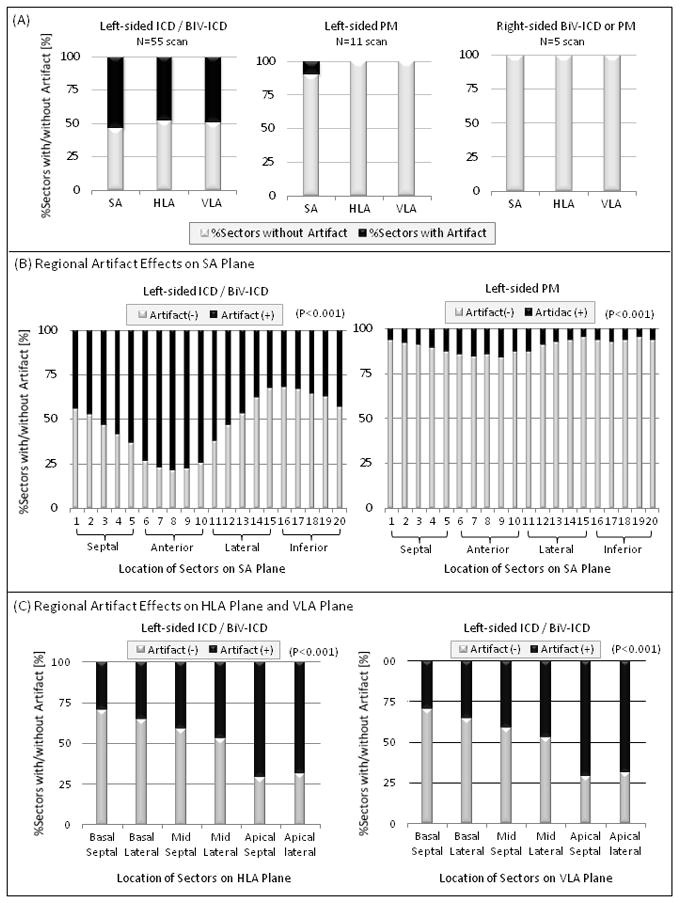
Artifact effects in each plane of LGE-CMR due to the generator were quantitatively assessed in patients with left and right-sided ICD/BiV-ICD or PM systems. (A) The greatest artifact on LGE-CMR was observed in patients with left-sided ICD/BiV-ICD. About 50% of the sectors were affected by the artifact. (B) Details about the regional artifact effects on SA planes are demonstrated in patients with left-sided ICD/BiV-ICD and PM systems. The anterior and apical regions were severely affected by artifacts due to the generator in patients with ICD/BiV-ICD systems. Smaller artifacts were observed in patients with left-sided PM systems. (C) The regional artifact effects on HLA and VLA plane are shown in patients with left-sided ICD/BiV-ICD systems. The apical regions on HLA and VLA planes were severely affected by the artifact.
BiV=biventricular; See abbreviations in Figure 1.
Figure 3. 17-Segment Model of Artifacts Effects on LGE-CMR.
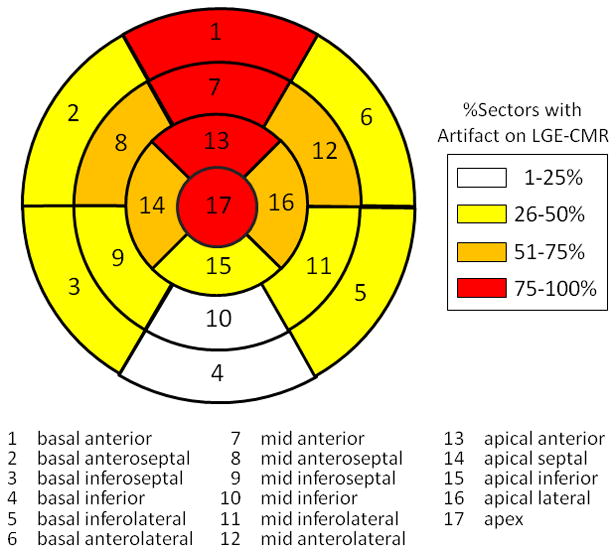
Artifact effects on LGE-CMR in patients with left-sided ICD/BiV-ICD are summarized using the 17-segment model. The percentages of sectors with artifact on LGE-CMR were divided into 4 groups (1–25, 26–50, 51–75, 76–100%).
