Abstract
Pancreatic ductal tumors invade local parenchyma and metastasize to distant organs. Src-mediated tyrosine kinase signaling pathways promote pancreatic ductal adenocarcinoma (PDAC) metastasis, though the molecular mechanisms supporting this invasive process are poorly understood and represent important and novel therapeutic targets. The large GTPase Dynamin2 (Dyn2), a Src-kinase substrate, regulates membrane-cytoskeletal dynamics although it is yet to be defined if this mechanoenzyme contributes to tumor cell migration and invasion. Therefore the goal of this study was to test if Dyn2 is upregulated in human pancreatic tumors and to define its role in cell migration and metastatic invasion using in vitro assays and nude mouse models. Histological analysis showed that 81% of the 85 patients tested had elevated Dyn2 in PDAC tissue. To test if Dyn2 overexpression alters metastatic properties of human pancreatic tumor cells, stable clones of BxPC-3 cells overexpressing either wild-type Dyn2 or a phosphorylation-deficient mutant Dyn2Y(231/597)F known to attenuate Dyn2 function, were generated and analyzed for migratory capacity. Importantly, tumor cells expressing 2-3 fold levels of Dyn2 protruded lamellipodia at twice the rate, migrated faster (180%) and farther (2.5-fold greater net distance) on glass and through transwell chambers (2-3 fold more cells through the filter) compared to cells expressing Dyn2Y(231/597)F or vector alone. Further, siRNA-mediated depletion of Dyn2 and dynamin inhibitors MiTMAB and Dynasore significantly reduced cell migration (>66%), wound healing (>75%) and invasion in transwell assays (>95%) compared to DMSO treated cells. To test the metastatic potential conferred by increased Dyn2 expression, the BxPC-3 clonal cell lines were implanted orthotopically into the pancreas of nude mice. Cells expressing Dyn2-GFP exhibited a 3-fold increase in large distal tumors compared to cells expressing Dyn2Y(231/597)F or vector alone. Finally, histological analysis of pancreatic metastases from human patients revealed that Dyn2 is upregulated in 60% of metastatic tumors examined. These findings are the first to implicate dynamin in any neoplastic condition and to directly demonstrate a role for this mechanoenzyme in invasive cell migration.
Keywords: Dynamin 2 (Dyn2), Pancreatic ductal adenocarcinoma (PDAC), Cell migration, Invasion, Metastasis, Src
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly invasive disease with an exceptionally poor prognosis (Nieto et al., 2008) making it the fourth leading cause of cancer deaths in America with an overall 5-year survival rate of only 5% (Jemal et al., 2010). These tumors are known to disseminate aggressively from the pancreas to invade peripheral tissues and organs including the surrounding mesentery, as well as the liver and lymph nodes (Mihaljevic et al., 2010). Currently little is known about the migratory mechanisms that support this metastatic process although it is well documented that the signaling cascades activating tumor cell migration are markedly over-expressed in these tumors. For example, the epidermal growth factor receptor family members ErbB-1 (EGFR/Her-1) and ErbB-2 (Her-2/neu) are amplified in over a third of PDACs, while important downstream effectors of these receptors such as Src and K-Ras are increased or mutated in the majority of these tumors (Bloomston et al., 2006; Dancer et al., 2007; Frame, 2002; Hakam et al., 2003; Harada et al., 2008; Kim et al., 2009; Lutz et al., 1998; Morton et al., 2010; Thybusch-Bernhardt et al., 2001).
The adherence and migratory behavior of tumor cells activated by these aberrant signaling cascades is supported by a complex cytoskeletal network composed of filamentous actin and scores of associated linker proteins and motors. The dynamics of this network support the extension of actin-membrane protrusions, or lamellipodia, at the leading edge of the tumor cells that facilitate migration (Etienne-Manneville, 2008; Machesky, 2008; Pollard and Borisy, 2003; Yamaguchi and Condeelis, 2007). How tumor cells coordinate lamellipodial extension with cell adhesion during invasion is currently a central focus of cancer cell biology. Recently, the dynamin family of large GTPases, known to deform and sever cellular membranes (Doherty and McMahon, 2009; Hansen and Nichols, 2009; Hinshaw, 2000; McNiven, 1998; Orth and McNiven, 2003; Praefcke and McMahon, 2004) has been shown to interact with the actin cytoskeleton and thus have been implicated in cell migration (Kessels et al., 2001; Kruchten and McNiven, 2006; McNiven et al., 2000; Qualmann et al., 2000; Schafer, 2004). Along with an established role in endocytosis and membrane traffic (Hinshaw, 2000; McNiven, 1998; Urrutia et al., 1997) the ubiquitously expressed Dyn2 isoform has been shown to function in actin polymerization and organization (Lee and De Camilli, 2002; Mooren et al., 2009; Orth et al., 2002; Schafer, 2004), focal adhesion regulation (Ezratty et al., 2009; Ezratty et al., 2005; Yoo et al., 2005), and lamellipodia extension (Krueger et al., 2003; Schlunck et al., 2004), possibly through interactions with actin-binding proteins. Thus, through participation in many different cellular processes it is likely that Dyn2 may act to accentuate the neoplastic condition by supporting invasive migration. Surprisingly, a role for Dyn2 in cell migration has yet to be demonstrated, and no information exists on the expression, function or regulation of this versatile mechano-enzyme in the metastatic process.
