Abstract
Background
African-Americans have a disproportionate burden of hypertension compared to Caucasians, while data on Hispanics is less well-defined. Mechanisms underlying these differences are unclear, but could be due in part to ancestral background and vascular function.
Methods and Results
660 African-Americans and 635 Hispanics from the Multi-Ethnic Study of Atherosclerosis (MESA) with complete data on genetic ancestry, pulse pressure (PP), and large and small arterial elasticity (LAE, SAE) were studied. LAE and SAE were obtained using the HDI PulseWave CR-2000 Research CardioVascular Profiling Instrument. Among African-Americans higher European ancestry was marginally associated with higher LAE (p=0.05) and lower PP (p=0.05) among African-Americans; results for LAE were attenuated after adjustment for potential mediators (p=0.30). Ancestry was not associated with SAE in African-Americans. Among Hispanics, higher Native American ancestry was associated with higher SAE (p=0.0006); higher African ancestry was marginally associated with lower SAE (p=0.07). Ancestry was not significantly associated with LAE or PP in Hispanics.
Conclusions
Among African-Americans, higher European ancestry may be associated with less large artery damage as measured by LAE and PP, although these associations warrant further study. Among Hispanics, ancestry is strongly associated with SAE. Future studies should consider information on genetic ancestry when studying hypertension burden in race/ethnic minorities, particularly among Hispanics.
Keywords: large artery elasticity, small artery elasticity, admixture, pulse pressure
Introduction
African-Americans have a disproportionate burden of hypertension when compared to Caucasians in the United States(1). In addition, control of hypertension may be more difficult to attain among Blacks and may require more medications(1). Data on the burden of hypertension among Hispanics is less clear. Among Mexican-Americans in National Health and Nutrition Examination Survey (NHANES), the prevalence of hypertension is similar compared to whites, but lower when compared to African-Americans(1). However, prevalence of hypertension varies by Hispanic country of origin, with Puerto Ricans and Dominicans having higher rates of hypertension than Mexicans-Americans(2). The mechanisms for these observed differences are unclear.
One possible factor to explain differences in rates of hypertension and hypertension control is differences in vascular function. For example, increased arterial stiffness and reduced arterial elasticity may limit the ability of arteries to withstand distending pressures. Increased arterial stiffness has been associated with mortality and cardiovascular events in healthy participants(3–6) as well as in subjects with diabetes(7), hypertension(8,9), and end stage renal disease(10,11). A recent meta-analysis of arterial stiffness showed significant associations with mortality, cardiovascular mortality, and total cardiovascular events in both high and low risk groups(12). Reduced small and large arterial elasticity also predicts the incidence of hypertension(13). Recent data suggest that African Americans may have increased arterial stiffness and decreased arterial elasticity as compared to whites(14–17). In the Multi-Ethnic Study of Atherosclerosis (MESA), African-Americans and Hispanics had significantly lower small artery elasticity (SAE) as compared to Caucasians or Chinese(15). In general, little is known about arterial elasticity in the Hispanic population or Hispanic subgroups compared with other racial/ethnic groups. In addition, it is unknown whether genetic ancestry may relate to differences in subclinical vascular dysfunction in either African-Americans or Hispanics.
Whether possible differences in vascular function by race/ethnicity may have a genetic component is unclear. Complex vascular phenotypes such as measures of arterial elasticity are likely associated with multiple genetic and environmental factors and their interactions. Assessing associations of self reported race/ethnicity with complex phenotypes is often complicated due to the heterogeneity within racial/ethnic groups(18,19). Genetic admixture provides a way to understand potential associations between genetic background due to a common ancestry and complex phenotypes such as vascular function. African-Americans are primarily a mixture of European and African ancestry, while Hispanics are generally a mixture of European, African, and Native American ancestral backgrounds. These admixed populations can be utilized to determine whether there is an association between ancestry and phenotypes such as the arterial elasticity measures, and may ultimately lead to identification of important differences in risk as well as clues to causal genetic loci(20,21). Moreover, it can lead to the study of mediators in the association of genetic ancestry and complex phenotypes.
Thus, we designed this study to examine the association of genetic ancestry with subclinical vascular disease (small and large arterial elasticity, and pulse pressure) among African-Americans and Hispanics from MESA. We also examined potential mediators of these associations. We hypothesized that higher European ancestry would be associated with higher elasticity and lower pulse pressure among African Americans and Hispanics. We further hypothesized that these associations of genetic ancestry and arterial elasticity differed by country of origin among Hispanics.
Methods
Participants
MESA participants were recruited from six field sites in the United States – Forsyth County, NC (Wake Forest), Northern Manhattan/Bronx, NY (Columbia), Baltimore/Baltimore County, MD (Johns Hopkins), St. Paul, MN (University of Minnesota), Chicago, IL (Northwestern), and Los Angeles County, CA (UCLA). Details of recruitment have been previously published(22). Briefly, MESA recruited 6,814 men and women ages 45 to 84 years free of cardiovascular disease. The cohort was 53% women with a racial/ethnic composition of approximately 38% white, 28% African American, 23% Hispanic and 11% Asian, primarily of Chinese descent. The MESA was approved by the Institutional Review Board of all participating field sites and reading centers and participants gave informed consent for participation and use of DNA specimens.
