Abstract
Objective
To investigate whether abnormal regional white matter architecture in the perisylvian region could be used as an easy and sensitive quantitative method to demonstrate language pathway abnormalities in children with developmental delay (DD).
Study design
We performed diffusion tensor imaging (DTI) in 15 DD subjects (age: 61.1± 20.9 months) and 15 age-matched typically developing (TD) children (age: 68.4± 19.2). Using DTI color-coded orientation maps, we quantified the fraction of fibers in the perisylvian region that are oriented in anteroposterior (AP) and mediolateral (ML) directions and their ratio(AP/ML) was calculated.
Results
The AP/ML ratio was more sensitive than tractography in characterizing perisylvian regional abnormalities in DD children. The AP/ML ratio of the left perisylvian region was significantly lower in DD children compared with TD children (p = 0.03). The ML component of bilateral perisylvian regions was significantly higher in DD children compared with TD children (p=0.01 (left) and p=0.004(right)). No significant difference was found in the AP component between the two groups. A significant negative correlation of the left ML component with Vineland communication skills was observed (r = −0.657, p=0.011).
Conclusions
The AP/ML ratio appears to be a sensitive indicator of regional white matter architectural abnormalities in the perisylvian region of DD children.
Keywords: Developmental delay, Arcuate Fasciculus, Diffusion tensor imaging, Mental retardation, perisylvian language pathways
Developmental delay (DD) in children is characterized by significantly delayed development in two or more of the following developmental domains: gross/fine motor, speech/language, cognition, social/personal skills, and activities of daily living1, 2. In particular, DD is almost always accompanied by significant speech/language delay. Although the neurologic substrates of normal speech development are complex and involve the interaction of left hemispheric perisylvian language networks with other white matter pathways3, the neurologic basis of speech delay in children with DD is less well studied. In a recent study using diffusion tensor imaging (DTI) tractography in children with DD, we found that the major language tract in the left (dominant) hemisphere, i.e., the arcuate fasciculus (AF), could not be identified in a significant proportion of the children4. This study demonstrated the usefulness of DTI tractography in identifying previously unrecognized abnormalities in language pathways of children with DD. This finding also suggested that aberrant development of the perisylvian region is a key factor in delayed development.
In a subsequent DTI study of children with Angelman syndrome (AS), a much more severe developmental disorder with cognitive delay, epilepsy, and virtually absent speech, the AF could not be identified in 6 out of 7 patients5. In the remaining AS patient with an identifiable AF, direct quantification of the color-coded DTI orientation map (instead of tractography) showed that the ratio of antero-posteriorly (AP) oriented fibers to medio-laterally (ML) oriented fibers in the perisylvian region was significantly lower compared with typically developing (TD) children. Tractography is not appropriate for demonstrating such quantitative abnormalities in the perisylvian region due to relatively lower reproducibility for tracking the short-range peripheral white matter (unlike central white matter tracts such as AF that can be reliably tracked). Thus, this study suggested that direct quantification of color-coded orientation maps may identify subtler abnormalities in the perisylvian region not detectable by tractography of AF.
We hypothesized that similar subtle abnormalities in the perisylvian region will also be found in children with DD and that these abnormalities can be identified by examining the distributions of fiber orientation directly from DTI color-coded orientation maps. Therefore, we sought to determine whether the proportion of fibers in the region that are oriented in anteroposterior (AP) and mediolateral (ML) directions and the AP/ML ratio can be used to identify these subtle abnormalities. In addition, we related these measures with their neuropsychological evaluation as measured by Vineland adaptive behavior scores.
Methods
Fifteen right-handed children with DD (age: 61.1± 20.9 months) and 15 right-handed TD children (age: 68.4± 19.2 months) underwent MRI with DTI and developmental-behavioral assessments. All the patients had been referred for neurologic evaluation in the Children's Hospital of Michigan and DD was confirmed by an experienced pediatric neurologist. Patients with the following conditions were excluded: history of seizures, history of prematurity or perinatal hypoxic-ischemic event, focal deficits on clinical examination by a pediatric neurologist, autism, dysmorphic features suggestive of a clinical syndrome, structural clinical MR imaging interpreted as abnormal by a pediatric neuro-radiologist, positive findings on cytogenetic and/or fragile X tests, or the presence of an inborn error of metabolism. Written informed consent was obtained from either the parents or legal guardians of the participants. The Human Investigations Committee at Wayne State University granted permission for the retrieval and analysis of the clinical and MR imaging data of children with DD.