Here we provide a novel, in depth, examination of the role of Dyn2 in pancreatic cancer. We find that Dyn2 expression is markedly upregulated in the majority of human patients with this disease and that elevated levels of this enzyme contribute substantially to lamellipodia extension that in turn enhances cell migration either along a planar substrate or through a 3-dimensional matrix. Conversely, inhibition of Dyn2 function by expression of phosphorylation-impaired mutant protein, treatment with Dyn2 inhibitory drugs, or depletion with siRNA all significantly reduce tumor cell dynamics. These experimental observations in isolated human tumor cell lines supported subsequent in vivo studies that show an enhanced dissemination and metastases of Dyn2-expressing tumor cells in an orthotopic nude mouse model. Finally, the phosphorylated state of Dyn2 is also an important factor in pancreatic cancer, as expression of Dyn2 phospho-mutants in cells leads to a marked attenuation of tumor cell migration and invasion.
Results
The expression of Dyn2 is markedly increased in patients with pancreatic cancer
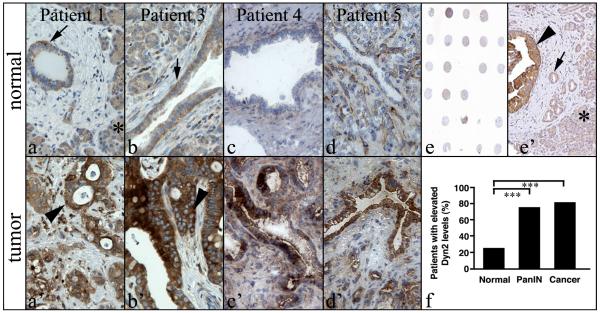
To determine the role of dynamin 2 (Dyn2) in pancreatic ductal adenocarcinoma (PDAC), tissue sections from both normal and cancerous regions of pancreatic cancer patients were probed for Dyn2 expression using immunohistochemistry. An initial immunohistochemical screening showed a marked increase in Dyn2 levels between normal and cancerous regions of pancreatic tissue from 9 of 11 patients diagnosed with PDAC. Modest Dyn2 expression was detected throughout the cytoplasm of non-malignant ductal epithelial cells and acinar cells of normal tissue from the diseased patients (Fig. 1a-1d). In comparison, Dyn2 levels were markedly elevated in the epithelium of cancerous lesions (Fig. 1a’-1d’). The increased Dyn2 levels were confined to the epithelial cells and did not increase noticeably in either stroma or acini.
Fig. 1. Dyn2 is markedly upregulated in pancreatic ductal carcinoma.
Immunohistochemistry staining of Dyn2 in matched normal (a-d) and cancer (a’-d’) tissue from PDAC patients. Dyn2 is expressed in the ductal epithelium (arrows) and in acinar cells (asterisk in a’) at moderate levels in non-cancerous regions of pancreatic tissue. In contrast, neoplastic ducts (arrowheads) from the same patients stain strongly for Dyn2. Ductular regions from four different patients are shown. A low (e) and high (e’) magnification image of 1 of the 3 tissue microarrays utilized in this study containing tissue from PDAC patients. Neoplastic ducts (arrowhead in e’) stain strongly for Dyn2 compared to normal ducts (arrow in e’) or acini (asterisk in e’). (f) Quantification of PDAC patients with elevated Dyn2 levels in regions of normal ducts, PanINs or cancer. Fisher’s Exact Test was used for statistics (*** represents p < 0.0001).
To expand the numbers of patients in the sample set, tissue microarrays (TMAs) of human PDAC from 85 patients were examined (Fig. 1e). The TMA sections contained normal ducts, pancreatic intraepithelial neoplasias (PanINs), and cancerous lesions. Consistent with the initial screen, Dyn2 levels were significantly higher in ductal epithelium during PanIN and cancerous stages, as compared to normal ductal epithelium within the TMA sections (Fig. 1e’). Notably, three times the number of pancreatic ducts that were scored as PanINs or cancer had elevated Dyn2 levels when compared to normal ducts within the TMA (Fig. 1f; p<0.0001). These data indicate that Dyn2 expression levels are upregulated in the majority of PDACs.