A subsample of 2847 MESA participants was selected for a candidate gene study from participants who gave informed consent for DNA extraction. Overall, the candidate gene study included 712 African American, 705 Hispanic, 718 Chinese, and 712 Caucasian participants and approximately equal numbers of men and women. For the current study, 660 African-Americans with information on genetic ancestry and vascular function measures were used. In addition, 635 Hispanics who had information available on genetic ancestry, vascular function, and country of origin, and were of Mexican (n=404), Dominican (n=89), Puerto Rican (n=87), South American or Central American descent (n=55) comprised the sample. Cuban Americans were excluded from all analyses due to small numbers (n=25).
Genetic ancestry and Country of Origin
To obtain individual genetic ancestry estimates and to determine the appropriate number of ancestral populations (K) for African Americans and Hispanics, STRUCTURE V2.2 was used, which employs a Bayesian Markov Chain Monte Carlo approach(23). A panel of 199 ancestry informative markers was used in ancestry estimation, 96 which were informative for differences in European, African, and Chinese descent, and 103 which were informative for differences in Hispanic ancestry. Pseudo-ancestral population genotype data was obtained from HapMap (60 Yoruban Nigerians, 60 Centre d’Etude du Polymorphisme Humain (CEPH)) and previous genotype data collected on 345 participants from Native American populations(19,24). For Hispanics, 3 populations were deemed adequate, and for African-Americans 2 populations were deemed adequate. Further details of ancestry estimation and the ancestry informative marker panel have been published elsewhere(25,26), and details of how percent ancestry estimates are modeled can be found in the statistical analysis section below.
Country of origin was obtained via self-report and participants categorized into Mexican, Dominican, Puerto Rican and Other Hispanic groups. Central Americans have been found to most resemble Mexicans(26), so these groups were categorized together. The Other Hispanic group included participants from South American countries.
Measurement of Arterial Elasticity
For large artery elasticity (LAE) and small artery elasticity (SAE), the HDI PulseWave CR-2000 Research CardioVascular Profiling Instrument (Eagan, MN, USA) was used to acquire and analyze pulse waveforms from the radial artery. This method derives from pressure fluctuations measures of changes in the pool of large arteries and of the pool of small arteries and their ability to withstand distending pressures throughout the cardiac cycle, by analyzing the diastolic pulse contour, and calculating an index using a Windkessel model, which is a solution to a 3rd order differential equation. Briefly, this model divides total systemic arterial compliance into large artery (capacitive) or small artery (oscillatory) compliances and then incorporates other parameters (age, heart rate, ejection time, weight and height) to estimate the systemic vascular resistance to form the elasticity indices. These measures have been compared to invasive methods in specific larger arteries, with high degrees of correlation(27).
Pulse pressure was assessed as the difference between systolic and diastolic blood pressures. A large pulse pressure can indicate loss of elasticity in the large arteries. During each examination, three blood pressure measurements were obtained five minutes apart in the seated position using an automated oscillometric sphygmomanometer (Dinamap). The mean of the second two measurements was used for analysis. The pulse pressure so derived was highly correlated (r>0.95) with the pulse pressure derived concurrently with measurement using the HDI device.
Covariates
Smoking and alcohol use were categorized into current versus never/former. Education was categorized as ≥ college education versus < college education and income was represented as ≥ $40,000 versus <$40,000. Height was measured by a stadiometer to the nearest 0.1 of a centimeter. Weight was measured to the nearest kilogram using a platform balance scale. Body mass index (BMI) was calculated as weight in kilograms per height in meters squared. Waist circumference was measured using a Gulick II anthropometric tape and was rounded to the nearest centimeter. Estimated glomerular filtration rate (eGFR) was calculated(28) using the equation 186*creatinine−1.154*age−0.203*0.742(if female)*1.21(if African-American). Prevalent diabetes was defined as fasting glucose ≥126 mg/dL, or use of insulin/oral diabetes medications. Systolic and diastolic blood pressures were determined by averaging the last two of three measurements taken with the Dinamap automated blood pressure device (GE Healthcare). Use of anti-hypertensive medications was ascertained via medication inventory. Heart rate was also measured with the Dinamap automated blood pressure device. Plasma HDL-cholesterol, plasma triglycerides, creatinine, and fasting plasma glucose were measured using standardized methods at a central laboratory after a 12 hour fast. LDL-cholesterol was calculated using the Friedewald formula(29).
Statistical Analysis
Baseline characteristics were compared between African-Americans and Hispanics using chi-square test, t-test, or Wilcoxon test as appropriate. Generalized additive models (GAMs) were used to construct splines in order to determine the most appropriate form for the associations of interest. SAE and LAE were not normally distributed and thus were natural log transformed. Percent genetic ancestry measures were divided by their standard deviations in order to facilitate comparisons. Multivariable linear regression was used to examine the association of percent genetic ancestry with SAE, LAE and PP. When natural log transforming the outcome variable in a linear regression, the transformation (eβ−1)*100 can be used for the beta coefficients for ease of interpretation. Thus, the beta coefficients from the LAE and SAE models were transformed, and this transformed beta coefficient can be interpreted as the percent higher or lower arterial elasticity measures per standard deviation higher ancestry. The association of percent European ancestry (per standard deviation) with SAE, LAE, and pulse pressure were examined within African-Americans; associations of percent European, African, and Native American ancestry per standard deviation with these arterial elasticity measures were assessed similarly among Hispanics, both overall and by country of origin. We studied associations by country of origin because Hispanics have been shown to differ in genetic ancestry and cardiovascular risk profile(30). In order to assess potential confounding and mediation effects, a staged modeling approach was used, first adjusting models for potential confounders then followed by potential mediators in the final stage. In model 1, we adjusted for age, gender, height and heart rate, model 2 adjusted additionally for income and education, model 3 additionally adjusted for waist, BMI, current smoking, lipids, and diabetes, and model 4 adjusted additionally for anti-hypertensive medications, systolic blood pressure, and eGFR. Of note, pulse pressure models did not adjust for systolic blood pressure, height, or heart rate. Additionally, the association of country of origin among Hispanics with SAE, LAE and pulse pressure was examined, adjusting for age and gender.