Vineland Adaptive Behavior Evaluation
The neuropsychological evaluation in the study included the Vineland Adaptive Behavior Scales, 2nd edn. (VABS) (Table I)6. The VABS is a caregiver-reported interview that yields measures of the child's adaptive behavior functioning in 4 domains (Communication, Daily Living, Socialization, and Motor skills), as well as an overall adaptive behavior composite (ABC). The measure has excellent reliability and validity6.
Table 1.
Table showing demographic data and Vineland adaptive behavior scores in DD group
| Age (months) | Sex | Vineland Communication | Vineland Daily living | Vineland Social | Vineland Motor | Vineland ABC | |
|---|---|---|---|---|---|---|---|
| 1 | 46 | M | 57 | 62 | 63 | 67 | 62.25 |
| 2 | 45 | M | 57 | 58 | 59 | 56 | 57.5 |
| 3 | 52 | F | 57 | 62 | 63 | 61 | 60.75 |
| 4 | 79 | F | 61 | 71 | 65 | 61 | 64.5 |
| 5 | 83 | F | 63 | 58 | 72 | 49 | 60.5 |
| 6 | 37 | M | 57 | 75 | 68 | 70 | 67.5 |
| 7 | 44 | M | 61 | 81 | 72 | 54 | 67 |
| 8 | 37 | M | 74 | 69 | 63 | 81 | 71.75 |
| 9 | 104 | M | 75 | 83 | 68 | 82 | 77 |
| 10 | 48 | M | 42 | 53 | 61 | 56 | 53 |
| 11 | 78 | F | 71 | 71 | 88 | 71 | 75.25 |
| 12 | 62 | F | 72 | 62 | 61 | 79 | 68.5 |
| 13 | 48 | M | 79 | 66 | 72 | 59 | 69 |
| 14 | 56 | M | 63 | 81 | 74 | 75 | 73.25 |
| 15 | 72 | F | 59 | 74 | 69 | 71 | 68.25 |
Data Acquisition and Preparation
All DTI scans were obtained on a 3T Signa scanner (GE Healthcare, Milwaukee, Wisconsin) equipped with an 8-channel head coil at TR = 1250 ms, TI = 88.7 ms, FOV = 240 cm, 128 × 128 acquisition matrix, contiguous 3-mm-thick sections to cover all the axial sections of the whole brain by using 55 isotropic gradient directions with b = 1000 s/mm2 and one b=0 acquisition. Double refocusing pulse was used to reduce eddy-current artifacts. In addition, array spatial sensitivity encoding technique was performed to further reduce geometric distortion because of the sequence design. Approximate scanning time for the DTI acquisition was 9 minutes.
All the children with DD were sedated for the scan. None of the typically developing children were sedated for the scan. However, the MRI scans were performed during the night time coinciding with their natural sleep cycle. After acquisition, all datasets were assessed for quality and deemed acceptable for analysis.
Color Map Quantification
Quantification procedure
The direction of individual fibers in diffusion tensor imaging data can be imaged by color-encoded orientation maps, where three components of the eigenvector v1, in association with the largest eigen value, are color coded using an RGB-color model which is symmetrical with respect to all color axes. This is given by the equation
(Equation 20 in Reference (7)) where r, g, and b represent red, green, and blue components of the voxel color, and (vx, vy, vz) is the normalized principal eigenvector. These measures can be directly obtained from DTI studio software.
The color axes are aligned with the patient coordinate system (green: anterior-to-posterior, red: medial-to-lateral, blue: superior-to-inferior). Thus, the measured green component is the same as anterior-posterior components (AP) and red component is the same as medial-lateral (ML) components that can be used as direct metric to quantify the proportion of fibers with different orientations in the perisylvian region. The total AP component value across all the voxels will give AP component quantification for that region. Same is true for ML component quantification.
ROI procedure
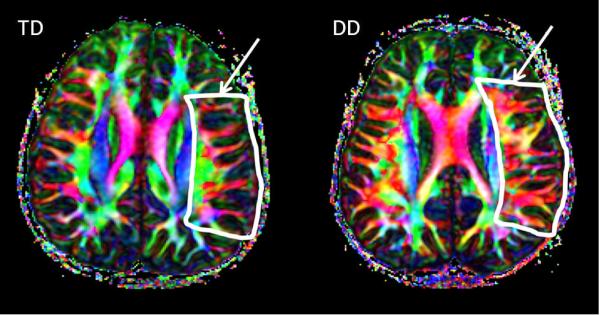
ROIs were drawn in the perisylvian region in a transaxial slice through which the bulk of arcuate fasciculus fibers pass through. Representative images of TD subjects and those with DD with drawn ROI are shown in the Figure. ROIs were drawn independently by two observers in all participants. Each participant's ROI was then placed on their respective diffusion tensor imaging color map. The mean values of red and green signal intensities for the perisylvian region were obtained using the DTIStudio software. These quantitative measures are automatically generated from the DTIStudio software. Given that the colors in the map represent the direction in which the white matter fibers travel, we defined this green/red ratio as the anterior-posterior/medial-lateral ratio(AP/ML ratio). An AP/MLratio was then calculated for all participants independently by two observers.