Elevated levels of phospho-Dyn2 promote a migratory phenotype
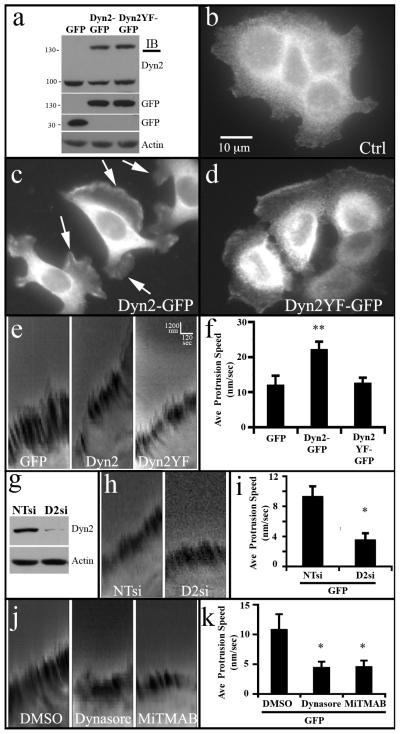
Dynamin expression and activity have been shown to regulate actin dynamics (Krueger et al., 2003; Lee and De Camilli, 2002; Mooren et al., 2009; Orth et al., 2002) while participating in the formation and extension of lamellipodia (McNiven et al., 2000; Schlunck et al., 2004). Importantly, it has been shown that the dynamins are phosphorylated by the tyrosine kinase c-Src (Ahn et al., 1999; Shajahan et al., 2004) that act downstream of membrane receptors such as EGFR (Ahn et al., 2002). Both EGFR and Src kinase have been shown to be markedly upregulated in PDACs (Bloomston et al., 2006; Dancer et al., 2007; Hakam et al., 2003; Lutz et al., 1998; Morton et al., 2010; Thybusch-Bernhardt et al., 2001) and activation of these signaling cascades have been shown to promote tyrosine phosphorylation of dynamin on Y231 and Y597 (Ahn et al., 2002; Ahn et al., 1999). Phosphorylation of these specific sites increases the GTPase activity and self-assembly of dynamin and regulates its functional roles in endocytosis and Golgi reorganization (Ahn et al., 2002; Cao et al., 2010; Vieira et al., 1996; Weller et al., 2010). From these past findings we hypothesized that elevated expression of Dyn2, combined with the activated signaling cascades observed in these tumors, could enhance invasive cell migration that would in turn promote the dissemination of PDAC cells. To test this premise we used a widely studied pancreatic cancer cell line (BxPC-3) to generate stable cell lines expressing either Dyn2-GFP, a phospho-deficient mutant Dyn2Y(231/597)F-GFP, or cells expressing GFP alone as a control. Western blot analysis of these cell lines revealed that expression levels of the stably-expressed WT or mutant Dyn2 were comparable to endogenous protein (Fig. 2a). Most remarkable was the change in phenotype of the WT Dyn2-GFP-expressing cells compared to the GFP-expressing cells. While the control cells were generally observed to clump together in non-motile colonies, the stable Dyn2-GFP-expressing cells displayed numerous, large lamellipodia (Fig. 2b,c). Moreover, the cell line expressing the mutant Dyn2Y(231/597)F-GFP exhibited some motile characteristics but not to the extent of the cells expressing the WT Dyn2-GFP (Fig. 2d), suggesting that phosphorylation of Dyn2 plays a role in promoting a motile phenotype of these tumor cells.
Fig. 2. Lamellipodia formation and dynamics in pancreatic tumor cells are dependent upon Dyn2 expression and phosphorylation.
(a) Western blot analysis of stable BxPC-3 cell lines expressing either GFP alone, Dyn2-GFP, or Dyn2Y(231,597)F-GFP. (b-d) Control BxPC-3 cells stained for cortactin (b) grow in tight clusters and appear non-motile, while stable lines expressing Dyn2-GFP (c) exhibit numerous dynamic lamellipodia. (d) In comparison, mutant Dyn2Y(231,597)F-GFP expressing cells appear loosely clustered with some motile morphology. (c-d) show GFP fluorescence of the Dyn2-GFP and Dyn2Y(231,597)F-GFP. (e) Kymographs generated from movies of the lamellipodia of BxPC-3 stable cells showing the rapid protrusion rate of lamellipodia in cells expressing WT Dyn2-GFP, relative to cells expressing GFP or Dyn2Y(231/597)F -GFP. (f) Average lamellipodia protrusion speed calculated from more than 11 cells per cell line based on collected kymographs shown in (e). (g) Western blot on lysates from BxPC-3-GFP cells treated with non-targeting siRNA (NTsi) or Dyn2 siRNA (D2si). Kymographs (i) and quantitation (i) showing reduced lamellipodia extension following Dyn2 knockdown. Kymographs (j) and quantitation (k) showing lamellipodia extension of BxPC-3-GFP cells following treatment with DMSO or dynamin inhibitors Dynasore (80 μM) or MiTMAB (20 μM). Error bars represent S.E.M. Student’s t-test was used for statistics (** represents p < 0.001 and * represents p < 0.05 compared to controls).
To quantify the effect of the exogenously expressed Dyn2 on lamellipodia dynamics, we performed live-time imaging of cells from each established cell line, collecting 1 image every 15 sec for 10 min. These collected images were assembled to form kymographs in which the phase-dense ruffles at the periphery of each lamellipodium could be tracked and measured for motility as performed by others (Bryce et al., 2005; Giannone et al., 2004). The extension of a lamellipodium recorded in each kymograph is reflected by the forward (upward) slope of the phase dense lines. This slope is converted to speed of protrusion in nm/sec using the formula (distance extended (nm)/time (sec). When comparing kymograph slopes of more than 11 cells from each cell line we found that Dyn2-GFP-overexpressing cells extended lamellipodia at a greater speed than did cells expressing GFP (Fig. 2e, 2f). These data are consistent with the observation that high levels of Dyn2 can contribute to enhanced lamellipodia protrusion rates, suggesting that Dyn2 could play a meaningful role in tumor cell migration. Further, Dyn2Y(231/597)F-GFP was not able to promote cell migration (Fig. 2e, 2f), suggesting that the role of Dyn2 in migration is likely dependent on its phosphorylation at known Src phosphorylation sites.
