Abstract
Objectives
Smoking is the single most preventable cause of perinatal morbidity. This study examines smoking behaviors during pregnancy in a high risk population of African Americans. The study also examines risk factors associated with smoking behaviors and cessation in response to a cognitive behavioral therapy (CBT) intervention.
Methods
This study is a secondary analysis of data from a randomized controlled trial addressing multiple risks during pregnancy. Five hundred African-American Washington DC residents who reported smoking in the six months preceding pregnancy were randomized to a CBT intervention. Psycho-social and behavioral data were collected. Self-reported smoking and salivary cotinine levels were measured prenatally and postpartum to assess the changes in smoking behavior. Comparisons were made between active smokers and those abstaining at baseline and follow-up in pregnancy and at postpartum.
Results
Sixty percent of participants reported quitting spontaneously during pregnancy. In regression models, smoking at baseline was associated with older age, < a high school education and illicit drug use. At follow-up closest to delivery, smoking was associated with lower education, smoking and cotinine level at baseline and depression. At postpartum, there was a relapse of 34%. Smokers postpartum were significantly more likely to smoke at baseline and use illicit drugs in pregnancy. Mothers in the intervention were less likely to relapse.
Conclusions
African-American women had a high spontaneous quit rate and no response to a behavioral intervention during pregnancy. Postpartum mothers’ resolve to maintain a quit status seems to wane despite their prolonged period of cessation. CBT reduced postpartum relapse rates.
Introduction
Smoking during pregnancy is associated with problems of placentation [1], low birthweight [2–8], prematurity [3,9,10], sudden infant death [10–12], infant mortality [12], and later physical [13], developmental [13] and behavioral [10,14,15] problems. A significant percentage of smokers continue to smoke during pregnancy and are unable to quit on their own despite their knowledge of the risks involved. Existing behavioral interventions are only modestly successful. Such interventions have had an attributable benefit of no more than 10% above that of spontaneous quit rates among pregnant women [16].
Success in smoking cessation during pregnancy could be different among different ethnic groups. In one study, Mexican-Americans were found to have three times higher cessation rates than non-Hispanic whites [17]. There are only a few studies in the literature describing African-American smoking behaviors during pregnancy and postpartum and even fewer testing the efficacy of smoking cessation interventions programs in that population. Although rates of smoking in pregnancy are lower among African Americans, genetically mediated differences in nicotine metabolism are associated with higher nicotine levels among African Americans compared to whites [18].
High rates of low birthweight and prematurity in African-Americans may be partly attributable to smoking in pregnancy [19], either independently or as a complicating factor for other medical risks including chronic hypertension. Although contested by some authors in the literature [20], an estimated effect of smoking on poor birth outcomes may be as high as 14.4% among black births [21]. In an effort to improve birth outcomes among African-Americans, there is a need to further understand smoking behaviors in pregnancy and postpartum including spontaneous cessation and relapse rates, associated variables impacting on these rates, and response to behavioral interventions.
This is a secondary analysis of a larger randomized controlled trial (RCT) addressing multiple risks during pregnancy. The main results of the RCT have been published elsewhere [22,23]. This paper investigates smoking cessation and relapse rates among African American women reporting smoking in the six months preceding pregnancy in Washington, D.C. Women were recruited during pregnancy and followed through the postpartum period and were randomized to an integrated cognitive behavioral intervention, addressing smoking, environmental tobacco smoke exposure (ETSE), depression and intimate partner violence (IPV).
Method
Study Population
The population recruited to this study was part of a larger cohort recruited to the District of Columbia Healthy Outcomes of Pregnancy Education (DC-HOPE), under the umbrella of the National Institutes of Health-District of Columbia Initiative to Reduce Infant Mortality in Minority Populations. DC-HOPE was a randomized controlled trial evaluating the efficacy of an integrated cognitive behavioral intervention targeting cigarette smoking, environmental tobacco smoke exposure (ETSE), intimate partner violence (IPV) and depression during pregnancy. Mothers were eligible if they were 18 years or older, English-speaking, less than 29 weeks gestation and Washington, DC residents. Women were recruited from six prenatal care sites and were screened using an audio-computer assisted self-interview (A-CASI) (For details see El-Khorazaty, et al. [24]). Recruitment occurred between July 2001 and October 2003 and followed through July 2004. Baseline interviews for eligible women occurred on average 9 days after screening. IRB approval was obtained from all participating institutions.
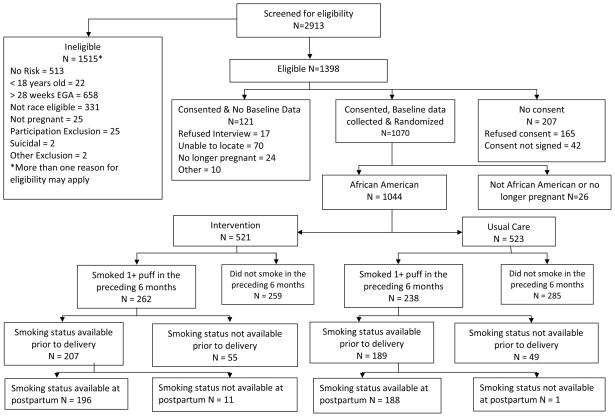
There were 2,913 women screened and 1,515 were ineligible. Of the 1,398 eligible women, 1,070 enrolled as eligible minority participants. (See Figure 1.) 1,044 women were included in these analyses; they self-identified as African-American. Eligible women were consented for randomization into the intervention or usual care group. Permuted block randomization was site- and risk-specific. The field staff were blinded with respect to the block size. Eight women (6 intervention and 2 usual care) were identified as suicidal during intervention or data collection and were referred immediately to mental health care and excluded from further participation. Five hundred women were screened into the study as having smoked a puff of a cigarette or more in the six months preceding pregnancy. This level was chosen to be as inclusive as possible because these women were at risk for continuing to smoke or relapsing if they had quit, early in pregnancy.