Comparisons of Artifact Effects on cine CMR, LGE-CMR, T2-weighted and Perfusion CMR images
Of 55 patients with left-sided ICD systems, 13 patients (23.6%) underwent all T2-weighted, perfusion, cine, and LGE-CMR sequences. Artifact effects on the three corresponding SA planes of cine, T2-weighted, perfusion and LGE-CMR images were compared in each myocardial region such as septal, anterior, lateral and inferior regions (Figure 4). Compared with other image sequences, susceptibility artifacts due to the ICD/BiV-ICD generator were most extensive on LGE-CMR and affected all regions except the inferior wall (LGE-CMR vs. cine CMR, T2-weighted and perfusion CMR in septal, anterior and lateral myocardial regions; P<0.001, respectively). Most artifacts on T2-weigthed images were due to cardiac motion or arrhythmia rather than the susceptibility artifacts from the PM/ICD generator.
Figure 4. Comparison of Artifact Effects on Cine, T2-weighted, Perfusion, and LGE-CMR.
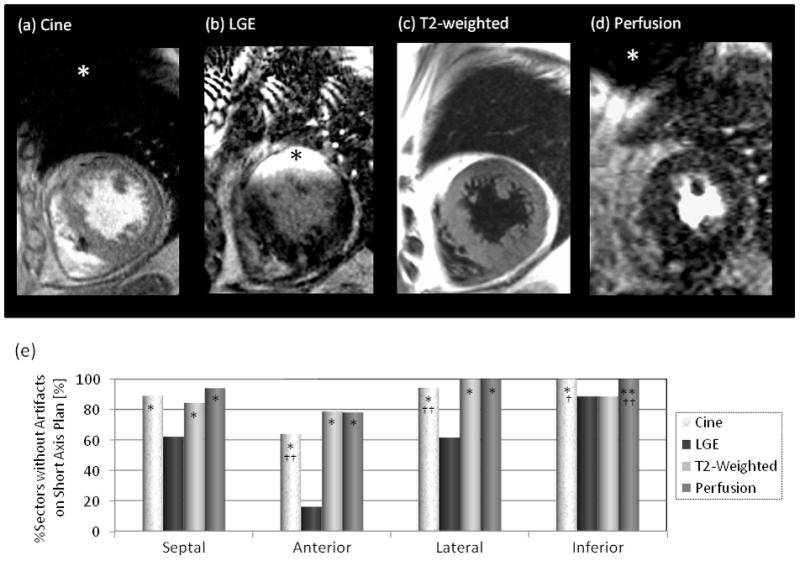
Comparison of artifact distribution and extent (asterisks) due to the ICD generator on (a) cine CMR, (b) LGE-CMR, (c) T2-weigthed and (d) perfusion CMR images. (e) The percentage of the sectors without any artifacts on short axis planes in each image sequence. Artifacts effects on LGE-CMR images were greater compared with the other images in all except the inferior myocardial regions (LGE-CMR vs. cine, T2-weighted and perfusion CMR in the septal, anterior and lateral myocardial regions; P<0.001, respectively).
*P<0.001 vs. LGE-CMR, **P<0.01 vs. LGE-CMR, †P<0.001 vs. T2-weighted CMR; ††P<0.01 vs. T2-weighted CMR.
See abbreviations in Figure 1.
Interpretability of CMR Images
The percentages of cine, perfusion, LGE-CMR, and MR angiography sequences with interpretable images have been summarized in Table 3. All CMR images were interpretable in patients with PM and right-sided ICD systems (16/16 scans; 100%). In contrast, interpretability of CMR images in patients with left-sided ICD/BiV-ICD systems was dependent upon the extent of susceptibility artifacts due to PM/ICD generators. Despite the presence of some artifact in most image sequences, images were completely (18/55 scans; 32.7%) or partially (31/55 scans, 56.4%) interpretable in most patients with left-side ICD/BiV-ICD systems.