Next, the role of Dyn2 function in efficient lamellipodia protrusion was tested by reducing Dyn2 levels via siRNA depletion or perturbing Dyn2 function with dynamin inhibitors in the control GFP-expressing BxPC-3 cell line. Notably, depletion of Dyn2 resulted in over 60% reduction in lamellipodia protrusion rate (Fig. 2g-i). Similarly, a 30 minute pretreatment of cells with the small molecule dynamin inhibitor MiTMAB (Myristyl trimethyl ammonium bromides), that attenuate dynamin GTPase activity by disrupting the interaction between the PH domain and phospholipids (Hill et al., 2004; Quan et al., 2007), or Dynasore, a non-competitive inhibitor of dynamin GTPase activity that locks dynamin in a GTP-bound state likely through association with the GTPase domain (Macia et al., 2006; Newton et al., 2006), also significantly attenuated lamellipodia protrusion to the same level as Dyn2 depletion (Fig. 2j, 2k). Taken together, these data indicate that Dyn2 expression is necessary for efficient lamellipodia protrusion and also that Dyn2 overexpression is sufficient for enhancing lamellipodia protrusion rate in pancreatic tumor cells.
Dynamin activity is required for cell migration and invasion
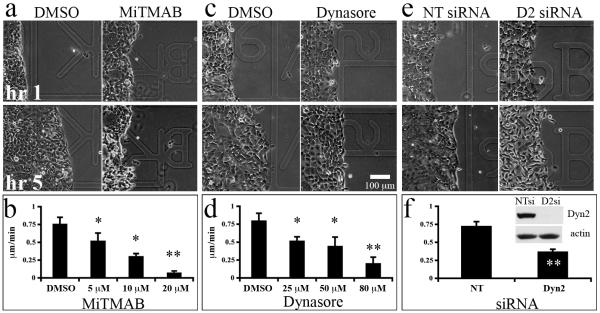
To test whether Dyn2 function is required for cell migration, we assessed the migratory capacity of pancreatic tumor cells in wound-healing assays. The BxPC-3 cell lines failed to close wounds consistently and when closure was realized, the rate was too slow to eliminate the confounding effects of cell division, precluding this assay from producing useful analysis of wound healing motility for these cell lines. Therefore, we utilized a highly motile pancreatic cancer cell line (Panc04.03) for wound-healing migration assays. Cells were plated to confluence and a wound was generated with a plastic pipette prior to the addition of 50 ng/mL EGF in the presence or absence of the pharmacological dynamin inhibitory compounds MiTMAB or Dynasore, or following Dyn2 depletion with siRNA. While Panc04.03 cells treated with the DMSO carrier migrated into the wounded area rapidly, cells in the presence of increasing concentrations of MiTMAB (Fig. 3a, 3b) or Dynasore (Fig. 3c, 3d) exhibited a dose-dependent reduction in cell migration into the wound, indicating that both dynamin’s GTPase activity and its association with the membrane contribute to cell migration in pancreatic cancer cells. Similarly, while Panc04.03 cells treated with non-targeting siRNA migrated rapidly into the wound, depletion of Dyn2 with siRNA resulted in a statistically significant attenuation of this directed cell migration, further supporting the requirement for Dyn2 in pancreatic cancer cell migration.
Fig. 3. Dynamin function is required for cell migration during wound healing.
Confluent monolayers of the motile pancreatic cancer cell line Panc04.03 were wounded and stimulated with 50 ng/mL EGF in the presence or absence of the indicated doses of the dynamin inhibitory compounds MiTMAB (a, b) or Dynasore (c, d), or after treatment with non-targeting (NT) or Dyn2 (D2) siRNA (e, f). Phase images of cell monolayers treated with DMSO (a,c), 20 μM MiTMAB (a), or 80 μM Dynasore (c) at 1 and 5 hrs post-EGF stimulation. Increasing the dosage of either drug resulted in a dose-dependent reduction in cell migration, contributing to slower wound closure. Similarly, depletion of Dyn2 by siRNA significantly reduced cell migration speed (e, f). Efficient knockdown of Dyn2 is shown in the inset in panel (f). The average speed of the wound edge from 3 experiments are shown. Error bars represent S.E.M. Student’s t-test was used for statistics (** represents p < 0.001, * represents p < 0.05 compared to DMSO).
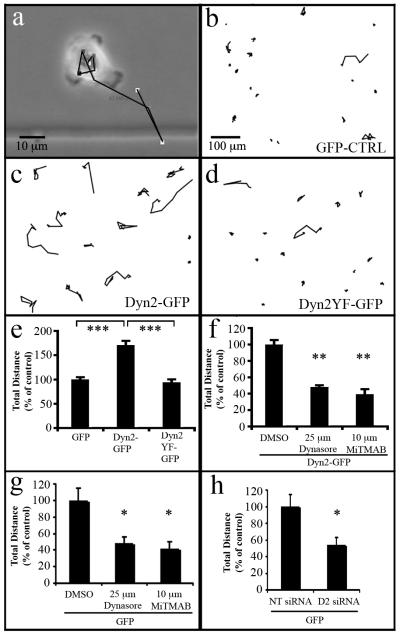
To define the contribution of Dyn2 expression and activity on the migratory behavior of individual tumor cells, the established stable BxPC-3 cell lines described in Fig. 2 were next analyzed in single-cell motility assays. Cells stably expressing either WT Dyn2-GFP, Dyn2Y(231/597)F-GFP or GFP were plated at low density, tracked over 11 hrs, and migration paths were generated. Consistent with the observation that Dyn2-overexpression enhances lamellipodia protrusion, the migration tracks indicate that Dyn2-GFP expressing cells migrated further than did cells expressing the phospho-deficient Dyn2Y(231/597)F-GFP or the control GFP-expressing cells (Fig. 4b-d). Quantification revealed a nearly 2-fold increase in total distance traveled for cells over-expressing Dyn2-GFP (Fig. 4e), suggesting that Dyn2 expression level enhances pancreatic cancer cell migration and that this enhancement requires phosphorylation at tyrosines 231/597.
