Abstract
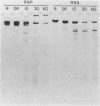
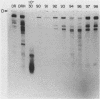
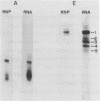
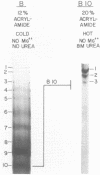
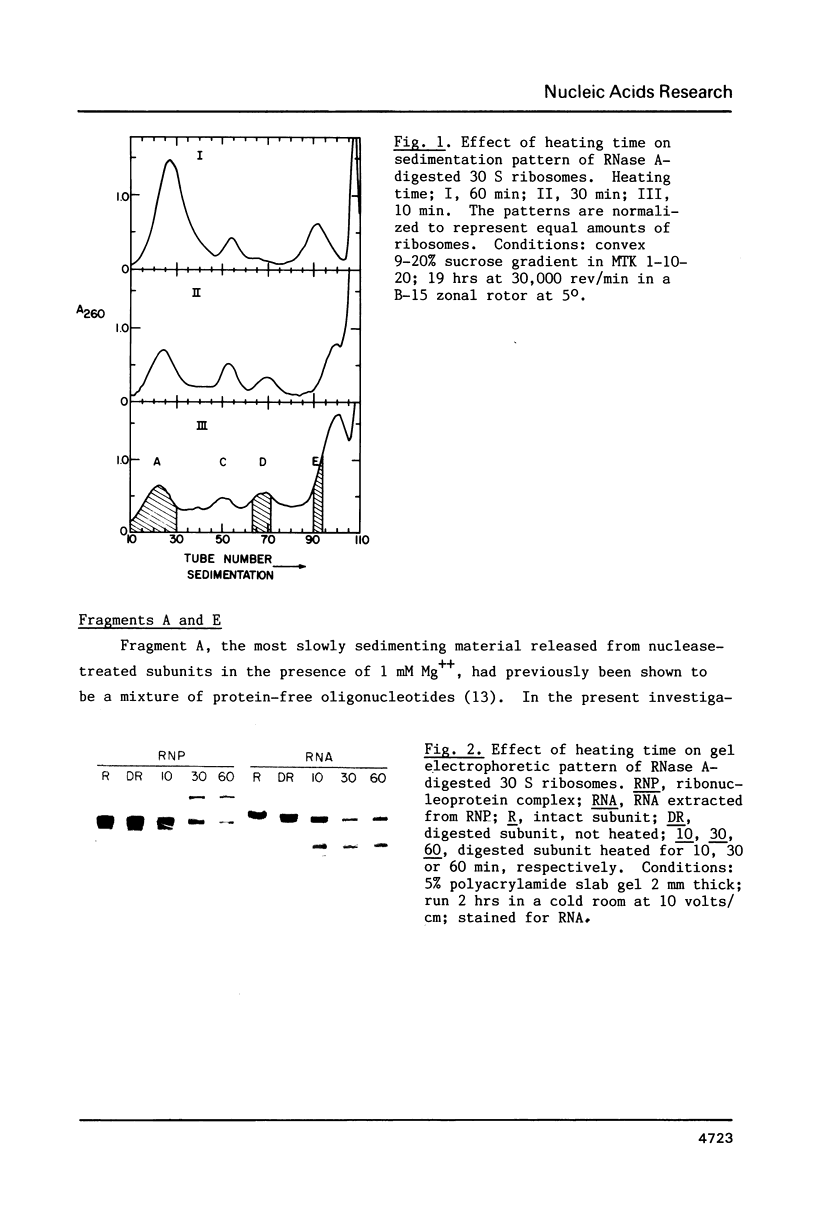
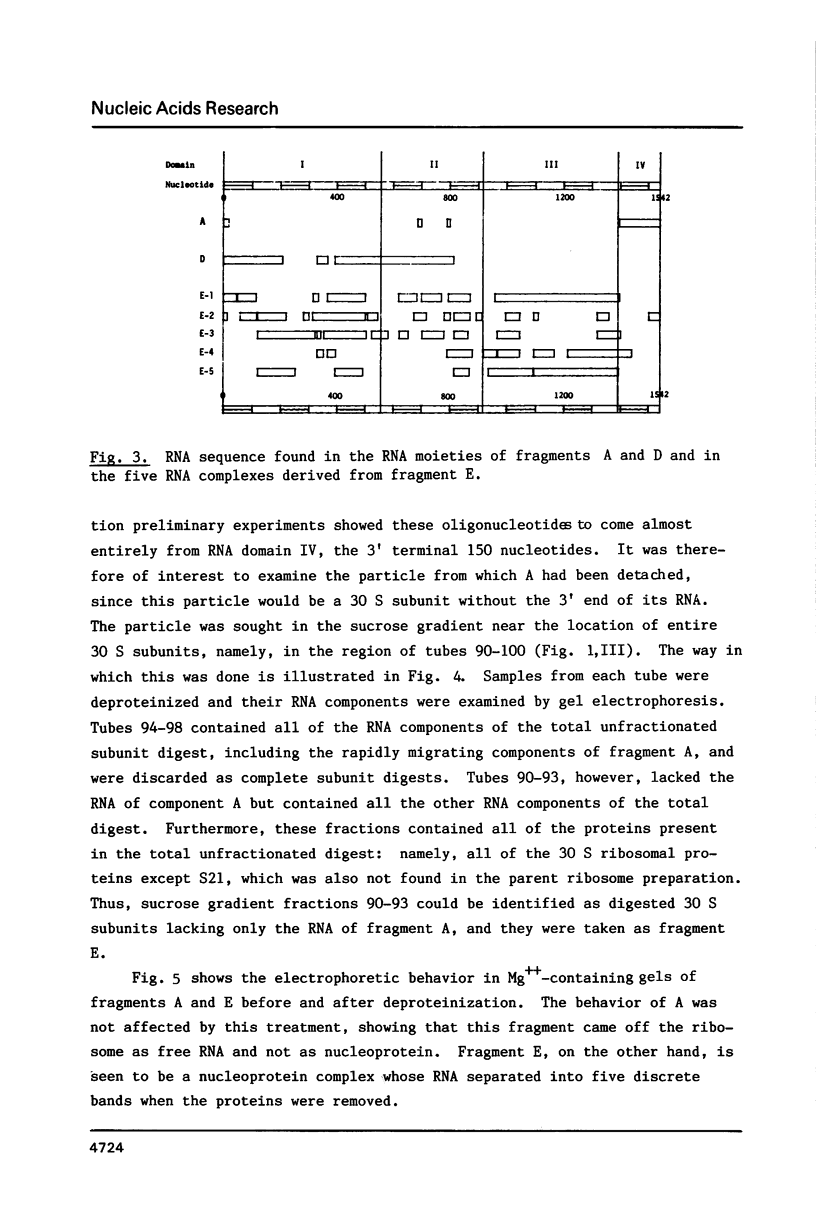
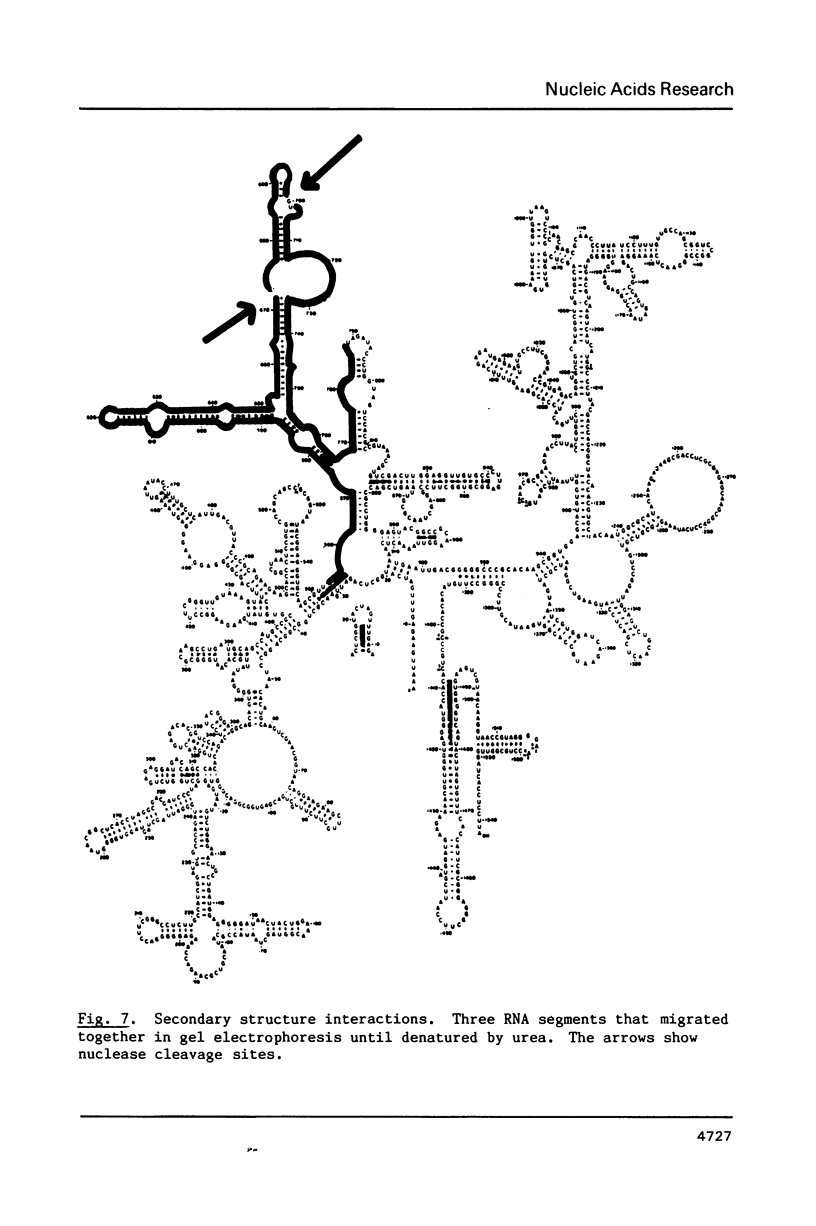
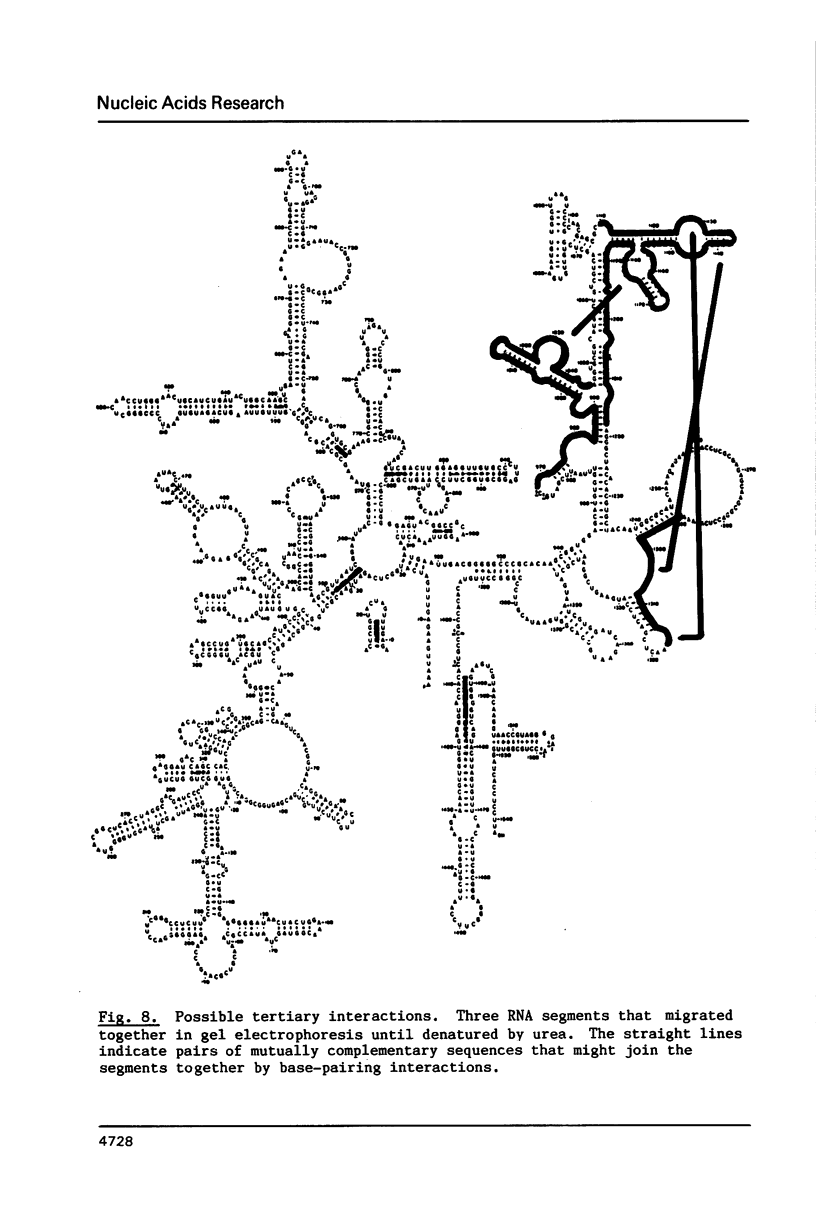
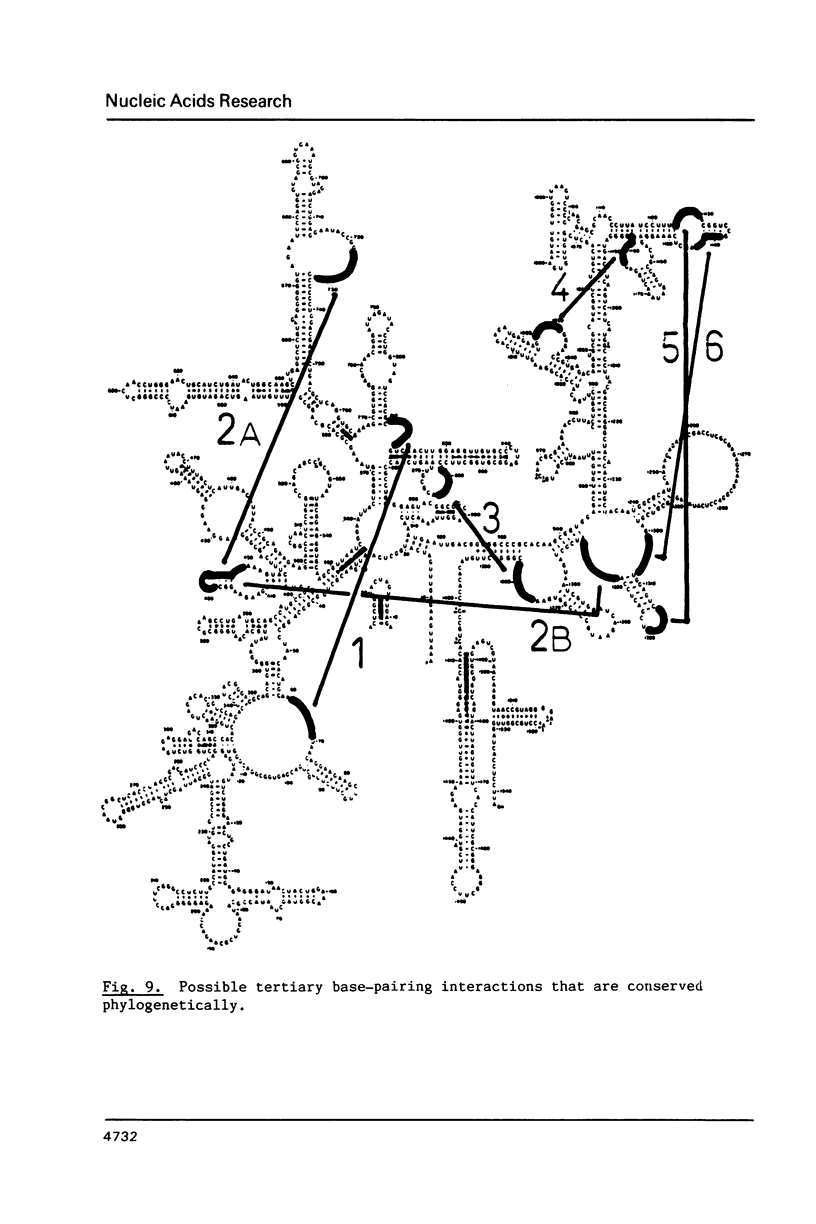
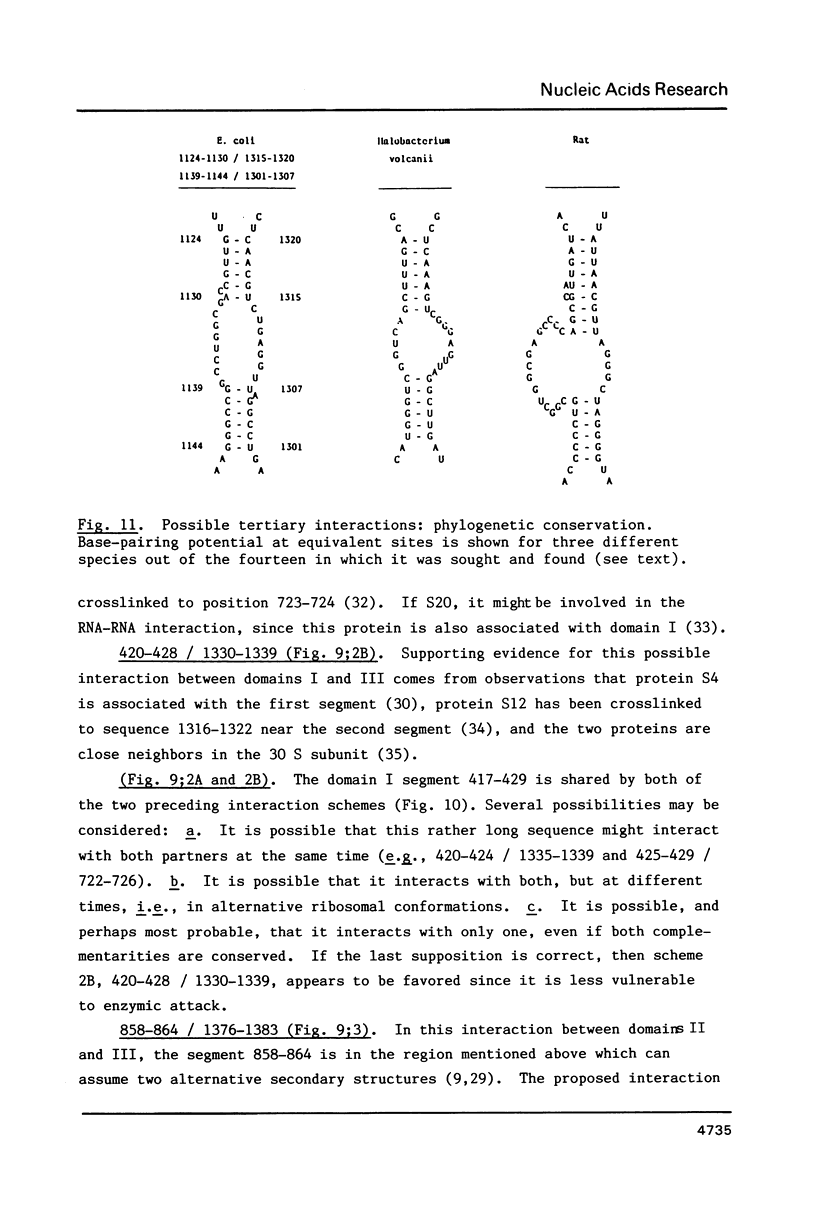
We have attempted to identify long-range interactions in the tertiary structure of RNA in the E. coli 30 S ribosome. Native subunits were cleaved with ribonuclease and separated into nucleoprotein fragments which were deproteinized and fractionated into multi-oligonucleotide complexes under conditions intended to preserve RNA-RNA interactions. The final products were denatured by urea and heat and their constituent oligonucleotides resolved and sequenced. Many complexes contained complementary sequences known to be bound together in the RNA secondary structure, attesting to the validity of the technique. Other co-migrating oligonucleotides, not joined in the secondary structure, contained mutually complementary sequences in locations that allow base-pairing interaction without disrupting pre-existing secondary structure. In seven instances the complementary relationship was found to have been preserved during phylogenetic diversification.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmadja J., Brimacombe R., Maden B. E. Xenopus laevis 18S ribosomal RNA: experimental determination of secondary structural elements, and locations of methyl groups in the secondary structure model. Nucleic Acids Res. 1984 Mar 26;12(6):2649–2667. doi: 10.1093/nar/12.