Figure 1.
Profile of Project DC-HOPE Randomized Controlled Trial
Data and Saliva Sample Collection
Data on sociodemographic and behavioral risk were collected during a baseline telephone interview, on average within nine days of screening. Follow-up telephone interviews were conducted during the second and third trimesters (22–26 weeks and 30–34 weeks, respectively) and 8–10 weeks postpartum to evaluate changes in the psycho-behavioral risks. Interviewers were blinded to whether women were in the intervention or usual care group. Smoking risks during pregnancy and postpartum were measured based on self-report. Saliva samples were collected at the prenatal care site on average within 19 days following the baseline interview, within a week from the follow-up telephone interview and 23 days following the postpartum interview. Salivary cotinine was measured by a radio-immune assay using gas chromatography-mass spectrometry (GC/MS) with lower detection limits of 10 ng/ml. IPV was measured using the Revised Conflict Tactics Scale physical assault and sexual coercion subscales [25]. Depression was measured using the 20-item Hopkins Symptom Checklist-Depression Scale [26].
Intervention
Of the 500 women included in these analyses, 262 were randomized to the intervention group and 238 were randomized to usual care. This integrated intervention was based on a conceptual framework of overlapping and interactive behavioral risks. Such risk factors are known to co-occur within a population of urban African-Americans living in communities with high poverty rates. The risks selected are all associated with poor pregnancy outcomes [27]. The smoking intervention was delivered to women who self-reported as smokers and not on a cutoff cotinine level since randomization was based on the initial response to the A-CASI.
The ten session intervention was delivered during prenatal (8 sessions) and postpartum (2 booster sessions) care visits. Four prenatal sessions were considered minimal adherence and the session duration was approximately 35 minutes. The smoking intervention was consistent with the Smoking Cessation or Reduction in Pregnancy Trial (SCRIPT) and the Counseling and Behavioral Interventions Work Group of the United States Preventive Services Task Force recommendations, a five-step behavioral counseling approach [28,29]. The intervention was tailored to the woman’s stage of change. Women were encouraged to avoid triggers and to use alternative coping and behavioral change strategies. The intervention included content to address both active smoking and ETSE, whether or not they met criteria for ETSE.
The intervention sessions also addressed the other associated risks. For depression, the intervention focused on secondary prevention of symptoms in pregnancy and extended into the postpartum period. Cognitive behavioral therapy strategies for mood management, increasing positive social interactions and pleasurable activities were emphasized. The IPV interventions used the Parker’s model to address the role of a negative partner support [30]. Danger assessment to identify risks for harm, and prevention options were considered along with the development of a safety plan. (For more details see Katz, et al. [27]). All measures were based on validated questionnaires.
Statistical Analysis
Women who were screened as having smoked a puff of a cigarette or more in the six months preceding pregnancy were compared according to their self-reported smoking status at baseline interview, last follow-up interview prior to delivery, and postpartum interview conducted 8–10 weeks after delivery. Comparisons were conducted by means of Chi-square tests for binary variables and t-tests for continuous variables.
Based on the results of these bivariate comparisons, we then used logistic regression procedures to model the probability of cigarette smoking at each of the three time points controlling for covariates with p-value <0.10 in the bivariate analyses. For control variables with a strong colinearity, we selected those with the highest level of significance or the greatest biological plausibility. Control variables included alcohol and illicit drug use during pregnancy, depression, IPV, prior smoking status, cotinine levels, and the intervention. In addition, variables descriptive of demographic and socioeconomic status (maternal age, education level, and Medicaid enrollment) were included as covariates so that their cumulative effects could be accounted for in the final logistic model. We used the LOGISTIC procedure in SAS version 9.1.3 (SAS Institute, Cary, NC) to conduct the analysis.
Results
Of the 500 mothers reporting cigarette smoking at screening, data were available on 396 at a follow-up interview prior to delivery and 384 mothers were interviewed in the postpartum period. No significant differences between the 500 and either the 396 or the 384 were seen in any of the sociodemographic or behavioral characteristics at baseline. (Data not shown) No significant differences were noted between the intervention and usual care groups regarding sociodemographic or behavioral characteristics at baseline. Among the 500 women who reported smoking at A-CASI screening and included in these analyses, 39% reported active smoking at baseline. An earlier paper from our study showed that women who reported smoking at A-CASI screening were significantly less likely to resolve risk (smoking, ETSE, depression and IPV) during pregnancy [22].
A significant difference was noted in salivary cotinine levels collected at baseline between mothers who self-identified as smokers and non-smokers (179 ± 156 ng/ml vs. 32 ± 59 ng/ml, p<0.001). At baseline 86.4% of women who reported themselves as non-smokers had a salivary cotinine level <50 ng/ml and 90.3% < 100 ng/ml.
In a logistic regression model, the factors that remain significantly associated with smoking at baseline are reviewed in Table 4A. Older maternal age, education at less than high school level and illicit drug use as reported by mothers at baseline were the factors significantly associated with smoking at baseline.
Table 4.
Logistic Regression Models to Predict Active Smoking among Pregnant Women at Baseline, Follow-up, and Postpartum
| Characteristic | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|
| A. Active Smoking at Baseline(1)
| |||
| Maternal age | 1.14 | 1.10, 1.18 | |
|
| |||
| Education level: | |||
|
|
|||
| < High school | 2.43 | 1.30, 4.54 | |
|
|
|||
| Completed high school or GED | 1.36 | 0.75, 2.47 | |
|
|
|||
| At least some college (Reference) | 1.00 | --- | |
|
| |||
| Illicit drug use | 2.09 | 1.27, 3.44 | |
|
| |||
| B. Active Smoking at Follow-up(2)
| |||
| Active smoking at baseline | 18.54 | 8.63, 39.84 | |
|
|
|||
| Cotinine level at baseline (10 ng/ml) | 1.09 | 1.05, 1.13 | |
|
|
|||
| Depression at follow-up prior to delivery | 2.69 | 1.27, 5.68 | |
|
| |||
| C. Active Smoking at Postpartum(3)
| |||
| Active smoking at baseline | 10.89 | 5.28, 22.47 | |
|
| |||
| Cotinine level at baseline (10 ng/ml) | 1.04 | 1.01,1.08 | |
|
| |||
| Illicit drug use | 2.38 | 1.11, 5.12 | |
|
| |||
| Intervention group | 0.45 | 0.25, 0.80 | |
This model also controlled for Medicaid status, alcohol use during pregnancy, depression at baseline and IPV at baseline.