Fig. 4. Elevated dynamin expression significantly increases individual tumor cell migration.
(a) Low magnification phase images of the BxPC-3 stable cell lines described in Fig 2 were acquired over 11 hrs and subsequently traced to produce migration paths. The black line depicts the migratory path of a cell over the observation period. (b) Migration tracks generated from stably transfected cells expressing GFP, (c) Dyn2-GFP and (d) Dyn2Y(231/597)F -GFP. (e) Graph depicting total migration distance of tumor cells stably overexpressing GFP, Dyn2-GFP, or Dyn2Y(231/597)F -GFP (e), Dyn2-GFP-expressing cells treated with the dynamin inhibitors MiTMAB or Dynasore at the indicated concentrations (f), or control GFP-expressing cells treated with dynamin inhibitors (g) or siRNA directed against Dyn2 (h). Compared to the other cell lines, the tumor cell line expressing Dyn2-GFP exhibited a significant increase in migration that was markedly reduced by inhibiting dynamin function. Further inhibition of endogenous Dyn2 in control cells using inhibitory compounds or Dyn2 depletion also reduced cell migration. (n=100 cells per condition, average of three experiments). Student’s t-test was used for statistics (*** represents p < 0.0001, ** represents p < 0.001, * represents p < 0.05 compared to control).
To confirm that the enhanced migration capacity of the Dyn2-GFP-overexpressing cells was due to Dyn2 activity, individual cell migration of BxPC-3 cells was examined in the presence of the dynamin inhibitors MiTMAB and Dynasore. Inhibition of dynamin function significantly reduced the migration of BxPC-3 cells overexpressing Dyn2-GFP in a dose-dependent manner (Fig. 4g) consistent with the inhibitory effect of these compounds on Panc04.03 migration (Fig. 3). Similarly, inhibition of dynamin function or depletion of Dyn2 with siRNA in control GFP-expressing cells significantly attenuated cell migration (Fig. 4g, 4h), consistent with the requirement of Dyn2 expression for cell migration (Fig. 3) and lamellipodia protrusion (Fig. 2).
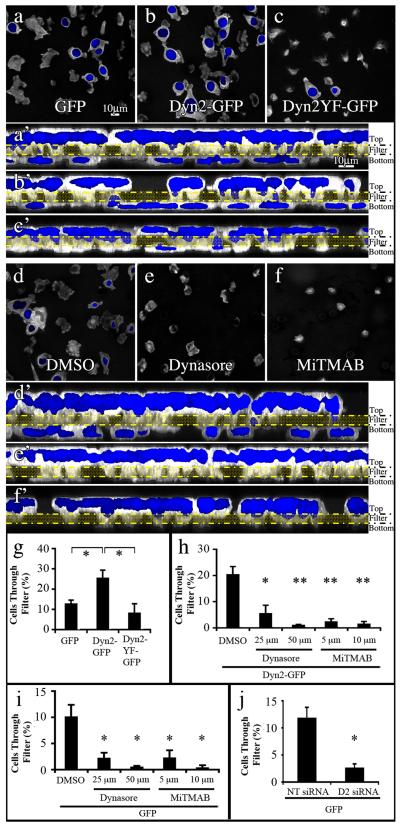
To link migratory activity with chemotactic invasion, blind-well chamber assays were performed using the BxPC-3 stable cell lines. Cells were plated in a low serum environment in the top chamber of a permeable barrier filter containing 8 micron diameter pores coated with 0.01% gelatin, while the bottom of the chamber contained high serum. Notably, over two-fold more Dyn2-GFP expressing tumor cells crossed the filter than did cells expressing Dyn2Y(231/597)F or GFP vector alone (Fig. 5a-c, 5g). Consistent with the migration assay findings from Fig. 4f, Dyn2 activity was required for this enhanced behavior as MiTMAB and Dynasore both significantly attenuated the ability of the Dyn2-GFP-expressing tumor cells to invade through the porous filter (Fig. 5d-f, 5h). To confirm that the basal level of migration in GFP-expressing control cells is dependent on Dyn2, the cells were treated with dynamin-targeting drugs or depleted of Dyn2 with siRNA. Consistent with Figs 2-4, Dyn2 inhibition or depletion significantly attenuated chemotactic invasion to levels equivalent to drug-treated Dyn2-expressing cells (Fig. 5i-j). Taken together, these experiments indicate that Dyn2 expression and activity are required for chemotactic invasion of BxPC-3 cells and that Dyn2 over-expression is sufficient to enhance this invasion process in a phosphorylation-dependent manner.
Fig. 5. Dyn2 function is essential for chemotactic invasion in vitro.