Table 3.
Percentages of Scans with Interpretable Images in Patients with Cardiac Implantable Devices
| Interpretable Images [%] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Underlying Heart Diseases | Patient Number | LGE-CMR (N=71 Scans) | Cine CMR (N=71 Scans) | Perfusion CMR (N=36 Scans) | MR Angiography (N=32 Scans) | |||||||
| ⌾ | Δ | × | ⌾ | Δ | × | ⌾ | Δ | × | ⌾ | × | ||
| Left-sided ICD/BiV-ICD | ||||||||||||
| Ischemic Cardiomyopathy | ||||||||||||
| Anteroseptal MI | 20 | 5 | 90 | 5 | 85 | 0 | 15 | 57 | 43 | 0 | 100 | 0 |
| Inferior MI | 14 | 0 | 85 | 15 | 77 | 8 | 15 | 17 | 83 | 0 | 100 | 0 |
| Diffuse | 4 | 25 | 75 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 100 | 0 |
| Dilated Cardiomyopathy | 10 | 10 | 80 | 10 | 100 | 0 | 0 | 0 | 50 | 50 | 100 | 0 |
| ARVC | 4 | 0 | 100 | 0 | 75 | 0 | 25 | 100 | 0 | 0 | 100 | 0 |
| Cardiac Sarcoidosis | 2 | 0 | 100 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 |
| Hypertrophic Cardiomyopathy | 1 | 0 | 100 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 |
| Idiopathic Ventricular Tachycardia | 1 | 100 | 0 | 0 | 100 | 0 | 0 | (−) | (−) | (−) | (−) | (−) |
| Left-sided PM, Right-sided PM/ICD | ||||||||||||
| Congenital heart disease | 7 | 86 | 14 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 |
| Myocardial Dystrophy | 3 | 67 | 33 | 0 | 100 | 0 | 0 | 67 | 33 | 0 | (−) | (−) |
| Dilated Cardiomyopathy | 1 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | (−) | (−) |
| ARVC | 1 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | (−) | (−) |
| Cardiac Sarcoidosis | 1 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | (−) | (−) |
| Hypertrophic Cardiomyopathy | 1 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | (−) | (−) |
| Neuromediated Syncope | 2 | 100 | 0 | 0 | 100 | 0 | 0 | (−) | (−) | (−) | (−) | (−) |
Values are shown as number or percentages. The interpretability of the CMR images were defined as “completely interpretable (⌾)”, “partially interpretable (Δ)”and “impossible to interpret (×)”.
CMR=cardiac magnetic resonance imaging; ARVC=arrhythmogenic right ventricular cardiomyopathy; LGE=late gadolinium enhanced; PM=PM. See abbreviations in Table 1.
Discussion
Despite prior demonstration of overall safety, MRI in the setting of PM/ICD systems may be associated with risks including heating, current induction leading to arrhythmia, generator movement, and/or PM/ICD malfunction.1–12 Therefore, the risks of CMR must be weighed against the potential clinical utility of images to be acquired in each case. By reporting the extent of artifact in each sequence and associations with generator size, location, and patient characteristics, this study enables improved patient selection for CMR. The extent of each plane involved by artifact on cine CMR was dependent upon the imaging plane. Artifacts were more pronounced in the SA plane compared to HLA and VLA planes largely due to the proximity between the PM/ICD generator and affected regions of the heart in each plane. Artifact size on cine CMR was also significantly associated with the size of the PM/ICD generator. We found that the artifact size due to ICD/BiV-ICD devices was greater than that with PM devices in proportion to the size of PM/ICD generator. It was possible to evaluate cardiac function using cine CMR in 86% of patients with left sided ICD. The most significant predictors of the capability to assess cardiac function were BMI and LVEDD. Both associations are likely mediated by the distance between the PM/ICD generator and the heart. Scans with right-sided ICD/BiV-ICD and PM systems had no effects on LGE-CMR images. In patients with left-sided ICD/BiV-ICD systems the artifacts on LGE-CMR images were most often localized to the anterior and apical myocardial regions. The artifact effects on LGE-CMR were significantly greater than cine, T2-weighted, and perfusion CMR images in patients with left-sided ICD systems. T2-weighted images scanned by the turbo spin echo sequence had less susceptibility artifacts due to PM/ICD generator compared with other image sequences.25,26 Additionally the distance between the PM/ICD generator and cardiac silhouette on frontal chest X-ray (AP) was inversely associated with artifact size on LGE-CMR.