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaruttini C., Expert-Bezançon A., Hayes D., Ehresmann B. Protein-RNA crosslinking in Escherichia coli 30S ribosomal subunits. Identification of a 16S rRNA fragment crosslinked to protein S12 by the use of the chemical crosslinking reagent 1-ethyl-3-dimethyl-aminopropylcarbodiimide. Nucleic Acids Res. 1982 Dec 11;10(23):7657–7676. doi: 10.1093/nar/10.23.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter R. I., McPhie P., Gratzer W. B. Internal organization of the ribosome. Nature. 1967 Dec 2;216(5118):864–868. doi: 10.1038/216864a0. [DOI] [PubMed] [Google Scholar]
- Cox R. A. Structure and function of prokaryotic and eukaryotic ribosomes. Prog Biophys Mol Biol. 1977;32(3):193–231. [PubMed] [Google Scholar]
- Dunn J. M., Wong K. P. Molecular mechanism of in vitro 30 S ribosome assembly. I. Conformational changes of the 16 S RNA. J Biol Chem. 1979 Aug 25;254(16):7705–7711. [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Carbon P., Ungewickell E., Garrett R. A. The topography of the 5' end of 16-S RNA in the presence and absence of ribosomal proteins S4 and S20. Eur J Biochem. 1980 Feb;103(3):439–446. doi: 10.1111/j.1432-1033.1980.tb05967.x. [DOI] [PubMed] [Google Scholar]
- Eilam Y., Elson D. Unfolding of the 30S ribosomal subunit of Escherichia coli and the conformation of the unfolded subunit. Parallel sedimentation and optical rotatory dispersion studies. Biochemistry. 1971 Apr 13;10(8):1489–1495. doi: 10.1021/bi00784a034. [DOI] [PubMed] [Google Scholar]