This model also controlled for maternal age, education, Medicaid enrollment status, alcohol and illicit drug use during pregnancy and IPV at follow-up.
This model also controlled for maternal age, education, Medicaid enrollment status, alcohol, depression at postpartum and IPV at baseline.
At follow-up prior to delivery, 34% of mothers reported smoking. A significant difference was noted in salivary cotinine levels collected at that time between those reporting smoking or not (135 ± 145 ng/ml vs. 28 ± 57 ng/ml, p<0.001). 83.5% of the women who reported themselves as non-smokers had a salivary cotinine level <50 ng/ml and 88.5% had a level <100 ng/ml. Of the women reporting smoking during the follow-up interview, 13.0% were not smoking at baseline and represented a relapse. Similarly, 12.8% of non-smokers had smoked at baseline but quit at a later stage of pregnancy. There was no significant interventional effect on smoking behavior as reported in the follow-up interviews during pregnancy. Women who continued to smoke during pregnancy were significantly older, had a lower level of education attainment, and had higher rates of enrollment in Medicaid. These women were also more likely to have reported alcohol and illicit drug use during the baseline interview and higher baseline cotinine levels. Depression at baseline was a predictor of smoking at follow-up while IPV was not. Depression and IPV confirmed during the follow-up interview were associated with smoking. No significant differences were seen between the characteristics of smokers randomized to the intervention group when compared to those in usual care. (Table 2)
Table 2.
Women Screening Positive for Smoking Before Pregnancy: Baseline Smokers Randomized to Intervention vs. Usual Care
| Characteristic | Intervention (n=105) | Usual Care (n=90) | p-value |
|---|---|---|---|
| Maternal age (Mean ± SD) | 26.9 ± 6.5 | 26.8 ± 6.1 | 0.930 |
|
| |||
| Pregnancies (incl. current) (Mean ± SD) | 4.8 ± 3.1 | 4.7 ± 2.6 | 0.948 |
|
| |||
| Previous live births (Mean ± SD) | 2.2 ± 2.1 | 2.2 ± 1.7 | 0.991 |
|
| |||
| Relationship status: | 0.522 | ||
| - Single/separated/widowed/divorced | 80 (76.2%) | 72 (80.0%) | |
| - Married/living with partner | 25 (23.8%) | 18 (20.0%) | |
|
| |||
| Education level: | 0.765 | ||
| - < High school | 51 (48.6%)
|
40 (44.4%)
|
|
| - High school/GED | 42 (40.0%)
|
37 (41.1%)
|
|
| - Some college or more | 12 (11.4%) | 13 (14.4%) | |
|
| |||
| Medicaid recipient | 95 (90.5%) | 79 (87.8%) | 0.545 |
|
| |||
| Alcohol use | 39 (37.1%) | 29 (32.2%) | 0.472 |
|
| |||
| Illicit drug use | 28 (26.7%) | 26 (28.9%) | 0.730 |
|
| |||
| Active smoking at follow-up | 62 (74.7%) | 54 (78.3%) | 0.607 |
|
| |||
| Active smoking at postpartum | 64 (83.1%) | 67 (91.8%) | 0.111 |
|
| |||
| Cotinine level at baseline (Mean ± SD) | 192.9 ± 165.0 | 162.4 ± 144.6 | 0.216 |
|
| |||
| Cotinine level at follow-up (Mean ± SD) | 146.0 ± 139.4 | 131.9 ± 117.6 | 0.528 |
|
| |||
| Cotinine level at postpartum (Mean ± SD) | 290.8 ± 182.7 | 236.1 ± 162.2 | 0.103 |
|
| |||
| ETSE at baseline | 89 (87.3%) | 75 (84.3%) | 0.555 |
|
| |||
| ETSE at follow-up | 66 (80.5%) | 52 (75.4%) | 0.448 |
|
| |||
| ETSE at postpartum | 58 (74.4%) | 56 (78.9%) | 0.516 |
|
| |||
| Depression at baseline | 57 (54.3%) | 45 (50.0%) | 0.550 |
|
| |||
| Depression at follow-up | 39 (47.0%) | 34 (49.3%) | 0.779 |
|
| |||
| Depression at follow-up postpartum | 23 (29.9%) | 23 (31.5%) | 0.828 |
|
| |||
| Intimate partner violence at baseline | 38 (36.2%) | 35 (38.9%) | 0.698 |
|
| |||
| Intimate partner violence at follow-up | 8 (9.8%) | 6 (8.7%) | 0.823 |
|
| |||
| Intimate partner violence at postpartum | 6 (7.8%) | 7 (9.6%) | 0.696 |
In a logistic regression model (Table 4B) the factors that retained significant association with continued smoking at follow-up were active smoking at baseline and salivary cotinine levels at baseline. Depression at the follow-up period preceding delivery was also predictive of active smoking during the same time period.