Fluorescence images of tumor cells plated on transwell filters. 2×105 stable BxPC-3 cells expressing GFP (a), Dyn2-GFP (b) or Dyn2Y(231/597)F -GFP (c), were plated in low serum medium (0.2%) in the top of a blind-well chamber, separated from high serum (10% FBS) in the bottom chamber by a gelatin-coated filter containing 8 μm pores and returned to the culture incubator for 4 hrs. After fixation, the cells on the filters were co-stained with phalloidin for actin (white) to visualize cell bodies and DAPI (blue) to visualize nuclei. The image focal plane in a-f represents the bottom of each filter and reveals cells that have successfully translocated by the emergence of a blue nucleus. While numerous Dyn2-GFP expressing cells have migrated through the filter (b), most cells expressing the GFP or Dyn2Y(231/597)F-GFP protein remain at the top of the chamber (a,c). These same confocal images were reoriented as brightest point projection Z-series, with the filter indicated by the yellow double lines (a’-c’). Note the marked increase in nuclei of Dyn2-GFP expressing cells below the filter compared to cells expressing Dyn2Y(231,597)F-GFP or the control. (g) Quantification of the percentage of cells that crossed a gelatin-coated filter in blind-well assays. Notably, twice as many Dyn2-GFP-expressing cells migrated across the filter as did cells expressing GFP, and more than 3 times the number compared to Dyn2Y(231/597)F-GFP. (d-f) Confocal images of the same Dyn2-GFP expressing cell line plated on filters as above and treated with DMSO as a control or the dynamin inhibitory compounds Dynasore (50 μM) and MiTMAB (10 μM). Migratory invasion through the filters was almost completely prevented in the presence of dynamin inhibitory compounds MiTMAB and Dynasore as shown clearly in the Z-series confocal images of these fields (d’-f’) and quantitated in (h). Similarly, the basal level of migration in GFP-expressing control cells was attenuated after depletion (i) or small-molecule inhibition of (j) endogenous Dyn2. Average +/− S.E.M of greater than 150 cells per line. Student’s t-test was used for statistics (**represents p < 0.001, * represents p < 0.05 compared to control).
Over-expression of Dyn2 enhances local dissemination and metastatic invasion of pancreatic cancer in vivo
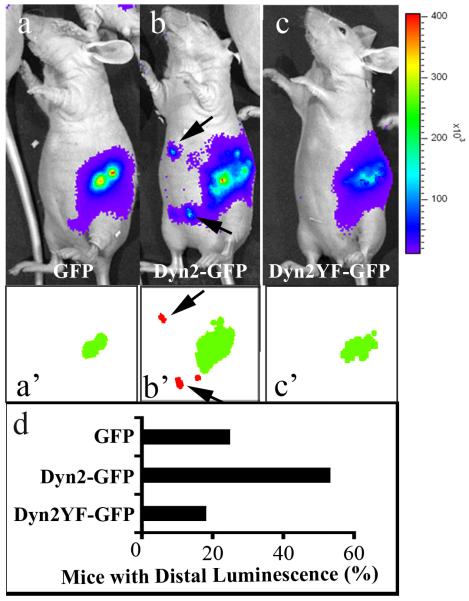
Based on the observations that increased levels of Dyn2 lead to the enhanced tumor cell migration and chemotactic invasion in vitro (Figs. 4 and 5), we next asked whether over-expression of Dyn2 promotes cell migration in vivo using a murine orthotopic implantation model (Alves et al., 2001). The stable BxPC-3 cell lines expressing WT Dyn2-GFP (two independent cell lines), Dyn2Y(231/597)F-GFP (one cell line) or GFP alone (two cell lines) were transduced with a lentivirus encoding a luciferase gene to monitor tumor cell metastasis in living animals, and subsequently the cells were injected directly into the pancreas of nude mice in at least three independent experiments per construct. Two weeks post-injection, these mice were anesthetized, injected with the luciferase substrate luciferin and imaged by bioluminescence to detect tumor cell dissemination away from the primary tumor (Fig. 6 a-c). Images obtained were auto-segmented with IVision software to detect peaks of luminescence (Fig. 6 a’-c’). Importantly, while luminescence emitted by the luciferin was detected at the site of injection in all mice, more than 2.5-fold more mice injected with the Dyn2-GFP expressing cells displayed luminescence distal from the injection area (arrows in Fig. 6b and 6b’) compared to GFP control cells. Most notable was the fact that markedly few mice injected with BxPC-3 cells expressing the phospho-deficient Dyn2Y(231/597)F-GFP mutation exhibited peripheral luminescence in comparison to mice injected with WT expressing Dyn2 cells (Fig. 6d), despite the fact that the same number of cells were injected into each mouse, there was no difference in the growth rates of the cell lines as performed with MTT assays and manual cell counts (unpublished data), and the resulting primary tumor was comparable across all cell lines (Fig. 7e). These data suggest that Dyn2 overexpression and phosphorylation contribute to tumor cell dissemination in vivo.
Fig. 6. Dyn2 overexpression enhances local dissemination of pancreatic cancer cells in vivo.