- Elson D., Spitnik-Elson P., Avital S., Abramowitz R. Binding of magnesium ions and ethidium bromide: comparison of ribosomes and free ribosomal RNA. Nucleic Acids Res. 1979 Sep 25;7(2):465–480. doi: 10.1093/nar/7.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotz C., Brimacombe R. An experimentally-derived model for the secondary structure of the 16S ribosomal RNA from Escherichia coli. Nucleic Acids Res. 1980 Jun 11;8(11):2377–2395. doi: 10.1093/nar/8.11.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotz C., Zwieb C., Brimacombe R., Edwards K., Kössel H. Secondary structure of the large subunit ribosomal RNA from Escherichia coli, Zea mays chloroplast, and human and mouse mitochondrial ribosomes. Nucleic Acids Res. 1981 Jul 24;9(14):3287–3306. doi: 10.1093/nar/9.14.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Chapman N. M., Noller H. F. Mechanism of ribosomal subunit association: discrimination of specific sites in 16 S RNA essential for association activity. J Mol Biol. 1979 Jun 5;130(4):433–449. doi: 10.1016/0022-2836(79)90433-9. [DOI] [PubMed] [Google Scholar]
- Klein B. K., King T. C., Schlessinger D. Structure of partially denatured Escherichia coli 23 S ribosomal RNA determined by electron microscopy. J Mol Biol. 1983 Aug 25;168(4):809–830. doi: 10.1016/s0022-2836(83)80076-x. [DOI] [PubMed] [Google Scholar]
- Korn A. P., Spitnik-Elson P., Elson D. The topology of the 30 S ribosomal subunit and a proposal for the surface distribution of its RNA by dark field electron microscopy. J Biol Chem. 1982 Jun 25;257(12):7155–7160. [PubMed] [Google Scholar]