In the postpartum period, 50% of participants self-reported as actively smoking. The salivary cotinine levels were significantly higher in women reporting active smoking (249 ± 176 ng/ml vs. 109 ± 149 ng/ml, p<0.001). Only 60.4% of the women who reported themselves as non-smokers had a salivary cotinine level <50 ng/ml, and 64.4% had cotinine levels below 100 ng/ml. The intensity of smoking in the postpartum was significantly higher than during the two preceding time points as confirmed by cotinine levels. Among postpartum smokers salivary cotinine levels were significantly higher than the levels at baseline (p<0.001) or the levels at follow-up closest to delivery (p<0.001). Of the women reporting smoking postpartum, 34% had not reported smoking during the follow-up interview during pregnancy. This group would be considered postpartum relapsers. A much smaller percentage (7.4%) of women not reporting smoking postpartum had reported smoking during pregnancy. A higher likelihood of smoking postpartum was associated in bivariate analysis with older age, higher gravidity and parity, lower educational attainment, higher Medicaid enrollment, other substance use, active smoking at baseline and follow-up (Table 3). Depression documented at baseline, during follow-up interviews or in the postpartum was significantly associated with active smoking. IPV did not show a similar association at any of the three time points. The intervention, for the first time, showed an association with reported smoking abstinence in the postpartum period, at a p-value of 0.053.
Table 3.
Women Screening Positive for Smoking Before Pregnancy: Postpartum Assessment
| Characteristic | Smoking (n=191) | Not Smoking (n=193) | p-value |
|---|---|---|---|
| Maternal age (Mean ± SD) | 25.9 ± 6.0 | 24.0 ± 4.9 | <0.001 |
|
| |||
| Pregnancies (incl. current) (Mean ± SD) | 4.5 ± 2.6 | 3.3 ± 2.2 | <0.001 |
|
| |||
| Previous live births (Mean ± SD) | 2.0 ± 1.9 | 1.2 ± 1.4 | <0.001 |
|
| |||
| Relationship status: | 0.957 | ||
| - Single/separated/widowed/divorced | 149 (78.0%) | 151 (78.2%) | |
| - married/living with partner | 42 (22.0%) | 42 (21.8%) | |
|
| |||
| Education level: | <0.001 | ||
| < High school | 89 (46.6%)
|
55 (28.5%)
|
|
| High school/GED | 79 (41.4%)
|
96 (49.7%)
|
|
| Some college or more | 23 (12.0%) | 42 (21.8%) | |
|
| |||
| Medicaid recipient | 172 (90.1%) | 149 (77.6%) | <0.001 |
|
| |||
| Alcohol use | 67 (35.1%) | 43 (22.4%) | 0.006 |
|
| |||
| Illicit drug use | 57 (29.8%) | 23(11.9%) | <0.001 |
|
| |||
| Active Smoking at baseline | 131 (68.6%) | 19 (9.8%) | <0.001 |
|
| |||
| Active Smoking at follow-up | 100 (64.5%) | 8 (4.7%) | <0.001 |
|
| |||
| ETSE at baseline | 159 (85.0%) | 134 (70.5%) | <0.001 |
|
| |||
| ETSE at follow-up | 115 (74.7%) | 95 (55.6%) | <0.001 |
|
| |||
| ETSE at postpartum | 147 (79.0%) | 97 (51.1%) | <0.001 |
|
| |||
| Cotinine level at baseline (Mean ± SD) | 143.3 ± 155.4 | 36.3 ± 73.1 | <0.001 |
|
| |||
| Cotinine level at follow-up (Mean ± SD) | 122.3 ± 128.6 | 33.7 ± 75.0 | <0.001 |
|
| |||
| Cotinine level at postpartum (Mean ± SD) | 248.8 ± 176.0 | 109.3 ± 149.2 | <0.001 |
|
| |||
| Depression at baseline | 98 (51.3%) | 82 (42.5%) | 0.008 |
|
| |||
| Depression at follow-up | 78 (50.3%) | 59 (34.3%) | 0.003 |
|
| |||
| Depression at postpartum | 62 (32.6%) | 42 (21.8%) | 0.017 |
|
| |||
| Intimate partner violence at baseline | 73 (38.2%) | 57 (29.5%) | 0.072 |
|
| |||
| Intimate partner violence at follow-up | 16 (10.3%) | 11 (6.4%) | 0.200 |
|
| |||
| Intimate partner violence at postpartum | 22 (11.6%) | 15 (7.8%) | 0.210 |
|
| |||
| Intervention group | 88 (46.1%) | 108 (56.0%) | 0.053 |
In a logistic regression model, factors that increased the likelihood of reported smoking in the postpartum were active smoking as reported by mothers and cotinine levels at baseline as well as illicit drug use during pregnancy. The intervention had a significant protective effect against smoking in the postpartum period. (Table 4C)
Discussion
The results of this study confirm the difficulty pregnant mothers who smoke have in quitting during pregnancy. Mothers included in our study that were less educated, depressed or using illicit substances were least likely to quit. The literature emphasizes the underlying demographic and psychosocial factors that impact smoking behaviors among African-American women [31]. In spite of findings that African-Americans, were significantly more likely than whites to express a desire to quit smoking [32]. it is not clear what barriers prevent them from doing so. None of the studies meeting the guidelines for inclusion in the Public Health Service Report, [33] specified abstinence rates by racial/ethnic group [34], and the only studies that report on the results of their smoking cessation interventions during pregnancy by race come to opposite conclusions [35,36]. Studies in the literature examining smoking cessation interventions during pregnancy published since the Public Health Service Report, either did not report their spontaneous cessation and relapse rates in pregnancy and postpartum by race/ethnicity or found no differences in rates by race [37–41].
The results of our study agree with previous authors showing a significant spontaneous cessation rate among smokers who become pregnant [42, 43]. The quit rate of more than 60% in our population of urban African-American pregnant women experiencing other socioeconomic and psychological stressors is encouraging. It is worthy of note that amongst this population of smokers, women who reported quitting during pregnancy experienced a high rate of depression (42%) and IPV (29%) throughout the pregnancy. Almost one-third of these women who reported quitting on their own had not completed high school and the majority were Medicaid enrollees. It is hard to determine whether the social desirability of quitting during pregnancy within this community, and/or the knowledge of the detrimental effects of smoking on the fetus could have been the main driving force.