Luminescence images of nude mice injected with 1×106 luciferase-transduced BxPC-3 stable cells expressing either GFP (a), Dyn2-GFP (b), or Dyn2Y(231/597)F-GFP (c) two weeks after orthotopic injection into the pancreas. Luminescent signals are displayed as pseudocolor heat-map images (blue, least intense; red most intense; ×103 photons/second) overlaid on gray-scale body images. (a’-c’) Luminescent primary tumors (green) and distally disseminated tumors (red) detected by automated segmentation of luminescence using IVision software (arrows in b and b’ indicate discrete foci of distal luminescence detected by IVision). (d) Quantification of the percentage of mice implanted with tumor cells expressing GFP, Dyn2-GFP or Dyn2Y(231/597)F-GFP that exhibited distal luminescence. At least 10 mice for each treatment group were used. Primary tumors expressing high levels of WT Dyn2-GFP disseminated more frequently than tumors expressing the mutant Dyn2Y(231/597)F-GFP or GFP.
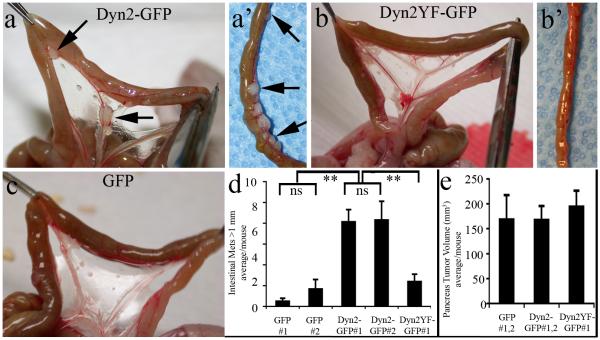
Fig. 7. Dyn2 overexpression promotes metastasis of pancreatic tumor cells to the small bowel.
Images of the small intestine of nude mice 8 wks after orthotopic pancreatic implantation of BxPC-3 stable cell lines expressing Dyn2-GFP (a), Dyn2Y(231/597)F-GFP (b, b’), or GFP (c). While tumors were rarely observed in mice injected with GFP-expressing tumor cells, exceptionally large tumors >1mm in diameter were apparent within the intestinal mesenchyme of mice injected with either Dyn2-GFP cell line (arrows in a, a’). (d) Quantitation of the number of large (>1 mm diameter) tumors per mouse injected with each cell line. Data from three separate experiments per treatment group resulting in at least 10 mice per construct show a marked increase in large intestinal tumors in mice bearing Dyn2-GFP-expressing BxPC-3 cell lines, even though the mice displayed with primary tumors of comparable volume (e). Data are shown as average +/− S.E.M. Statistics were performed using a Student’s t-test (** represents p < 0.001).
To assess whether Dyn2 overexpression might increase metastatic potential of the injected tumor cells, mice implanted with the BxPC-3 cell lines were sacrificed 8 weeks post orthotopic injection. Macroscopic inspection of mice injected with either of the GFP-expressing control cell lines revealed locally advanced, regionally invasive tumors on the spleen and the retroperitoneum consistent with the observations of others using parental BxPC-3 cells (Bouvet et al., 2000). Few large tumors were observed outside of the injection site and no tumors were observed on the liver. In marked contrast to control mice, all 18 mice injected with Dyn2-GFP expressing cell lines had large (>1mm) tumors within the body cavity, particularly on the intestinal wall and within the intestinal mesentery (Fig. 7a-a’, 6b’). In addition, 3 of 18 of these mice had tumors on the liver. Quantification revealed a >7-fold increase in the average number of large intestinal tumors when compared to mice injected with GFP-expressing BxPC-3 cells and a >3-fold increase compared to mice injected with cells expressing Dyn2Y(231/597)F-GFP (Fig. 7d), even though the volume of the primary pancreas tumors were equivalent (Fig. 7e). These findings provide a compelling in vivo correlation to the cellular migration studies shown in Figs. 2-5 and further implicate Dyn2 expression and its phosphorylation state in invasive migration of pancreatic tumor cells.
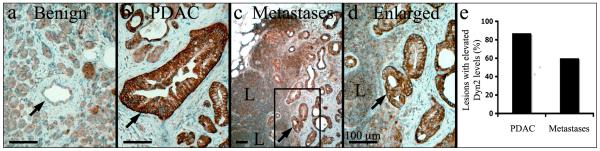
To assess the status of Dyn2 expression in human metastates, the relative Dyn2 expression levels were compared on histological samples representing benign tissue, PDAC and metastatic tissue from 39 patients (Fig. 8). Consistent with the TMA data described in Fig.1, greater than 80% of the PDAC samples displayed elevated Dyn2 levels when compared to benign tissue from the same patient (Fig. 8a-b, 8e). Notably, elevated Dyn2 expression was retained in metastatic lesions of 60% of the patients, even after the tumors had reorganized into duct-like structures (Fig. 8c, enlarged in Fig. 8d).
Fig. 8. Dyn2 is over-expressed in human PDAC metastases.
Immunohistochemistry staining of Dyn2 in benign (a), pancreatic ductal adenocarcinoma (PDAC) (b) and lymph node metastases (boxed region in lower magnification image c is enlarged in d). Dyn2 is expressed in the ductal epithelium (arrow in a) at moderate levels in benign regions of pancreatic tissue. In contrast, strong staining of Dyn2 was observed in neoplastic pancreatic ducts (arrow in b) and in duct-like structures that were formed in lymph nodes by metastatic pancreatic ductal cells (arrow in c, d). ‘L’ demarks lymphocytes in the lymph node. (e) Quantitation from 39 patients showing that greater than 80% of PDAC and 60% of metastases expressed higher levels of Dyn2 when compared to benign ducts from the same patient. All scale bars represent 100 μM.