- Laughrea M., Moore P. B. On the relationship between the binding of ribosomal protein S1 to the 30 S subunit of Escherichia coli and 3' terminus of 16 S RNA. J Mol Biol. 1978 Jun 5;121(4):411–430. doi: 10.1016/0022-2836(78)90391-1. [DOI] [PubMed] [Google Scholar]
- Lührmann R., Stöffler-Meilicke M., Stöffler G. Localization of the 3' end of 16S rRNA in Escherichia coli 30S ribosomal subunits by immuno electron microscopy. Mol Gen Genet. 1981;182(3):369–376. doi: 10.1007/BF00293924. [DOI] [PubMed] [Google Scholar]
- Mackie G. A., Zimmermann R. A. Characterization of fragments of 16 S ribonucleic acid protected against pancreatic ribonuclease digestion by ribosomal protein S4. J Biol Chem. 1975 Jun 10;250(11):4100–4112. [PubMed] [Google Scholar]
- Maly P., Brimacombe R. Refined secondary structure models for the 16S and 23S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1983 Nov 11;11(21):7263–7286. doi: 10.1093/nar/11.21.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles L., Fang B. L., Volckaert G., Vandenberghe A., De Wachter R. Nucleotide sequence of a crustacean 18S ribosomal RNA gene and secondary structure of eukaryotic small subunit ribosomal RNAs. Nucleic Acids Res. 1984 Dec 11;12(23):8749–8768. doi: 10.1093/nar/12.23.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Woese C. R. Secondary structure of 16S ribosomal RNA. Science. 1981 Apr 24;212(4493):403–411. doi: 10.1126/science.6163215. [DOI] [PubMed] [Google Scholar]