The underlying depressive symptoms in our study population may have interfered with their ability to control their smoking habit. There is a growing awareness of the prevalence of depressive symptoms within the smoking population, with a range of 22% to 61%, amongst those entering smoking cessation programs [44–46]. The current literature is mixed regarding the effect of depression on the success of smoking cessation, and one may infer that depression interferes with short term quit rates and not long-term success in smoking cessation [47,48]. In the logistic analyses we conducted at three time points, depression was predictive of smoking during the follow-up period during pregnancy but neither at baseline nor postpartum.
Other studies have confirmed the effect of psychosocial challenges as a mediator of smoking in pregnancy [49,50]. Alcohol and illicit drug use in the DC residents we recruited may have also interfered with their ability to quit. The relationship between alcohol use and smoking during pregnancy has been previously confirmed, although not in an exclusively African-American population [51]. Studies have shown smoking cessation in alcohol drinkers to be more difficult due to reactivity between alcohol and nicotine withdrawal [52,53]. In our logistic models, alcohol effect was not seen to be significantly associated with smoking at any of the three time points. Illicit drug use significantly increased the chances of smoking at baseline and during the postpartum period. Illicit drug use may have served as a surrogate for the severity of addiction to nicotine, a reliable marker for maintenance of smoking and failure of cessation attempts among African-Americans [54]. This is confirmed by our findings that active smoking at baseline and cotinine level at baseline, markers for intensity of smoking, were both predictors of smoking during the follow-up interview closest to delivery and postpartum. Other studies have shown similar results using reported number of cigarettes smoked early in pregnancy as a marker for intensity of smoking [40].
The literature has shown an association between poverty and smoking during pregnancy and postpartum [51,55,56]. In this study we used Medicaid enrollment as a marker for poverty. In bivariate analyses, Medicaid was a significant predictor of smoking during the three time points. In the logistic models, Medicaid lost its significance and a low level of education was only significantly associated with smoking at baseline. However, other studies have shown that education is negatively associated with smoking during pregnancy and with relapse after delivery [57]. It is plausible that level of education may influence the knowledge base mothers may draw upon in their decision making during pregnancy. A more compelling argument would be a high resilience in mothers attaining higher educational levels under significantly challenging living conditions in an environment of urban poverty. Such women may also possess a higher level of self-efficacy proven to impact significantly on successful smoking cessation [40]. Women with higher levels of education may have been a product of a more supportive social environment which is known to influence successful quitting during pregnancy as well [39].
A percentage of women may quit early in pregnancy due to physical aversion to tobacco smoke during the first trimester [58]. These pregnant mothers may then be susceptible to relapse at a later stage. Our results show relapse during pregnancy as reported by the population we studied (13%) to be lower than previously reported in the Canadian study (21%) [43]. In fact, the reported smoking rates in our population declined from 39% at baseline to 33% during follow-up.
The postpartum period represents a different challenge, where a high percentage of women resume their smoking habit after a prolonged period of cessation. The literature cites mediators to resumed smoking such as postpartum depression and concerns related to weight gain [59,60]. Although this has not been studied in populations that are predominately black, in a study based on the Pregnancy Risk Assessment Monitoring system, postpartum relapse was significantly more likely among black mothers.[59] It is also possible that mother’s have a lower level of awareness of the harms of ETSE to the infant as compared to in utero exposure. The added stress of parenting a newly born infant, especially within a population of mainly single mothers with limited social network and community support, may trigger the need for stress relief associated with the smoking habit [49]. Sixty-five percent of women who quit during pregnancy will have relapsed by three months and an additional 10% by six months postpartum [61]. Other studies showed that of those who quit smoking during pregnancy, half relapsed at 2–6 months [62] and 60% to 70% relapsed within one year [63] after delivery. In our study, the percentage of women who reported actively smoking increased from 33% to 50% during our follow-up period of ten weeks postpartum.
Few studies address the efficacy of interventions targeting reduction of postpartum relapse [64,65]. We were encouraged that this integrated intervention did impact on relapse rates reported postpartum. This could be explained by a longer exposure to the intervention, but also the emphasis on ETSE as a significant risk to the newborn infant which may have encouraged mothers to maintain their quit status. In addition, emphasis on mood regulation could have assisted mothers in dealing with postpartum depression as well as the stress associated with caring for a newborn. Previous studies showing similar success postpartum emphasized interventions including partners and close friends, and encouraging the social networks to support the mother in her decision [66,67]. Although our intervention did not address either of these strategies directly, it encouraged women to establish a supportive social network, and as such may have had similar effects. Furthermore studies have emphasized the postpartum success of interventions if they start earlier in pregnancy [67], which was our case.
The strength and the limitation of our study is that it was conducted with high risk African American women. The results cannot be generalizable to other populations without corroboration through similar studies in other diverse groups. Although the intervention did not influence smoking behavior significantly during the pregnancy, it had a protective effect against relapse during the postpartum. This study also confirmed the importance of associated addictions to illicit drugs and co-occurring depression as important associations with smoking during pregnancy in this population. More qualitative research to examine why African American women may or may not be inclined to stop smoking in pregnancy may inform research in the future in the design of appropriate interventions with efficacy in this population.
The results of this study support the importance of screening early in pregnancy and providing mothers with opportunities for behavioral modification through culturally informed interventions. Behavioral interventions for smoking should be available but cannot be relied upon alone as the intervention of choice for mothers who continue to smoke during pregnancy. More research is needed to test efficacy and safety of pharmacological therapy with proven efficacy in non-pregnant populations. Studies such as ours emphasize the importance of expanding prenatal care beyond the medical model in order to respond to the complex health risks of minority populations during pregnancy.
Table 1.