Results
Study Participants
In the overall sample including both African-Americans and Hispanics, mean age was 62 ± 10 years, mean eGFR was 77 ± 18 ml/min/1.73m2, with 49% having hypertension, and 17% having diabetes. African-Americans and Hispanics in this sample did not differ by age, gender, diabetes or heart rate, but did differ on other major cardiovascular risk factors including hypertension and kidney function (Table 1). Mean ± SD European ancestry in African-Americans was 24% ± 15%. Among Hispanics overall, mean ± SD European ancestry was 53% ± 16%, African ancestry was 15% ± 18%, and Native American ancestry was 32% ± 17%. Ancestral proportions varied by country of origin (p <0.001). Mean ± SD for Native American ancestry was 41% ± 13%, 12% ± 5%, 16% ± 8%, and 32% ± 20% for Mexican, Dominican, Puerto Rican and Other Hispanic groups, respectively. For African ancestry, Mean ± SD were 7% ± 4%, 39% ± 21%, 23% ± 16%, and 13% ± 19%, and for European ancestry mean ± SD were 51% ± 13%, 49% ± 19%, 61% ± 15%, and 55% ± 22% for Mexican, Dominican, Puerto Rican, and Other Hispanic groups, respectively.
Table 1.
Baseline characteristics of MESA candidate gene study Hispanic and African-American participants with available vascular function measures*
| Characteristic | African-American (n=660) | Hispanic (n=635) | P Value† |
|---|---|---|---|
| Age, years | 61.8±9.7 | 61.3±10.1 | 0.38 |
| Male Gender | 300 (45.5) | 296 (46.6) | 0.64 |
| Income ≥$40,000 | 305 (46.2) | 160 (25.2) | <0.001 |
| Education ≥High School | 323 (48.9) | 136 (21.4) | <0.001 |
| Hypertension | 370 (56.1) | 267 (42.1) | <0.001 |
| Hypertension Medication Use | 307 (46.4) | 187 (29.5) | <0.001 |
| Diabetes | 112 (17.0) | 111 (17.5) | 0.80 |
| Current Smoking | 127 (19.2) | 93 (14.7) | 0.02 |
| Height, cm | 168.5±9.5 | 161.3±9.2 | <0.001 |
| Heart Rate, beats per minute | 62.9±10.1 | 63.2±9.2 | 0.60 |
| eGFR, ml/min/1.73m2 | 85.8±19.3 | 82.5±18.1 | <0.001 |
| LDL cholesterol, mg/dL | 116.1±32.3 | 120.0±32.9 | 0.04 |
| HDL cholesterol, mg/dL | 52.6±15.4 | 47.2±12.2 | <0.001 |
| Triglycerides, mg/dL | 91.0 (67.0, 123.0) | 137.0 (101.0, 188.0) | <0.001 |
| Fasting glucose, mg/dL | 100.0±32.8 | 103.9±40.5 | 0.06 |
| Body Mass Index, kg/m2 | 30.2±5.7 | 29.6±5.1 | 0.05 |
| Waist circumference, cm | 101.1±14.5 | 100.3±12.9 | 0.36 |
| Systolic Blood Pressure, mmHg | 129.3±20.9 | 125.9±21.5 | 0.005 |
| Diastolic Blood Pressure, mmHg | 73.9±9.9 | 71.3±10.3 | <0.001 |
| Pulse Pressure, mmHg | 60.1±14.4 | 59.5±14.2 | 0.51 |
| Large Artery Elasticity, ml/mmHgx10 | 12.7 (9.8, 16.3) | 12.3 (9.2, 16.0) | 0.06 |
| Small Artery Elasticity, ml/mmHgx100 | 3.62 (2.31, 5.33) | 3.71 (2.39, 6.00) | 0.15 |
Mean ± SD for continuous variables, and n (%) are presented for dichotomous variables
P-values by t-test, Wilcoxon or chi-square test as appropriate
Genetic Ancestry and Arterial Elasticity
Median (interquartile range) for SAE was 3.62 (2.31, 5.33) for African-Americans and 3.71 (2.39, 6.00) for Hispanics, p=0.15 (Table 1). Median (interquartile range) for LAE for African-Americans was 12.7(9.8, 16.3), and for Hispanics was 12.3 (9.2, 16.0), p=0.06 (Table 1). Splines indicated that ancestry was approximately linearly related to ln(LAE) and ln(SAE) among both African-Americans and Hispanics (data not shown).
Among African Americans, higher European ancestry was marginally associated with higher LAE (Table 2). However, adjustment for potential mediators, including anti-hypertensive medication use, systolic blood pressure and eGFR further attenuated this association. Among African Americans, European ancestry was not significantly associated with SAE.
Table 2.