- Olson H. M., Glitz D. G. Ribosome structure: localization of 3' end of RNA in small subunit by immunoelectronmicroscopy. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3769–3773. doi: 10.1073/pnas.76.8.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politz S. M., Glitz D. G. Ribosome structure: localization of N6,N6-dimethyladenosine by electron microscopy of a ribosome-antibody complex. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1468–1472. doi: 10.1073/pnas.74.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan V., Capel M., Kjeldgaard M., Engelman D. M., Moore P. B. Positions of proteins S14, S18 and S20 in the 30 S ribosomal subunit of Escherichia coli. J Mol Biol. 1984 Apr 5;174(2):265–284. doi: 10.1016/0022-2836(84)90338-3. [DOI] [PubMed] [Google Scholar]
- Ross A., Brimacombe R. Experimental determination of interacting sequences in ribosomal RNA. Nature. 1979 Sep 27;281(5729):271–276. doi: 10.1038/281271a0. [DOI] [PubMed] [Google Scholar]
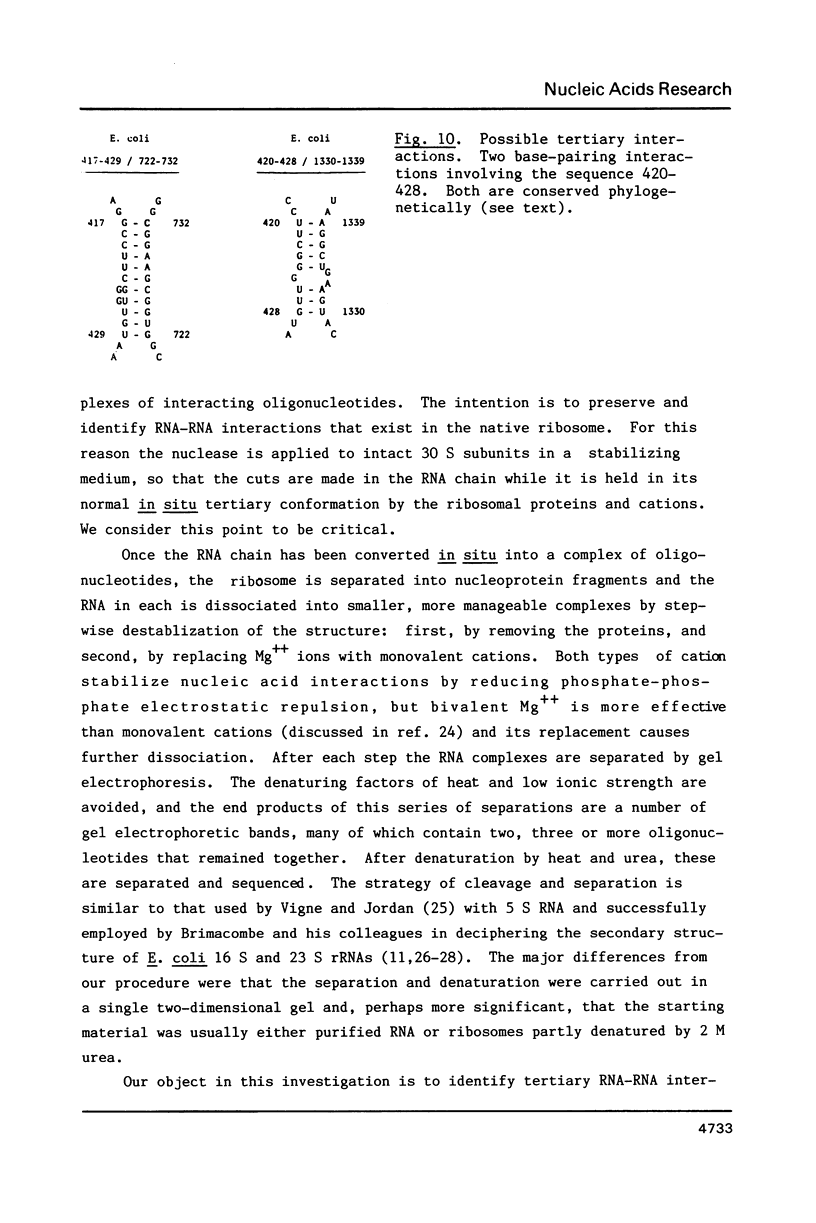
- Santer M., Chung S. C., Harmon G., Estner M., Hendrick J. P., Hopper J., Brecht C., Pandhi P. Comparative surfact structure of 16S ribosomal ribonucleic acid of 30S ribosomes of procaryotic cells. J Bacteriol. 1979 Oct;140(1):131–140. doi: 10.1128/jb.140.1.131-140.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer M., Santer U. Action of ribonuclease T1 on 30S ribosomes of Escherichia coli and its role in sequence studies on 16S ribonucleic acid. J Bacteriol. 1973 Dec;116(3):1304–1313. doi: 10.1128/jb.116.3.1304-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer M., Shane S. Area of 16S ribonucleic acid at or near the interface between 30S and 50S ribosomes of Escherichia coli. J Bacteriol. 