Women Screening Positive for Smoking Before Pregnancy: Baseline Assessment
| Characteristic | Smoking (n=195) | Not Smoking (n=305) | p-value |
|---|---|---|---|
| Maternal age (Mean ± SD) | 26.9 ± 6.3 | 23.6 ± 4.5 | <0.001 |
|
| |||
| Pregnancies (incl. current) (Mean ± SD) | 4.8 ± 2.9 | 3.5 ± 2.2 | <0.001 |
|
| |||
| Previous live births (Mean ± SD) | 2.2 ± 1.9 | 1.2 ± 1.4 | <0.001 |
|
| |||
| Relationship status: | 0.770 | ||
| - Single/separated/widowed/divorced | 152 (78.0%) | 241 (79.0%) | |
| - Married/living with partner | 43 (22.0%) | 64 (21.0%) | |
|
| |||
| Education level: | 0.002 | ||
| - < High school | 91 (46.7%) | 97 (31.8%) | |
| - High school/GED | 79 (40.5%) | 147 (48.2%) | |
| - Some college or more | 25 (12.8%) | 61 (20%) | |
|
| |||
| Medicaid recipient | 174 (89.2%) | 241 (79.3%) | 0.004 |
|
| |||
| Alcohol use | 68 (34.9%) | 72 (23.7%) | 0.007 |
|
| |||
| Illicit drug use | 54 (27.7%) | 47 (15.4%) | <0.001 |
|
| |||
| Depression | 102 (52.3%) | 129 (42.3%) | 0.029 |
|
| |||
| Intimate partner violence | 73 (37.4%) | 89 (29.2%) | 0.054 |
Acknowledgments
Funding
This work was supported by grants no. 3U18HD030445; 3U18HD030447; 5U18HD31206; 3U18HD031919; 5U18HD036104, Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center on Minority Health and Health Disparities, National Institutes of Health, Department of Health and Human Services. These analyses were supported, in part, by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
The authors wish to thank the field work staff, the interviewers, and data management staff. We wish to thank the participants who welcomed us into their lives in hopes of helping themselves and their children.
Footnotes
Competing Interests
None of the authors have any competing interests to declare.
References
- 1.Zdravkovic T, Genbacev O, McMaster MT, Fisher SJ. The adverse effects of maternal smoking on the human placenta: a review. Placenta. (26) 2005;(Suppl A):S81–S86. doi: 10.1016/j.placenta.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein IM, Mongeon JA, Badger GJ, Solomon L, Heil SH, Higgins ST. Maternal smoking and its association with birth weight. Obstet Gynecol. 2005;106(5):986–991. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]
- 3.Jaddoe VW, Troe EJ, Hofman A, Mackenbach JP, Moll HA, Steegers EA, Witteman JC. Active and passive maternal smoking during pregnancy and the risks of low birth weight and preterm: the generation R study. Paediatr Perinat Epidemiol. 2008;22:162–171. doi: 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 4.Li CQ, Windsor RA, Perkins L, Goldenberg RL, Lowe JB. The impact on infant birth weight and gestational age of cotinine-validated smoking reduction during pregnancy. JAMA. 1993;269:1519–1524. [PubMed] [Google Scholar]
- 5.Magee BD, Hattis D, Kivel NM. Role of smoking in low birth weight. J Reprod Med. 2004;49:23–27. [PubMed] [Google Scholar]
- 6.Peacock JL, Cook DG, Carey IM, Jarvis MJ, Bryant AE, Anderson HR, Bland JM. Maternal cotinine level during pregnancy and birth weight for gestational age. Int J Epidemiol. 1998;27:647–656. doi: 10.1093/ije/27.4.647. [DOI] [PubMed] [Google Scholar]
- 7.Secker-Walker RH, Vacek PM, Flynn BS, Mead PB. Smoking in pregnancy, exhaled carbon monoxide, and birth weight. Obstet Gynecol. 1997;89:648–653. doi: 10.1016/s0029-7844(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Tager IB, Van Vunakis H, Speizer FE, Harahan JP. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int J Epidemiol. 1997;26:978–988. doi: 10.1093/ije/26.5.978. [DOI] [PubMed] [Google Scholar]
- 9.Burns L, Mattick RP, Wallace C. Smoking patterns and outcomes in a population of pregnant women with other substance use disorders. Nicotine Tob Res. 2008;10:969–974. doi: 10.1080/14622200802097548. [DOI] [PubMed] [Google Scholar]
- 10.Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine Tob Res. 2008;10:267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- 11.Hunt CE, Hauck FR. Sudden infant death syndrome. CMAJ. 2006;174(13):1861–1869. doi: 10.1503/cmaj.051671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83:713–720. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Jaakkola JJK, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health. 2004;94(1):136–140. doi: 10.2105/ajph.94.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day NL, Richardson GA, Goldschmidt L, Cornelius MD. Effects of prenatal tobacco exposure on preschoolers’ behavior. J Dev Behav Pediatr. 2000;21:180–188. [PubMed] [Google Scholar]
- 15.Wakschlag LS, Han SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: A developmental framework. Dev Psychopathol. 2002;14:351–369. doi: 10.1017/s0954579402002092. [DOI] [PubMed] [Google Scholar]
- 16.Lumley J, Chamberlain C, Dowswell T, Oliver SS, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Systems Review. 2009 Jul;8(3):CD001055. doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camilli AE, McElroy LF, Reed KL. Smoking and pregnancy: A comparison of Mexican-American and non-Hispanic white women. Obstet Gynecol. 1994;84:1033–1037. [PubMed] [Google Scholar]
- 18.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal Cigarette Smoking, Metabolic Gene Polymorphism, and Infant Birth Weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 19.Moore ML, Zaccaro DJ. Cigarette smoking, low birth weight, and preterm births in low-income African American women. J Perinatol. 2000;3:176–180. doi: 10.1038/sj.jp.7200336. [DOI] [PubMed] [Google Scholar]
- 20.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2003 period linked birth/infant death data set. Natl Vital Stat Rep. 2006;54:1–29. [PubMed] [Google Scholar]