Association of European ancestry (per standard deviation higher) with arterial elasticity among African-Americans*
| Small Arterial Elasticity | P Value | Large Arterial Elasticity | P Value | |
|---|---|---|---|---|
| Percent (95% CI) | Percent (95% CI) | |||
| Age, gender, height, heart rate | 2.32 (−1.51, 6.30) | 0.24 | 2.69 (0.02, 5.43) | 0.05 |
| +Income, education | 1.76 (−2.18, 5.85) | 0.39 | 2.72 (−0.04, 5.56) | 0.05 |
| +Smoking, BMI, waist, lipids, diabetes | 2.66 (−1.20, 6.66) | 0.18 | 2.78 (−0.01, 5.64) | 0.05 |
| +HTN medications, systolic BP, eGFR | 0.44 (−3.14, 4.15) | 0.81 | 1.39 (−1.22, 4.07) | 0.30 |
Standard deviation is 15%
Among Hispanics overall, higher Native American ancestry was significantly associated with higher SAE. Adjustment for potential mediators did not attenuate this association (Table 3). Interestingly, higher African ancestry was associated with a lower SAE among Hispanics. This association was modestly attenuated and was no longer significant (p=0.07) after the final stage of adjustment for anti-hypertensive medication use, systolic blood pressure, and eGFR (Table 3). European ancestry was not significantly associated with SAE among Hispanics. Ancestral background was not associated with LAE among Hispanics (Table 3).
Table 3.
Association of percent ancestry (per standard deviation higher) with arterial elasticity among Hispanics*
| Small Arterial Elasticity | P Value | Large Arterial Elasticity | P Value | |
|---|---|---|---|---|
| Percent (95% CI) | Percent (95% CI) | |||
| European Ancestry | ||||
| Age, gender, height, heart rate | −2.05 (−5.73, 1.78) | 0.29 | −0.45 (−2.74, 1.89) | 0.70 |
| +Income, education | −2.25 (−5.96, 1.61) | 0.25 | −0.27 (−2.60, 2.11) | 0.82 |
| +Smoking, BMI, waist, lipids, diabetes | −1.62 (−5.40, 2.31) | 0.41 | −0.42 (−2.79, 2.01) | 0.73 |
| +HTN medications, systolic BP, eGFR | −2.96 (−6.52, 0.74) | 0.12 | −1.67 (−3.84, 0.55) | 0.14 |
| Native American Ancestry | ||||
| Age, gender, height, heart rate | 6.87 (2.84, 11.06) | 0.0007 | −0.44 (−2.76, 1.93) | 0.71 |
| +Income, education | 7.22 (3.12, 11.48) | 0.0005 | −1.03 (−3.37, 1.38) | 0.40 |
| +Smoking, BMI, waist, lipids, diabetes | 6.78 (2.56, 11.18) | 0.0015 | −1.23 (−3.67, 1.27) | 0.33 |
| +HTN medications, systolic BP, eGFR | 7.12 (3.03, 11.37) | 0.0006 | −1.01 (−3.30, 1.34) | 0.40 |
| African Ancestry | ||||
| Age, gender, height, heart rate | −4.36 (−7.96, −0.61) | 0.02 | 0.83 (−1.50, 3.22) | 0.49 |
| +Income, education | −4.40 (−8.03, −0.63) | 0.02 | 1.22 (−1.15, 3.65) | 0.32 |
| +Smoking, BMI, waist, lipids, diabetes | −4.47 (−8.21, −0.59) | 0.02 | 1.56 (−0.91, 4.09) | 0.22 |
| +HTN medications, systolic BP, eGFR | −3.48 (−7.12, 0.31) | 0.07 | 2.56 (0.24, 4.94) | 0.03 |
Per country of origin-specific standard deviation increase in ancestry
Most interestingly, we found that SAE differed by country of origin among Hispanics (p=0.003), with adjusted geometric mean (adjusted for age, gender, and height) SAE highest for Mexicans (3.92, 95% CI (3.74, 4.11)), and lowest for Dominicans (3.24, 95% CI (2.93, 3.58)); for Puerto Ricans adjusted mean SAE was 3.86 (95% CI (3.48, 4.27)), and for the Other Hispanics group it was 3.42 (95% CI (3.01, 3.89)), after adjustment for gender, age, height, and heart rate. LAE did not vary by country of origin (p=0.87).
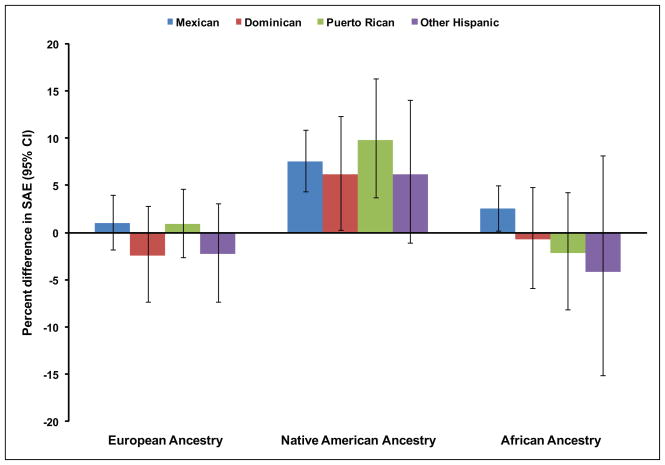
The association between genetic ancestry and arterial elasticity varied slightly by country of origin (Figure 1). Higher Native American ancestry was significantly associated with higher SAE for Mexicans (7.6%, 95% CI (4.3%, 10.9%), p<0.001), Dominicans (6.1%, 95% CI (0.3%, 12.4%), p=0.04), and Puerto Ricans (9.8%, 95% CI (3.8%, 16.3%), p=0.001); however, this association was not significant among the Other Hispanic group (6.2%, 95% CI (−1.1%, 14.1%), p=0.10) (Figure 1). African or European ancestry was not significantly associated with SAE within any country of origin. Native American, African or European ancestries were not associated with LAE within any Hispanic country of origin (data not shown).