1977 May;130(2):900–910. doi: 10.1128/jb.130.2.900-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdyuk I. N., Agalarov S. C., Sedelnikova S. E., Spirin A. S., May R. P. Shape and compactness of the isolated ribosomal 16 S RNA and its complexes with ribosomal proteins. J Mol Biol. 1983 Sep 15;169(2):409–425. doi: 10.1016/s0022-2836(83)80058-8. [DOI] [PubMed] [Google Scholar]
- Shatsky I. N., Mochalova L. V., Kojouharova M. S., Bogdanov A. A., Vasiliev V. D. Localization of the 3' end of Escherichia coli 16 S RNA by electron microscopy of antibody-labelled subunits. J Mol Biol. 1979 Oct 9;133(4):501–515. doi: 10.1016/0022-2836(79)90404-2. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitnik-Elson P., Elson D., Abramowitz R. A purified nucleoprotein fragment of the 30 S ribosomal subunit of Escherichia coli. Biochim Biophys Acta. 1979 Feb 27;561(2):435–444. doi: 10.1016/0005-2787(79)90151-5. [DOI] [PubMed] [Google Scholar]
- Spitnik-Elson P., Elson D., Avital S., Abramowitz R. A ribonucleoprotein fragment of the 30 S ribosome of E. coli containing two contiguous domains of the 16 S RNA. Nucleic Acids Res. 1982 Aug 11;10(15):4483–4492. doi: 10.1093/nar/10.15.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitnik-Elson P., Elson D., Avital S., Abramowitz R. A ribonucleoprotein fragment of the 30 S ribosome of E. coli: evidence for long range RNA-RNA interactions. Nucleic Acids Res. 1982 Mar 25;10(6):1995–2006. doi: 10.1093/nar/10.6.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitnik-Elson P., Elson D. The fragmentation of ribosomes. Methods Enzymol. 