Artifacts Effects on CMR due to PM/ICD Leads
Artifacts on CMR created by PM/ICD leads are smaller than those by PM/ICD generators. Artifacts due to PM/ICD leads did not affect image interpretation in any patient regardless of the image sequence or the type of lead. The conducting wires and ICD coils of PM/ICD leads, although ferromagnetic, are thin and therefore associated with significantly less artifact compared with PM/ICD generators. In addition, the lead tips are made from non-ferromagnetic materials such as platinum or other alloys which result in minimal artifact on CMR images. Artifacts of PM/ICD leads on cardiac CT are qualitatively larger than those on CMR (Figure 5).27 Based upon our experience, CMR appears to be the superior modality for evaluation of myocardium near PM/ICD leads (e.g to rule out perforation).
Figure 5. Artifacts Effects on CMR due to PM/ICD Leads.
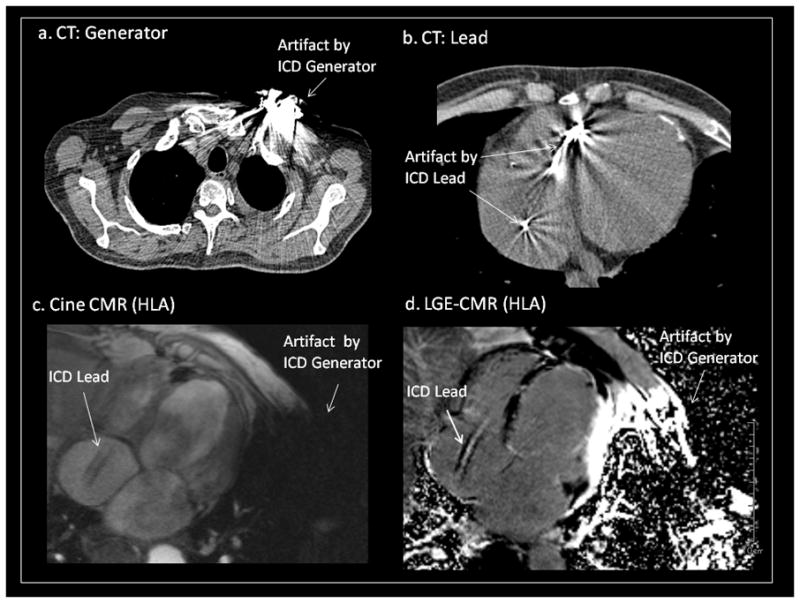
Comparison of artifacts due to the ICD generator and lead in both CT (a, b) and MRI (c, d) in a patient with single chamber ICD. Artifacts due to the lead were smaller on MRI compared with CT. In contrast, artifacts due to the generator were larger with MRI.
CT=computed tomography. See abbreviations in Figure 1.
Mechanism of PM/ICD Artifacts on MRI
Metallic PM/ICD components have magnetic susceptibilities that are very different from human tissue. Such disparities in magnetic susceptibility lead to significant distortion of the MRI magnetic field and result in image artifacts (Figure 5). Various metallic PM/ICD components contribute differently to the observed artifact. For example, ferromagnetic components such as stainless steel made from iron alloys result in significantly larger artifacts than components made from materials such as titanium which have much lower relative magnetic susceptibility.25,26 The size and orientation of the artifact are associated with the direction and strength of the magnetic field, the relative magnetic susceptibility of the PM/ICD, and the type of pulse sequence being used. SSFP gradient echo and inversion recovery sequences with longer echo times are associated with increased magnetic susceptibility artifacts compared to gradient echo and spin echo sequences.25,26 Artifacts due to PM/ICD generators can be reduced by use of lower magnetic field strength and shorter echo times, however such adjustments may compromise the image signal intensity and contrast. The use of spin echo techniques produces black blood contrast which is not always desirable and is typically associated with high SAR which may reduce safety.1–12,24 Importantly, PM and ICD/BiV-ICD systems substantially differ from other metal implants such as orthopedic artificial joints26 and dental implants.28 PM/ICD are intricate electronic devices, and their dysfunction can be directly associated with life-threatening events. These limitations must be taken into account when adjusting parameters to reduce artifact size on CMR.