Figure 1. Association of European, Native American, and African ancestry with small artery elasticity by country of origin among Hispanics*.
*Per country of origin-specific standard deviation increase in European, Native American, or African ancestry in models adjusted for age, gender, education, income, current smoking, body mass index, waist circumference, height, heart rate, prevalent diabetes, anti-hypertensive medication use, estimated glomerular filtration rate, systolic blood pressure, HDL cholesterol, LDL cholesterol, and triglycerides
Genetic Ancestry and Pulse Pressure
Mean pulse pressure for African-Americans was 60.1±14.4 mmHg and for Hispanics was 59.5 ± 14.2 mmHg, p=0.51 (Table 1). Splines indicated that ancestry was approximately linearly related to pulse pressure among both African-Americans and Hispanics (data not shown).
Among African-Americans, each SD higher European ancestry was associated with a 1.31 mmHg lower pulse pressure (95% CI (−2.38, −0.23), p=0.02). This association was somewhat attenuated by adjustment for potential mediators, including anti-hypertensive medications and eGFR, and was of borderline significance, p=0.06 (Table 4). In contrast to findings among African Americans, genetic ancestry did not appear to be significantly associated with pulse pressure among Hispanics; however, eGFR and anti-hypertensive medication use did appear to somewhat mediate the association between African ancestry and pulse pressure (p=0.01) as well as the association between Native American ancestry and pulse pressure (p=0.07) in the final stage of adjustment (Table 4). Pulse pressure did not differ by country of origin for Hispanics (p=0.85). Native American, African or European ancestries were not associated with pulse pressure within any Hispanic country of origin (data not shown). Results were not materially changed for any model when adjusting for separate classes of medications (beta blockers, diuretics, calcium channel blockers, and angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARB)) instead of any anti-hypertensive medication use overall.
Table 4.
Association of percent ancestry (per standard deviation higher) with pulse pressure among African-Americans and Hispanics*
| African-American Pulse Pressure Beta (95% CI) | P Value | Hispanic Pulse Pressure Beta (95% CI) | P Value | |
|---|---|---|---|---|
| European Ancestry | ||||
| Age, gender, heart rate | −1.46 (−2.52, −0.40) | 0.007 | 0.23 (−0.72, 1.17) | 0.64 |
| +Income, education | −1.31 (−2.37, −0.25) | 0.02 | 0.22 (−0.74, 1.17) | 0.66 |
| +Smoking, BMI, waist, lipids, diabetes | −1.25 (−2.32, −0.17) | 0.02 | 0.37 (−0.60, 1.34) | 0.45 |
| +HTN medications, eGFR | −1.02 (−2.09, −0.05) | 0.06 | 0.40 (−0.55, 1.34) | 0.41 |
| Native American Ancestry | ||||
| Age, gender, heart rate | -- | -- | 0.55 (−0.40, 1.49) | 0.26 |
| +Income, education | -- | -- | 0.57 (−0.38, 1.52) | 0.24 |
| +Smoking, BMI, waist, lipids, diabetes | -- | -- | 0.49 (−0.49, 1.48) | 0.33 |
| +HTN medications, eGFR | -- | -- | 0.92 (−0.06, 1.89) | 0.07 |
| African Ancestry | ||||
| Age, gender, heart rate | -- | -- | −0.73 (−1.67, 0.21) | 0.13 |
| +Income, education | -- | -- | −0.74 (−1.68, 0.21) | 0.13 |
| +Smoking, BMI, waist, lipids, diabetes | -- | -- | −0.80 (−1.78, 0.17) | 0.11 |
| +HTN medications, eGFR | -- | -- | −1.24 (−2.20, −0.27) | 0.01 |
Standard deviation is 15% for African-Americans; per country of origin-specific standard deviation increase in ancestry for Hispanics
Discussion
In this study of African Americans and Hispanic middle aged adults free of cardiovascular disease, we found that genetic ancestry was associated with measures of vascular function and these associations vary by ethnic group and vessel caliber. In particular, we found that higher European ancestry was marginally associated with lower pulse pressure among African Americans. Higher European ancestry was marginally associated with higher large arterial elasticity; however, this association was attenuated after adjustment for mediators. Among Hispanics, higher Native American ancestry was significantly associated with higher small arterial elasticity, both overall and in Hispanic subgroups. These results require further study and confirmation for both groups.
Our results in African Americans are in accordance with prior literature suggesting that in general African Americans may have increased arterial stiffness and decreased arterial elasticity compared with Caucasians(14–17). It should, however, be noted that pulse wave velocity and small and large artery elasticity are different measures of arterial compliance and may provide different information. Our results expand on these findings by highlighting the heterogeneity within the African Americans and suggest that higher European ancestry may be associated with lower burden of decreased large artery elasticity. These findings are important because measures of arterial elasticity or stiffness have been shown to be independent predictors of mortality and cardiovascular events(3,5–12,31). Moreover, the fact that the associations between ancestry and elasticity appear to be mediated by hypertension is in accordance to our prior study showing that levels of large arterial elasticity predict incident hypertension among healthy subjects(13). Our findings of an association between European ancestry and large artery elasticity among African Americans may suggest that there may be a genetic predisposition to vascular dysfunction among African-Americans. However, other factors such as socio-economic status and the interaction of genes with these factors also likely play a significant role. Future studies should investigate whether these associations may help explain the higher rates of hypertension and lower blood pressure control among African Americans compared with Caucasians.