Figure.
Representative diffusion tensor color-coded orientation map of the left perisylvian area in TD and DD children. One can see that in the region of interest (shown in arrow), proportion of green and red components of principal eigenvector are dramatically different in the two groups of children.
Once the AP/ML ratio was determined for all subjects, the appropriate threshold value that best separates the two groups was determined by a leave-one-out cross-validation procedure to ensure that the thresholds will generalize to an independent dataset. In this procedure, an observation from the original sample is used as the validation data, and the remaining observations are used for deriving threshold. This process is then repeated iteratively so that each observation in the sample is used once as the validation data.
Tractography
Tensor calculation and tractography were performed using DTI-Studio software7. Tractography was carried out based on the Fiber Assignment by Continuous Tracking algorithm8 with fiber propagation starting at an fractional anisotropy threshold value of >0.2. The fiber propagation was stopped at a fractional anisotropy threshold <0.2 or angle threshold >60 degrees. The tracking protocol followed to isolate the arcuate fasciculus was described previously4.
Statistical analysis
The DD and TD groups did not differ with respect to age and sex. Two-sample unpaired t-tests were applied to compare the DD and TD groups. Chi square tests were used to compare the compare the sensitivities of tractography and color map quantification. A p-value of less than 0.05 was considered as statistically significant. Inter-observer error was calculated by 2×(R2−R1)/(R2+R1) (R=Rater ROI measure) and percent difference was averaged.
Two observers knowledgeable in the neuroanatomy of perisylvian region and DTI methodology drew the region of interest. The inter-observer error was calculated for AP component, ML component and AP/ML ratio on both sides.
Results
The mean ML component in the left hemisphere in the DD group was 60.18 ± 15.64 and in the TD group was 53.07±13.70; this difference was significant (p=0.01). Similarly, the mean ML component in the right hemisphere in the DD group was 61.61 ± 15.9 and in the TD group was 52.17 ± 13.47; this difference also was significant (p=0.04). However, no significant difference was found in the AP components between the two groups for either hemisphere. The mean AP/ML ratio of left perisylvian region in DD group was 1.21 ± 0.31 and in TD group was 1.03 ± 0.26 and this difference was significant (p=0.03). The mean AP/ML ratio of right perisylvian region in the DD group was 1.22 ± 0.32 and in the TD group was 1.01 ± 0.26; this difference was not significant (Table II). A significant negative correlation of the left ML component with Vineland communication skills was observed (r = −0.657, p=0.011).
Table 2.
Table showing perisylvian AP and ML components in DD and TD groups
| Left side | Right side | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TD | DD | p-value | TD | DD | p-value | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| A-P | 51.16 | 13.21 | 48.68 | 12.57 | NS | 52.53 | 13.56 | 51.9 | 13.4 | NS |
| M-L | 53.07 | 13.70 | 60.18 | 15.64 | 0.01 | 52.17 | 13.47 | 61.61 | 15.9 | .004 |
| A-P / M-L ratio | 1.03 | .26 | 1.21 | .31 | 0.03 | 1.01 | .26 | 1.22 | .32 | NS |
By conventional tractography, the AF could not be identified in 5 out of the 15 patients (33%) in the left hemisphere in the DD group. We performed a leave one out cross-validation procedure where the derivation of the AP/ML ratio threshold is separated from determination of the abnormality. The ratio was obtained in a subset of patients and applied back to the untested patients in an iterative manner. The AP/ML threshold obtained by this procedure was 0.82. We also evaluated this classification procedure and found an overall accuracy of 73.3% (evaluated using the leave-one-out cross-validation procedure described above). The sensitivity of the left hemisphere AP/ML ratio (10/15) was significantly higher than that of tractography (5/15) (p=0.03 by chi square test). The inter-observer error averaged across both sides was 1.8 ± 0.34% for AP component, 2.5 ± 0.47% for ML component and 1.2 ± 0.30% for AP/ML ratio. Even though the inter-observer variation is low, it has to be noted that the coefficient of variation (SD/Mean) in left AP/ML ratio was 23.6% in the population with DD and 16.6% in the TD population.