Study Limitations
In the current study, we analyzed the artifacts on the most commonly utilized sequences in CMR including SSFP cine, LGE, T2-weighted, perfusion and contrast-enhanced MR angiography. Artifacts in other image sequences were not evaluated. Additionally, newer “MRI conditional devices” were not studied. However, the modifications incorporated into such systems primarily focuses on safety, rather than artifact reduction. This study included only 1 patient with a right-sided BiV-ICD system, therefore artifact effects in this setting could not be sufficiently evaluated. All PM/ICD generators were implanted in the infraclavicular pre-pectoral area; therefore, artifact effects due to submuscular devices were not investigated. Although interpretability was assessed by two independent observers and disagreements were rare, the measure is subjective and an inter-observer reliability analysis was not performed. Distance between the generator and the cardiac silhouette on frontal chest X-ray was considered the most readily available quantifiable parameter prior to CMR and was therefore used as predictor of artifact effects in this study. However, the distance on frontal x-ray is an uni-dimensional surrogate of the true distance and 3-dimensional imaging techniques may improve the association.
Conclusions
This study demonstrated artifact characteristics on SSFP cine, T2-weighted, perfusion, LGE-CMR and MR angiography in patients with PM/ICD. The utility of CMR in patients with left-sided ICD/BiV-ICD systems may be limited due to larger PM/ICD artifacts than in patients with PM or right-sided ICD/BiV-ICD systems. Artifact reduction methodologies for CMR in the setting of left-sided PM/ICD systems warrant further investigation.
Supplementary Material
Clinical Implication.
The decision to perform CMR in patients with cardiac pacemakers (PMs) and implantable cardiac defibrillators (ICDs) depends on the balance of risks versus benefits of imaging in each individual. While considerable work has focused on safety considerations, this work evaluated the potential limitations imposed on CMR by susceptibility artifacts in patients with PM/ICDs. In patients with left-sided PM, and right-sided PM/ICD systems, CMR images had minimal artifacts regardless of the image sequence and were completely interpretable. In contrast, in patients with left-sided ICD/BiV-ICD systems, artifact effects on LGE-CMR were greater than those on MR angiography, cine, T2-weighted, and perfusion CMR. We found it particularly difficult to evaluate the anterior and apical regions on LGE-CMR of patients with left sided ICD/BiV-ICD systems. Lower BMI, larger generator size, larger LVEDD, and shorter distance between the PM/ICD generator and the cardiac silhouette on chest X-ray are associated with greater artifact size on CMR. The results of this study may improve patient selection for CMR in the setting of PM/ICD systems.
Acknowledgments
Sources of Funding
Dr. Sasaki is funded by the Francis Chiaramonte MD Private Foundation. Dr. Nazarian is supported by Career Development Award K23HL089333 and Dr. Halperin by Grant R01-HL65795 from the National Institutes of Health.