Our findings that higher Native American ancestry was associated with higher SAE while African ancestry was associated with lower SAE are noteworthy. Hispanics are a heterogeneous group and their risk for hypertension and its sequalae are likely to differ by country of origin(1). This particular finding may explain why Hispanics of Mexican origin have lower hypertension rates compared to the other Hispanic subgroups that have lower Native American ancestry but higher African ancestry. Hispanics also have increasing burden of cardiovascular disease(1), and it is possible these findings represent the known association of particular risk factors for CVD (i.e. obesity, diabetes) among Hispanics rather than arterial elasticity.
The significant association of genetic ancestry with arterial elasticity in this study is supported by several studies and reviews of genetic variants associated with measures of vascular dysfunction(32–50), including variants in the angiotensinogen, beta-adrenergic receptor, and advanced glycation endproducts receptor genes, as well as several genes involved in cardiac function and structure; however, these studies were in participants of European descent. Our study suggests that there is genetic heterogeneity among both Hispanics and African-Americans, and that this heterogeneity is important in determining measures of vascular dysfunction. It also suggests that there are underlying genetic factors that are important to vascular dysfunction for these groups. However, our findings must be interpreted with caution because an association of ancestry and complex phenotypes such as arterial elasticity may also be explained by environmental differences or gene-environment interactions, i.e. sodium intake plus genetic predisposition to salt-sensitive hypertension.
Our study has a number of strengths, but also some limitations to note. It is the first report associations of ancestry with measures of arterial elasticity among African-Americans and Hispanics, and to investigate differences in Hispanics by country of origin. MESA has a group of well-characterized African-Americans and Hispanics with no known cardiovascular disease at the baseline examination. In this study, ancestry is well-characterized due to use of pseudo-ancestral groups which helps to provide unbiased estimates of ancestry(51), and adequate marker panel for both African-Americans and Hispanics. Furthermore, the measures of vascular function have been validated and been compared to invasive methods in specific larger arteries with high degrees of correlation(27). Since this is an observational study, unknown confounders or residual confounding could be present. Our study is also limited by using measures of blood pressure from the Dinamap PRO-100 device which may have lower accuracy compared to blood pressure measurement by a trained technician with a manual sphygmomanometer. However, MESA measured blood pressure three times and we used the average of the last two measures. Measures of blood pressure in MESA have consistently been associated with adverse outcomes and other traditional cardiovascular risk factors. Country of origin is by self-report so misclassification could have occurred. Due to small numbers of some Hispanic subgroups or some Hispanic subgroups not being present in MESA, we were restricted to using Mexican, Dominican, Puerto Rican, and Other Hispanic (South American) as our classifications. Finally, it is possible that the genetic ancestry in this study is not representative of ancestry in the US, as we do not know to what extent those of mixed ancestral background would or would not be inclined to participate.
In summary, we found that European ancestry was significantly associated with lower LAE and higher pulse pressure among African-Americans. Moreover, we found that SAE differed by country of origin among Hispanics, and that Native American ancestry was an important determinant of SAE both overall and by country of origin. These findings have important implications for genetic and epidemiologic studies which include racial/ethnic groups of mixed ancestral background, namely that studies should account for differences even within ethnic group. These findings also indicate that SAE, LAE and pulse pressure may be good traits for admixture mapping which capitalizes on traits that differ by admixed groups to find significant genetic variants for that trait. Further study is needed to elucidate genetic variants for vascular dysfunction, and whether or not they differ by racial/ethnic group, either through genetic association or admixture mapping studies. Future studies should also focus on potential gene-environment interactions which may be additionally important in describing differences in vascular dysfunction.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Support: CLW was supported by the NIH training grant in cardiovascular genetic epidemiology (T32 HL097972) and cardiovascular epidemiology and prevention (T32HL07779) during a portion of this research. CAP is currently supported by 1K23DK082793-01 and the Robert Wood Johnson Harold Amos award. This research was supported by contracts N01-HC-95159 through N01-HC-95169 and grants R01HL071051, R01HL071205, R01HL071250, RO1HL071251, R01HL071252, R01HL071258, and R01HL071259 from the National Heart, Lung, and Blood Institute.