1979;59:461–481. doi: 10.1016/0076-6879(79)59108-3. [DOI] [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Zuker M., Ebel J. P., Ehresmann C. Structural organization of the 16S ribosomal RNA from E. coli. Topography and secondary structure. Nucleic Acids Res. 1981 May 11;9(9):2153–2172. doi: 10.1093/nar/9.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teterina N. L., Kopylov A. M., Bogdanov A. A. Segments of the 16 S RNA located on the surface of the 30 S subunits of Escherichia coli ribosomes. FEBS Lett. 1980 Jul 28;116(2):265–268. doi: 10.1016/0014-5793(80)80659-4. [DOI] [PubMed] [Google Scholar]
- Thammana P., Cantor C. R., Wollenzien P. L., Hearst J. E. Crosslinking studies on the organization of the 16 S ribosomal RNA within the 30 S Escherichia coli ribosomal subunit. J Mol Biol. 1979 Nov 25;135(1):271–283. doi: 10.1016/0022-2836(79)90352-8. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Hamilton M. G. Raman spectra and conformational properties of ribosomes during various stages of disassembly. Biochemistry. 1980 Jul 22;19(15):3604–3613. doi: 10.1021/bi00556a029. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Hearst J. E. Structure of E. coli 16S RNA elucidated by psoralen crosslinking. Cell. 1983 Apr;32(4):1355–1365. doi: 10.1016/0092-8674(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Trifonov E. N., Bolshoi G. Open and closed 5 S ribosomal RNA, the only two universal structures encoded in the nucleotide sequences. J Mol Biol. 1983 Sep 5;169(1):1–13. doi: 10.1016/s0022-2836(83)80172-7. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Garrett R., Ehresmann C., Stiegler P., Fellner P. An investigation of the 16-S RNA binding sites of ribosomal proteins S4, S8, S15, and S20 FROM Escherichia coli. Eur J Biochem. 1975 Feb 3;51(1):165–180. doi: 10.1111/j.1432-1033.1975.tb03917.x. [DOI] [PubMed] [Google Scholar]