Abbreviations
- BiV-ICD
biventricular ICD
- BMI
body mass index
- CMR
cardiac magnetic resonance imaging
- CT
computed tomography
- HLA
horizontal long axis
- ICD
Implantable cardioverter defibrillator
- LGE-CMR
late gadolinium enhanced CMR
- LVEDD
left ventricular end-diastolic diameter
- MRI
magnetic resonance imaging
- PM
pacemaker
- SA
short axis
- SAR
specific absorption rate
- SSFP
steady-state free precession
- VLA
vertical long axis
Footnotes
Disclosures
Dr. Nazarian has received honoraria for lectures from St. Jude Medical Inc., Boston Scientific Inc., and Biotronic Inc. Dr. Halperin has received research grant and consultant fees from Zoll Circulation Inc., and has ownership interests in MRI International Inc. and IMRICOR Medical Systems Inc. Dr. Calkins has received honoraria from Biosense Webster Inc. and Medtronic Inc. Dr. Berger has received research grants from St. Jude Medical Inc. and Medtronic Inc. and consultant fees from Boston Scientific Crop. and Cameron Health Inc. The Johns Hopkins University Conflict of Interest Committee manages all commercial arrangements.
References
- 1.Nazarian S, Roguin A, Zviman MM, Lardo AC, Dickfeld TL, Calkins H, Weiss RG, Berger RD, Bluemke DA, Halperin HR. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent PMs and ICDs at 1.5 tesla. Circulation. 2006;114:1277–1284. doi: 10.1161/CIRCULATIONAHA.105.607655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roguin A, Zviman MM, Meininger GR, Rodrigues ER, Dickfeld TM, Bluemke DA, Lardo A, Berger RD, Calkins H, Halperin HR. Modern PM and ICD systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation. 2004;110:475–482. doi: 10.1161/01.CIR.0000137121.28722.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazarian S, Halperin HR. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart Rhythm. 2009;6:138–143. doi: 10.1016/j.hrthm.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Pulver AF, Puchalski MD, Bradley DJ, Minich LL, SUJT, Saarel EV, Whitaker P, Etheridge SP. Safety and imaging quality of MRI in pediatric and adult congenital heart disease patients with PMs. PACE. 2009;32:450–456. doi: 10.1111/j.1540-8159.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 5.Naehle CP, Strach K, Thomas D, Meyer C, Linhart M, Bitaraf S, Litt H, Schwab JO, Schild H, Sommer T. Magnetic resonance imaging at 1.5-T in patients with ICDs. J Am Coll Cardiol. 2009;54:549–555. doi: 10.1016/j.jacc.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 6.Tian J, Smith MF, Jeudy J, Dickfeld T. Multimodality fusion imaging using delayed-enhanced cardiac magnetic resonance imaging, computed tomography, positron emission tomography, and real-time intracardiac echocardiography to guide ventricular tachycardia ablation in ICD patients. Heart Rhythm. 2009;6:825–828. doi: 10.1016/j.hrthm.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Roguin A, Donahue JK, Bomma CS, Bluemke DA, Halperin HR. Cardiac magnetic resonance imaging in a patient with ICD. Pacing Clin Electrophysiol. 2005;28:336–338. doi: 10.1111/j.1540-8159.2005.40032.x. [DOI] [PubMed] [Google Scholar]
- 8.Sommer T, Naehle CP, Yang A, Zeijlemaker V, Hackenbroch M, Schmiedel A, Meyer C, Strach K, Skowasch D, Vahlhaus C, Litt H, Schild H. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac PMs in non-PM-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114:1285–1292. doi: 10.1161/CIRCULATIONAHA.105.597013. [DOI] [PubMed] [Google Scholar]
- 9.Levine GN, Gomes AS, Arai AE, Bluemke DA, Flamm SD, Kanal E, Manning WJ, Martin ET, Smith JM, Wilke N, Shellock FS. Safety of magntic resonance imaging in patients with cardiovascular devices: An American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: Endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878–2891. doi: 10.1161/CIRCULATIONAHA.107.187256. [DOI] [PubMed] [Google Scholar]
- 10.Martin ET, Coman JA, Shellock FG, Pulling CC, Fair R, Jenkins K. Magnetic resonance imaging and cardiac pacemaker safety at 1.5-Tesla. J Am Coll Cardiol. 2004;43:1315–24. doi: 10.1016/j.jacc.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Mollerus M, Albin G, Lipinski M, Lucca J. Magnetic resonance imaging of pacemakers and implantable cardioverter-defibrillators without specific absorption rate restrictions. Europace. 12:947–51. doi: 10.1093/europace/euq092. [DOI] [PubMed] [Google Scholar]