Discussion
In the present study, we found disorganized white matter in the bilateral perisylvian regions by direct quantification of DTI color-coded orientation maps. We also found that this method is more robust and sensitive than tractography in identifying abnormalities in the perisylvian region in children with DD. The proportion of fibers in the left perisylvian region that are oriented in the AP direction compared with the ML direction was decreased in children with DD compared with TD children. The ML component of bilateral perisylvian regions was significantly higher in children with DD compared with TD children. In addition, the left ML component of children with DD was negatively correlated with their communication skills.
Children with DD are difficult to evaluate because of limitations in our diagnostic approach. Standard neuroimaging, in particular, has been disappointing in its ability to reveal specific abnormalities9 In our previous studies, we used DTI tractography to extract language related pathways in the white matter of children with DD who had been evaluated with standard tests which had failed to reveal positive results4. Our major finding was the unidentifiability of the AF in approximately half of the patient4. Although this finding is replicated in the present study, unidentifiability of the AF in about one-third of patients is slightly lower than reported previously. This slight discrepancy is most likely due to relatively small sample sizes used in both studies. Nevertheless, the demonstration of unidentifiable AF in two independent datasets confirms the central importance of abnormal connectivity in perisylvian white matter pathways, such as the AF, in children with DD. Such unidentifiable AF has also been observed in severe syndromic conditions causing developmental delay such as Angelman syndrome5,10 and congenital bilateral perisylvian syndrome11. For example, in our previous study of seven children with Angelman syndrome, the AF could not be identified in 6 children5. In the remaining AS patient with identifiable AF, we noticed a decreased AP/ML ratio in left the perisylvian region; this suggested to us that a broader examination of fiber orientation may be a more sensitive approach to examine the perisylvian region. Although tractography is a powerful tool, it is only helpful where the tracts are long and the anatomic trajectory of tracts is well defined. Thus, we have now shown that even when the AF can be identified with tractography, a direct quantification of the proportion of fibers in the AP and ML orientations in color coded map is likely to be more useful.
In another study of children with DD, there was an atypical pattern of age-related maturity of AP and ML pathways12, further supporting the broader involvement of the perisylvian region in these children. In fact, functional MRI studies suggest that the neural substrate of language development is not just confined to the arcuate fasciculus but is more complex and dependent upon the interaction of perisylvian pathways with fronto-striatal and cerebellar pathway3. An earlier preliminary DTI study on patients with DD reported abnormal diffusivity changes in several areas such as the centrum semiovale, corona radiata, internal capsule, corpus callosum, and subcortical white matter of the frontal and parieto-occipital lobe13. Even though neither tractography nor color map quantification was performed in that study, the finding of abnormalities in various white matter regions is consistent with the complex neurological substrate of language and development3. Our present study is another step in demonstrating additional structural connectivity abnormalities underlying idiopathic developmental delay12.
The changes in microstructure of the perisylvian region in children with DD suggested by our findings could be due to a number of different processes. Many reasons are cited for this kind of abnormality like agenesis, excessive pruning, poor myelination, or an aberrant course of the white matter tracts due to regional abnormalities along the tract path resulting from genetic abnormalities in molecular pathways involved in axonal guidance or scarring from brain injury blocking axonal growth4. In light of our AP and ML abnormalities we think that regional abnormalities in perisylvian region resulting from genetic abnormalities in molecular pathways involved in axonal guidance could be the predominant factor, particularly those controlling dual projections of callosal projection neurons that have been shown to give rise to both AP and ML oriented fibers14. During prenatal and early postnatal development, there are exuberant dual projections from callosal projection neurons that not only cross the midline but also bifurcate in the ipsilateral white matter to develop association tracts14. Subsequent genetic factors and activity-dependent pruning determine the relative balance of ipsilaterally projecting (AP fibers) and contralaterally projecting (ML fibers) fibers. Mutations in the genes involved in the developmental control of callosal projection neurons or variations in the subsequent activity-dependent pruning of these fibers may alter this balance. Further studies are needed to identify the molecular mechanisms of perisylvian white matter disorganization in children with DD.