Footnotes
The authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010 May 26;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Pabon-Nau LP, Cohen A, Meigs JB, Grant RW. Hypertension and diabetes prevalence among U.S. Hispanics by country of origin: the national health interview survey 2000–2005. J Gen Intern Med. 2010 Aug;25(8):847–852. doi: 10.1007/s11606-010-1335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duprez DA, Jacobs DR, Jr, Lutsey PL, Bluemke DA, Brumback LC, Polak J, Kronmal RA. Large and Small Artery Elasticty, but Not Coronary Calcium Score and Carotid Intima-Media Thickness, Predict Congestive Heart Failure Events in an Asymptomatic Population: Results of the Multiethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2009;53(Suppl A A382):1028. [Google Scholar]
- 4.Duprez DA, Jacobs DR, Jr, Lutsey PL, Bluemke DA, Brumback LC, Polak JF, et al. Association of Small Artery Elasticity With Incident Cardiovascular Disease in Older Adults: The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2011 Jun 27; doi: 10.1093/aje/kwr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010 Feb 2;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005 Jun 28;111(25):3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 7.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002 Oct 15;106(16):2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 8.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002 Jan;39(1):10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 9.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003 May;34(5):1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 10.Zoungas S, Asmar RP. Arterial stiffness and cardiovascular outcome. Clin Exp Pharmacol Physiol. 2007 Jul;34(7):647–651. doi: 10.1111/j.1440-1681.2007.04654.x. [DOI] [PubMed] [Google Scholar]
- 11.Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension. 2005 Apr;45(4):592–596. doi: 10.1161/01.HYP.0000159190.71253.c3. [DOI] [PubMed] [Google Scholar]
- 12.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010 Mar 30;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 13.Peralta CA, Adeney KL, Shlipak MG, Jacobs D, Jr, Duprez D, Bluemke D, et al. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2010 Jan 1;171(1):63–71. doi: 10.1093/aje/kwp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhuiyan AR, Li S, Li H, Chen W, Srinivasan SR, Berenson GS. Distribution and correlates of arterial compliance measures in asymptomatic young adults: the Bogalusa Heart Study. Am J Hypertens. 2005 May;18(5 Pt 1):684–691. doi: 10.1016/j.amjhyper.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Duprez DA, Jacobs DR, Jr, Lutsey PL, Herrington D, Prime D, Ouyang P, et al. Race/ethnic and sex differences in large and small artery elasticity--results of the multi-ethnic study of atherosclerosis (MESA) Ethn Dis. 2009 Summer;19(3):243–250. [PMC free article] [PubMed] [Google Scholar]
- 16.Madero M, Wassel CL, Peralta CA, Najjar SS, Sutton-Tyrrell K, Fried L, et al. Cystatin C associates with arterial stiffness in older adults. J Am Soc Nephrol. 2009 May;20(5):1086–1093. doi: 10.1681/ASN.2008030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valappil NI, Jacobs DR, Duprez DA, Gross MD, Arnett DK, Glasser S. Association between Endothelial Biomarkers and Arterial Elasticity in Young Adults - The CARDIA Study. J Am Soc Hypertens. 2008 Apr;2(2):70–79. doi: 10.1016/j.jash.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003 Mar 20;348(12):1170–1175. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 19.Choudhry S, Coyle NE, Tang H, Salari K, Lind D, Clark SL, et al. Population stratification confounds genetic association studies among Latinos. Hum Genet. 2006 Jan;118(5):652–664. doi: 10.1007/s00439-005-0071-3. [DOI] [PubMed] [Google Scholar]
- 20.McKeigue PM. Prospects for admixture mapping of complex traits. Am J Hum Genet. 2005 Jan;76(1):1–7. doi: 10.1086/426949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeigue PM. Mapping genes underlying ethnic differences in disease risk by linkage disequilibrium in recently admixed populations. Am J Hum Genet. 1997 Jan;60(1):188–196. [PMC free article] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002 Nov 1;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000 Jun;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seldin MF, Tian C, Shigeta R, Scherbarth HR, Silva G, Belmont JW, et al. Argentine population genetic structure: large variance in Amerindian contribution. Am J Phys Anthropol. 2007 Mar;132(3):455–462. doi: 10.1002/ajpa.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wassel CL, Pankow JS, Peralta CA, Choudhry S, Seldin MF, Arnett DK. Genetic ancestry is associated with subclinical cardiovascular disease in African-Americans and Hispanics from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Genet. 2009 Dec;2(6):629–636. doi: 10.1161/CIRCGENETICS.109.876243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peralta CA, Li Y, Wassel C, Choudhry S, Palmas W, Seldin MF, et al. Differences in Albuminuria between Hispanics and Whites: An Evaluation by Genetic Ancestry and Country of Origin: The Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Genet. 2010 May 5; doi: 10.1161/CIRCGENETICS.109.914499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension. 1999 Jun;33(6):1392–1398. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 30.Peralta CA, Li Y, Wassel C, Choudhry S, Palmas W, Seldin MF, et al. Differences in albuminuria between Hispanics and whites: an evaluation by genetic ancestry and country of origin: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Genet. 2010 Jun 1;3(3):240–247. doi: 10.1161/CIRCGENETICS.109.914499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010 Mar;55(3):799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akasaka H, Katsuya T, Saitoh S, Sugimoto K, Ohnishi H, Congrains A, et al. A promoter polymorphism of lamin A/C gene is an independent genetic predisposition to arterial stiffness in a Japanese general population (the Tanno and Sobetsu study) J Atheroscler Thromb. 2009 Aug;16(4):404–409. doi: 10.5551/jat.no1271. [DOI] [PubMed] [Google Scholar]