- 21.Barnett E. Race differences in the proportion of low birth weight attributable to maternal cigarette smoking in a low-income population. Am J Health Promot. 1995;10(2):105–110. doi: 10.4278/0890-1171-10.2.105. [DOI] [PubMed] [Google Scholar]
- 22.Joseph JG, El-Mohandes AAE, Kiely M, El-Khorazaty MN, Gantz MG, Johnson AA, Katz K, Blake SM, Rossi MW, Subramanian S. Reducing psychosocial and behavioral pregnancy risk factors: Results of a randomized clinical trial among high-risk pregnant African American women. Am J Public Health. 2009;99(6):1053–1061. doi: 10.2105/AJPH.2007.131425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Mohandes AA, Kiely M, Joseph JG, Subramanian S, Johnson AA, Blake SM, Gantz MG, El-Khorazaty MN. An intervention to improve postpartum outcomes in African-American mothers: a randomized controlled trial. Obstet Gynecol. 2008;112(3):611–20. doi: 10.1097/AOG.0b013e3181834b10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El- Khorazaty MN, Johnson AA, Kiely M, El-Mohandes AAE, Subramanian S, Laryea HA, Murray KB, Thornberry JS, Joseph JG. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. BMC Public Health. 2007;7:233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scale (CTS2): development and preliminary psychometric data. Journal of Family Issues. 1996;17:283–316. [Google Scholar]
- 26.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 27.Katz KS, Blake SM, Milligan RA, Sharps PW, White DB, Rodan MF, Rossi M, Murray KB. The design, implementation and acceptability of an integrated intervention to address multiple behavioral and psychosocial risk factors among pregnant African American women. BMC Pregnancy Childbirth. 2008;8:22. doi: 10.1186/1471-2393-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Windsor RA. Counseling smokers in Medicaid maternity care: The SCRIPT project. Tob Control. 2000;9(Suppl 1):i62. doi: 10.1136/tc.9.suppl_1.i62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitlock EP, Orleans CT, Pender N, Allan J. Evaluating primary care behavioral counseling interventions: an evidence-based approach. Am J Prev Med. 2000;22(4):267–284. doi: 10.1016/s0749-3797(02)00415-4. [DOI] [PubMed] [Google Scholar]
- 30.Parker B, McFarlane J, Soeken K, Silva C, Reel S. Testing an intervention to prevent further abuse to pregnant women. Res Nurs Health. 1999;22:59–66. doi: 10.1002/(sici)1098-240x(199902)22:1<59::aid-nur7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Webb MS, Carey MP. Tobacco smoking among low-income Black women: Demographic and psychosocial correlates in a community sample. Nicotine Tob Res. 2008;10:219–229. doi: 10.1080/14622200701767845. [DOI] [PubMed] [Google Scholar]
- 32.Royce JM, Hymowitz N, Corbett K, Hartwell TD, Orlandi MA. Smoking cessation factors among African-Americans and whites. COMMIT Research Group. Am J Public Health. 1993;83:220–226. doi: 10.2105/ajph.83.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaen CR, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Treating Tobacco Use and Dependence. AHRQ Publication No. 00–0032. Washington DC: U.S. Department of Health and Human Services; 2000. [Google Scholar]
- 34.Piper ME, Fox BJ, Welsch SK, Fiore MC, Baker TB. Gender and racial/ethnic differences in tobacco-dependence treatment: a commentary and research recommendations. Nicotine Tob Res. 2001;3:291–297. doi: 10.1080/14622200110050448. [DOI] [PubMed] [Google Scholar]
- 35.Gebauer C, Kwo CY, Haynes E, Wewers M. A nurse-managed smoking cessation intervention during pregnancy. J Obstet Gynecol Neonatal Nurs. 1998;27(1):47–53. doi: 10.1111/j.1552-6909.1998.tb02590.x. [DOI] [PubMed] [Google Scholar]
- 36.Windsor RA, Lowe J, Perkins L, Smith-Yoder D, Artz L, Crawford M, Amburgy K, Boyd NR., Jr Health education for pregnant smokers: Its behavioral impact and cost benefit. Am J Public Health. 1993;83:201–206. doi: 10.2105/ajph.83.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Händel G, Hannöver W, Röske K, Thyrian JR, Rumpf HJ, John U, Hapke U. Naturalistic changes in the readiness of postpartum women to quit smoking. Drug Alcohol Depend. 2009;101(3):196–201. doi: 10.1016/j.drugalcdep.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug Alcohol Depend. 2009;104(Suppl 1):S100–5. doi: 10.1016/j.drugalcdep.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y, Goins KV, Pbert L, Ockene JK. Predictors of smoking cessation in pregnancy and maintenance postpartum in low-income women. Matern Child Health J. 2005;9:393–402. doi: 10.1007/s10995-005-0020-8. [DOI] [PubMed] [Google Scholar]
- 40.Morasco BJ, Dornelas EA, Fischer EH, Oncken CA, Lando HA. Spontaneous smoking cessation during pregnancy among ethnic minority women: A preliminary investigation. Addict Behav. 2006;31:203–210. doi: 10.1016/j.addbeh.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Ruger JP, Weinstein MC, Hammond SK, Kearney MH, Emmons KM. Cost-effectiveness of motivational interviewing for smoking cessation and relapse prevention among low-income pregnant women: A randomized controlled trial. Value Health. 2008;11(2):191–198. doi: 10.1111/j.1524-4733.2007.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canadian Task Force on the Periodic Health Examination. The Canadian guide to clinical preventive health care. Ottawa: Canada Communication Group Publishing; 1994. p. 28. [Google Scholar]
- 43.Kirkland SA, Dodds LA, Brosky G. The natural history of smoking during pregnancy among women in Nova Scotia. CMAJ. 2000;163:281–282. [PMC free article] [PubMed] [Google Scholar]