There are some limitations to the present study. The manual drawing of ROIs in this study could potentially increase the variability of the measurements. However, in order to minimize this potential error, the ROIs were carefully drawn by two observers experienced in analyzing DTI data. The inter-observer variation was 1.8 ± 0.34% for AP component, 2.5 ± 0.47% for ML component and 1.2 ± 0.30% for AP/ML ratio. Even though the inter-observer variation is low, it has to be noted that the coefficient of variation (SD/Mean) in AP/ML ratio was 23.6% in the population with DD and 16.6% in the TD population. In addition, the overall classification accuracy of AP/ML ratio was 73.3% (sensitivity of 60% and specificity of 86%) suggesting that there is significant overlap between the two populations. Another issue is that the sample size of the study is relatively small. Nevertheless, the significant findings observed in the present study suggest that the AP/ML ratio method could be a clinically useful and practical biomarker of language impairment in children with DD. An additional issue is that these findings are not unique to developmental delay. The abnormalities in the perisylvian region are observed in a variety of populations. Angelman syndrome, congenital bilateral perisylvian syndome are some of the well established conditions with dramatic abnormalities in this region5,10,11,15.
This new imaging method is promising in understanding the neurologic basis of developmental delay. However, given the heterogeneity and complexity of developmental disorders, it has to be emphasized that imaging procedures demonstrate only specific aspects of these disorders. More work is needed in this area but the current study is a step forward in demonstrating broader perisylvian structural connectivity abnormalities underlying idiopathic developmental delay.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Shevell M, Ashwal S, Donley D, Flint J, Gingold M, Hirtz D, et al. Practice parameter: evaluation of the child with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society. Neurology. 2003;60(3):367–80. doi: 10.1212/01.wnl.0000031431.81555.16. [DOI] [PubMed] [Google Scholar]
- 2.Crawford TO, Comi A, Freeman JM, Kossoff EH, Singer H, Vining EP, et al. Practice parameter: evaluation of the child with global developmental delay. Neurology. 2003;61(9):1315. author reply. [PubMed] [Google Scholar]
- 3.Kotz SA, Schwartze M. Cortical speech processing unplugged: a timely subcortico-cortical framework. Trends Cogn Sci. 2010;14(9):392–9. doi: 10.1016/j.tics.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Sundaram SK, Sivaswamy L, Makki MI, Behen ME, Chugani HT. Absence of arcuate fasciculus in children with global developmental delay of unknown etiology: a diffusion tensor imaging study. J Pediatr. 2008;152(2):250–5. doi: 10.1016/j.jpeds.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 5.Wilson BJ, Sundaram SK, Huq AHMM, Chugani HT. Abnormal Language Pathway in Children with Angelman Syndrome: A Diffusion Tensor Imaging (DTI) Study. Annals of Neurology. 2009;66:S109–S. doi: 10.1016/j.pediatrneurol.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparrow SS, Balla DA, Cicchetti DV, Doll EA. Vineland Adaptive Behavior Scales. American Guidance Service; Circle Pines (MN): 1984. [Google Scholar]
- 7.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81(2):106–16. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–9. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Yeargin-Allsopp M, Drews CD, Decoufle P, Murphy CC. Mild mental retardation in black and white children in metropolitan Atlanta: a case-control study. Am J Public Health. 1995;85(3):324–8. doi: 10.2105/ajph.85.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters SU, Kaufmann WE, Bacino CA, Anderson AW, Adapa P, Chu Z, Yallampalli R, Traipe E, Hunter JV, Wilde EA. Alterations in white matter pathways in Angelman syndrome. Dev Med Child Neurol. 2011;53:361–367. doi: 10.1111/j.1469-8749.2010.03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saporta ASD, Kumar A, Govindan RM, Chugani HT. Arcuate Fasciculus Fiber-tracking Identification and Speech Development in Patients with Congenital Bilateral Perisylvian Malformations. Annals of Neurology. 2009;66:S116–S7. [Google Scholar]
- 12.Jeong JW, Sundaram SK, Kumar A, Chugani DC, Chugani HT. Aberrant Diffusion and Geometric Properties in the Left Arcuate Fasciculus of Developmentally Delayed Children: A Diffusion Tensor Imaging Study. AJNR Am J Neuroradiol. 2010 doi: 10.3174/ajnr.A2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filippi CG, Lin DD, Tsiouris AJ, Watts R, Packard AM, Heier LA, et al. Diffusion-tensor MR imaging in children with developmental delay: preliminary findings. Radiology. 2003;229(1):44–50. doi: 10.1148/radiol.2291020049. [DOI] [PubMed] [Google Scholar]
- 14.Fame RM, MacDonald JL, Macklis JD. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011;34(1):41–50. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson BJ, Sundaram SK, Huq AHM, Jeong JW, Halverson SR, Behen ME, Bui DQ, Chugani HT. Abnormal Language Pathway in Children With Angelman Syndrome. Pediatr Neurol. 2011;44:350–356. doi: 10.1016/j.pediatrneurol.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]