- 33.Baker M, Rahman T, Hall D, Avery PJ, Mayosi BM, Connell JM, et al. The C-532T polymorphism of the angiotensinogen gene is associated with pulse pressure: a possible explanation for heterogeneity in genetic association studies of AGT and hypertension. Int J Epidemiol. 2007 Dec;36(6):1356–1362. doi: 10.1093/ije/dym213. [DOI] [PubMed] [Google Scholar]
- 34.Bjorck HM, Lanne T, Alehagen U, Persson K, Rundkvist L, Hamsten A, et al. Association of genetic variation on chromosome 9p21.3 and arterial stiffness. J Intern Med. 2009 Mar;265(3):373–381. doi: 10.1111/j.1365-2796.2008.02020.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Srinivasan SR, Boerwinkle E, Berenson GS. Beta-adrenergic receptor genes are associated with arterial stiffness in black and white adults: the Bogalusa Heart Study. Am J Hypertens. 2007 Dec;20(12):1251–1257. doi: 10.1016/j.amjhyper.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Engelen L, Ferreira I, Gaens KH, Henry RM, Dekker JM, Nijpels G, et al. The association between the -374T/A polymorphism of the receptor for advanced glycation endproducts gene and blood pressure and arterial stiffness is modified by glucose metabolism status: the Hoorn and CoDAM studies. J Hypertens. 2010 Feb;28(2):285–293. doi: 10.1097/HJH.0b013e3283330931. [DOI] [PubMed] [Google Scholar]
- 37.Kelley-Hedgepeth A, Peter I, Montefusco MC, Levy D, Benjamin EJ, Vasan RS, et al. The KCNMB1 E65K variant is associated with reduced central pulse pressure in the community-based Framingham Offspring Cohort. J Hypertens. 2009 Jan;27(1):55–60. doi: 10.1097/HJH.0b013e328317c8ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacolley P, Challande P, Osborne-Pellegrin M, Regnault V. Genetics and pathophysiology of arterial stiffness. Cardiovasc Res. 2009 Mar 1;81(4):637–648. doi: 10.1093/cvr/cvn353. [DOI] [PubMed] [Google Scholar]
- 39.Levy D, Larson MG, Benjamin EJ, Newton-Cheh C, Wang TJ, Hwang SJ, et al. Framingham Heart Study 100K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Med Genet. 2007 Sep 19;8( Suppl 1):S3. doi: 10.1186/1471-2350-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer O, Jr, Filipovsky J, Pesta M, Cifkova R, Dolejsova M, Simon J. Synergistic effect of angiotensin II type 1 receptor and endothelial nitric oxide synthase gene polymorphisms on arterial stiffness. J Hum Hypertens. 2008 Feb;22(2):111–118. doi: 10.1038/sj.jhh.1002279. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell GF, Guo CY, Kathiresan S, Vasan RS, Larson MG, Vita JA, et al. Vascular stiffness and genetic variation at the endothelial nitric oxide synthase locus: the Framingham Heart study. Hypertension. 2007 Jun;49(6):1285–1290. doi: 10.1161/HYPERTENSIONAHA.106.085266. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher W, Cockcroft J, Timpson NJ, McEniery CM, Gallacher J, Rumley A, et al. Association between C-reactive protein genotype, circulating levels, and aortic pulse wave velocity. Hypertension. 2009 Feb;53(2):150–157. doi: 10.1161/HYPERTENSIONAHA.108.117622. [DOI] [PubMed] [Google Scholar]
- 43.Sie MP, Isaacs A, de Maat MP, Mattace-Raso FU, Uitterlinden AG, Kardys I, et al. Genetic variation in the fibrinogen-alpha and fibrinogen-gamma genes in relation to arterial stiffness: the Rotterdam Study. J Hypertens. 2009 Jul;27(7):1392–1398. doi: 10.1097/HJH.0b013e32832a95b0. [DOI] [PubMed] [Google Scholar]
- 44.Sie MP, Mattace-Raso FU, Uitterlinden AG, Arp PP, Hofman A, Hoeks AP, et al. TGF-beta1 polymorphisms and arterial stiffness; the Rotterdam Study. J Hum Hypertens. 2007 Jun;21(6):431–437. doi: 10.1038/sj.jhh.1002175. [DOI] [PubMed] [Google Scholar]
- 45.Sie MP, Mattace-Raso FU, Uitterlinden AG, Arp PP, Hofman A, Pols HA, et al. The interleukin-6-174 G/C promoter polymorphism and arterial stiffness; the Rotterdam Study. Vasc Health Risk Manag. 2008;4(4):863–869. doi: 10.2147/vhrm.s1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sie MP, Yazdanpanah M, Mattace-Raso FU, Uitterlinden AG, Hofman A, Hoeks AP, et al. Genetic variation in the renin-angiotensin system and arterial stiffness. The Rotterdam Study. Clin Exp Hypertens. 2009 Jul;31(5):389–399. doi: 10.1080/10641960802668706. [DOI] [PubMed] [Google Scholar]
- 47.Tarasov KV, Sanna S, Scuteri A, Strait JB, Orru M, Parsa A, et al. COL4A1 is associated with arterial stiffness by genome-wide association scan. Circ Cardiovasc Genet. 2009 Apr;2(2):151–158. doi: 10.1161/CIRCGENETICS.108.823245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vlachopoulos C, Terentes-Printzios D, Dima I, Aznaouridis K, Stefanadis C. Polymorphisms of inflammatory markers/mediators and arterial stiffness. Hypertension. 2009 Jun;53(6):e39. doi: 10.1161/HYPERTENSIONAHA.109.129361. author reply e40. [DOI] [PubMed] [Google Scholar]
- 49.Yasmin, McEniery CM, O’Shaughnessy KM, Harnett P, Arshad A, Wallace S, et al. Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1799–1805. doi: 10.1161/01.ATV.0000227717.46157.32. [DOI] [PubMed] [Google Scholar]
- 50.Zhou S, Feely J, Spiers JP, Mahmud A. Matrix metalloproteinase-9 polymorphism contributes to blood pressure and arterial stiffness in essential hypertension. J Hum Hypertens. 2007 Nov;21(11):861–867. doi: 10.1038/sj.jhh.1002244. [DOI] [PubMed] [Google Scholar]
- 51.Tang H, Peng J, Wang P, Risch NJ. Estimation of individual admixture: analytical and study design considerations. Genet Epidemiol. 2005 May;28(4):289–301. doi: 10.1002/gepi.20064. [DOI] [PubMed] [Google Scholar]