- 12.Baker KB, Tkach JA, Nyenhuis JA, Phillips M, Shellock FG, Gonzalez-Martinez J, Rezai AR. Evaluation of specific absorption rate as a dosimeter of MRI-related implant heating. J Magn Reson Imaging. 2004;20:315–20. doi: 10.1002/jmri.20103. [DOI] [PubMed] [Google Scholar]
- 13.Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol. 2009;54:1407–1424. doi: 10.1016/j.jacc.2009.04.094. [DOI] [PubMed] [Google Scholar]
- 14.McCrohon JA, Prasad SK, McKenna WJ, Lorenz CH, Coats AJS, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using Galinium-Enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 15.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 16.Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, Meininger GR, Roguin A, Calkins H, Tomaselli GF, Weiss RG, Berger RD, Lima JAC, Halperin HR. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–2825. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marbán E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tandri H, Castillo E, Ferrari VA, Nasir K, Dalal D, Bomma C, Calkins H, Bluemke DA. Magnetic resonance imaging of arrhythmogenic right ventricular dysplasia: sensitivity, specificity, and observer variability of fat detection versus functional analysis of the right ventricle. J Am Coll Cardiol. 2006;48:2277–2284. doi: 10.1016/j.jacc.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Roes SD, Borleffs CJ, van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA, Reiber JH, Zeppenfeld K, Lamb HJ, de Roos A, Schalij MJ, Bax JJ. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and ICD. Circ Cardiovasc Imaging. 2009;2:183–190. doi: 10.1161/CIRCIMAGING.108.826529. [DOI] [PubMed] [Google Scholar]
- 20.Cobelli FD, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, Canu T, Perseghin G, Gaudio C, Maseri A, Frustaci A, Maschio AD. Delayed Gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol. 2006;47:1649–54. doi: 10.1016/j.jacc.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 21.Cheong BYC, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, Elayda MA, Lee VV, Flamm SD. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–2076. doi: 10.1161/CIRCULATIONAHA.109.852517. [DOI] [PubMed] [Google Scholar]
- 22.Bogun FM, Desjardins B, Good E, Gupta S, Crawford T, Oral H, Ebinger M, Pelosi F, Chugh A, Jongnarangsin K, Morady F. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol. 2009;53:1138–1145. doi: 10.1016/j.jacc.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desjardins B, Crawford T, Good E, Oral H, Chugh A, Pelosi F, Morady F, Bogun F. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009;6:644–651. doi: 10.1016/j.hrthm.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schueler BA, Parrish TB, Lin JC, Hammer BE, Pangrle BJ, Ritenour ER, Kucharczyk J, Truwit CL. MRI compatibility and visibility assessment of implantable medical devices. J Magn Reson Imaging. 1999;9:596–603. doi: 10.1002/(sici)1522-2586(199904)9:4<596::aid-jmri14>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Suh JS, Jeong EK, Shin KH, Cho JH, Na JB, Kim DH, Han CD. Minimizing artifacts caused by metallic implants at MR imaging: experimental and clinical studies. AJR Am J Roentgenol. 1998;171:1207–1213. doi: 10.2214/ajr.171.5.9798849. [DOI] [PubMed] [Google Scholar]
- 26.Stradiotti P, Curti A, Castellazzi G, Zerbi A. Metal-related artifacts in instrumented spine. Techniques for reducing artifacts in CT and MRI: state of the art. Eur Spine J. 2009;18:102–108. doi: 10.1007/s00586-009-0998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiFilippo FP, Brunken RC. Do implanted PM leads and ICD leads metal-related artifact in cardiac PET/CT? J Nucl Med. 2005;46:436–443. [PubMed] [Google Scholar]
- 28.Eggers G, Rieker M, Kress B, Fiebach J, Dickhaus H, Hassfeld S. Artifacts in magnetic resonance imaging caused by dental material. MAGMA. 2005;18:103–111. doi: 10.1007/s10334-005-0101-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