- Vassilenko S. K., Carbon P., Ebel J. P., Ehresmann C. Topography of 16 S RNA in 30 S subunits and 70 S ribosomes accessibility to cobra venom ribonuclease. J Mol Biol. 1981 Nov 15;152(4):699–721. doi: 10.1016/0022-2836(81)90123-6. [DOI] [PubMed] [Google Scholar]
- Vigne R., Jordan B. R. Conformational analysis of RNA molecules by partial RNAse digestion and two dimensional acrylamide gel electrophoresis. Application to E. coli 5S RNA. Biochimie. 1971;53(9):981–986. doi: 10.1016/s0300-9084(71)80066-4. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. Nuclease mapping of the secondary structure of the 49-nucleotide 3' terminal cloacin fragment of Escherichia coli 16s RNA and its interactions with initiation factor 3. Nucleic Acids Res. 1983 Apr 11;11(7):2035–2052. doi: 10.1093/nar/11.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenzien P. L., Cantor C. R. Gel electrophoretic technique for separating crosslinked RNAs. Application to improved electron microscopic analysis of psoralen crosslinked 16 S ribosomal RNA. J Mol Biol. 1982 Jul 25;159(1):151–166. doi: 10.1016/0022-2836(82)90036-5. [DOI] [PubMed] [Google Scholar]
- Wower I., Brimacombe R. The localization of multiple sites on 16S RNA which are cross-linked to proteins S7 and S8 in Escherichia coli 30S ribosomal subunits by treatment with 2-iminothiolane. Nucleic Acids Res. 1983 Mar 11;11(5):1419–1437. doi: 10.1093/nar/11.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]