- 44.Hall SM, Muñoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- 45.Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, Hartz DT. Mood management and nicotine gum in smoking treatment: A therapeutic contact and placebo-controlled study. J Consult Clin Psychol. 1996;64:1003–1009. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- 46.Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: Characteristics of depressed smokers and effects of nicotine dependence. J Consult Clin Psychol. 1996;64:791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- 47.Glassman AH, Covey LS, Dalack GW, Stetner F, Rivelli SK, Fleiss JF, Cooper TB. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther. 1993;54:670–679. doi: 10.1038/clpt.1993.205. [DOI] [PubMed] [Google Scholar]
- 48.Ginsberg D, Hall SM, Reus VI, Muñoz RF. Mood and depression diagnosis in smoking cessation. ExpClin Psychopharmacol. 1995;3:389–395. [Google Scholar]
- 49.Goedhart G, van der Wal MF, Cuijpers P, Bonsel GJ. Psychosocial problems and continued smoking during pregnancy. Addict Behav. 2009;34:403–406. doi: 10.1016/j.addbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 50.al’Absi M, Carr SB, Bongard S. Anger and psychobiological changes during smoking abstinence and in response to acute stress: Prediction of smoking relapse. Int J Psychophysiol. 2007;66:109–115. doi: 10.1016/j.ijpsycho.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin LT, McNamara M, Milot A, Bloch M, Hair EC, Halle T. Correlates of smoking before, during, and after pregnancy. Am J Health Behav. 2008;32:272–282. doi: 10.5555/ajhb.2008.32.3.272. [DOI] [PubMed] [Google Scholar]
- 52.McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl) 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmerman RS, Warheit GJ, Ulbrich PM. The relationship between alcohol use and attempts and success at smoking cessation. Addict Behav. 1990;15(3):197–207. doi: 10.1016/0306-4603(90)90063-4. [DOI] [PubMed] [Google Scholar]
- 54.Choi WS, Okuyemi KS, Kaur H, Ahluwalia JS. Comparison of smoking relapse curves among African-American smokers. Addict Behav. 2004;29:1679–1683. doi: 10.1016/j.addbeh.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 55.Jun H-J, Acevedo-Garcia D. The effect of single motherhood on smoking by socioeconomic status and race/ethnicity. Soc Sci Med. 2007;65:653–666. doi: 10.1016/j.socscimed.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 56.Everett-Murphy K, Steyn K, Mathews C, Petersen Z, Odendaal H, Gwebushe N, Lombard C. The effectiveness of adapted, best practice guidelines for smoking cessation counseling with disadvantaged, pregnant smokers attending public sector antenatal clinics in Cape Town, South Africa. Acta Obstet Gynecol Scand. 2010;89:478–89. doi: 10.3109/00016341003605701. [DOI] [PubMed] [Google Scholar]
- 57.Kahn RS, Certain L, Whittaker RC. A reexamination of smoking before, during, and after pregnancy. Am J Public Health. 2002;92:1801–1808. doi: 10.2105/ajph.92.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pletsch PK, Pollak KI, Peterson BL, Park J, Oncken CA, Swamy GK, Lyna P. Olfactory and gustatory sensory changes to tobacco smoke in pregnant smokers. Res Nurs Health. 2008;31:31–41. doi: 10.1002/nur.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen AM, Prince CB, Dietz PM. Postpartum depressive symptoms and smoking relapse. Am J Prev Med. 2009;36:9–12. doi: 10.1016/j.amepre.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 60.Quinn G, Ellison BB, Meade C, Roach CN, Lopez E, Albrecht T, Brandon TH. Adapting smoking relapse prevention materials for pregnant and postpartum women: Formative research. Matern Child Health J. 2006;10:235–245. doi: 10.1007/s10995-005-0046-y. [DOI] [PubMed] [Google Scholar]
- 61.US Department of Health and Human Services. DHHS Publication No. (CDC) 90–8416. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease prevention and Health Promotion, Office on Smoking and Health; 1990. The Health Benefits of Smoking Cessation. [Google Scholar]
- 62.Carmaichael SL, Ahluwalia IB. Correlates of postpartum smoking relapse: Results from Pregnancy Risk Assessment Monitoring System (PRAMS) Am J Prev Med. 2000;19:193–196. doi: 10.1016/s0749-3797(00)00198-7. [DOI] [PubMed] [Google Scholar]
- 63.Severson HH, Andrews JA, Lichtenstein E, Wall M, Akers L. Reducing maternal smoking and relapse: long-term evaluation of a pediatric intervention. Prev Med. 1997;26:120–130. doi: 10.1006/pmed.1996.9983. [DOI] [PubMed] [Google Scholar]
- 64.Fang WL, Goldstein AO, Butzen AY, Hartsock SA, Hartmann KE, Helton M, Lohr JA. Smoking cessation in pregnancy: A review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17(4):264–275. doi: 10.3122/jabfm.17.4.264. [DOI] [PubMed] [Google Scholar]
- 65.Reitzel LR, Vidrine JI, Businelle MS, Kendzor DE, Costello TJ, Li Y, Daza P, Mullen PD, Velasquez MM. Preventing postpartum smoking relapse among diverse low-income women: A randomized clinical trial. Nicotine Tob Res. 2101;12:326–335. doi: 10.1093/ntr/ntq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gielen AC, Windsor RA, Faden R, O’Campo P, Repke J, Davis M. Evaluation of smoking cessation intervention for pregnant women in an urban prenatal clinic. Health Educ Res. 1997;12:247–254. doi: 10.1093/her/12.2.247. [DOI] [PubMed] [Google Scholar]
- 67.Mullen PD, Richardson MA, Quinn VP, Ershoff DH. Postpartum return to smoking: who is at risk and when. Am J Health Promot. 1997;11:323–330. doi: 10.4278/0890-1171-11.5.323. [DOI] [PubMed] [Google Scholar]