Discussion
In this study, we have presented some of the first observations linking the expression levels, function, and regulation of the large mechanoenzyme dynamin to the neoplastic condition. Dyn2 expression is upregulated in greater than 80% of PDAC in patients tested (Fig. 1, Fig. 8) and 60% of metastases (Fig. 8). Further, we show that overexpression of Dyn2 in the PDAC cell line BxPC-3 induces a motile phenotype characterized by large and protrusive lamellipodia that is dependent on the phosphorylation state of Dyn2, as the Dyn2 phospho-deficient mutant Dyn2Y(231,597)F failed to induce the motile phenotype (Fig. 2d, 2f). In support of these findings, dynamin inhibitors or Dyn2 depletion by siRNA attenuated PDAC cell migration (Fig. 3, Fig. 4g-h) and chemotactic invasion (Fig. 5d-j) while overexpression of WT, but not phospho-deficient, Dyn2 enhanced these events (Fig. 4a-f, 5a-c’, 5g). Finally, this accentuation of invasion by Dyn2 function was observed by the marked dissemination and metastasis of orthotopically injected pancreatic tumor cells into nude mice (Figs. 6-7).
The initial premise of this study was based on the hypothesis that Dyn2 would participate in promoting cell migration and metastasis. We did not predict the amplification of Dyn2 levels in cancer patients. Histological analysis revealed elevated Dyn2 protein levels in a remarkably high percentage of the ductal epithelium of neoplastic ducts, though there were no obvious changes in acinar Dyn2 expression (Fig. 1). Interestingly, the Dyn2 levels were increased in the early PanIN stages as well as the later cancer stages, suggesting that Dyn2 upregulation is an early event that precedes or coincides with cell shape changes observed in the neoplastic condition. Most relevant is the fact that 60% of metastases exhibit Dyn2 overexpression while 80% of the PDAC samples excised from the pancreas exhibited Dyn2 overexpression. Thus, Dyn2 overexpression appears to contribute to the initial stages of neoplasia while also driving the metastatic process.
An essential role of dynamin in cell migration and invasion is further supported by the capacity of siRNA-mediated Dyn2 depletion or dynamin inhibitory compounds MiTMAB and Dynasore to attenuate the Dyn2-mediated enhancement of these migratory processes (Fig. 2-5). These observations are similar to Schwartz and colleagues (Schlunck et al., 2004) who observed that siRNA-mediated knockdown of Dyn2 reduces lamellipodia extension in glioblastoma cells. Marcia and Kirchhausen showed that Dynasore is a non-competitive inhibitor of dynamin GTPase, likely locking it in a GTP-bound state (Macia et al., 2006). In contrast, MiTMAB inhibits the GTPase activity of dynamin as well as interaction between the dynamin PH domain and lipids (Quan et al., 2007), suggesting that lipid-mediated interactions as well as GTPase of Dyn2 are required for the formation of lamellipodia.
How Dyn2 synergizes with the action of the actin cytoskeleton to augment migration remains unclear. Dynamin has been shown to bind directly to actin-associated proteins and influence actin organization (Itoh et al., 2005; Kim et al., 2005; Kruchten and McNiven, 2006; Lee and De Camilli, 2002; McNiven et al., 2000; Mooren et al., 2009; Orth et al., 2002; Qualmann et al., 2000; Schafer, 2004; Wu et al., 2010). In particular, dynamin directly binds the actin-binding protein cortactin and together this complex regulates actin remodeling (McNiven et al., 2000; Mooren et al., 2009). This specific interaction appears to play an important role in the organization of actin bundles as dynamin and cortactin cooperate to crosslink actin into bundles that are remodeled and potentially turned over following dynamin GTPase activity (Mooren et al., 2009), as well as the formation of dendritic actin networks as demonstrated graphically by experiments with actin comet formation by Listeria monocytogenes (Lee and De Camilli, 2002; Orth et al., 2002). Thus, remodeled actin bundles and enhanced actin network branching facilitated by a Dyn2-cortactin complex would be expected to augment lamellipodial extension and cell migration. In addition, Dyn2 phosphorylation and GTPase activity could influence downstream signaling to the actin cytoskeleton as Dyn2 activity is required to traffic and activate the small GTPase Rac1 (Schlunck et al., 2004), perhaps in association with the Rac guanine exchange factor Vav1 (Gomez et al., 2005). Further, it has been shown that Dyn2 activity plays an important role in the remodeling of focal adhesions, multiprotein complexes that link the internal actin cytoskeleton to the extracellular matrix (Ezratty et al., 2009; Ezratty et al., 2005; Yoo et al., 2005). In this model, elevated expression of Dyn2 would enhance turnover of focal complexes near the leading edge, allowing for more rapid transport of integrins (Chao and Kunz, 2009) and other focal adhesion molecules to the front of the cell. This model is consistent with the observation that cells expressing phospho-deficient Dyn2 continued to ruffle yet fail to extend lamellipodia at the same rate as Dyn2-overexpressing cells (Fig. 2e-f) potentially due to the failure to lay down new focal contacts due to less turnover of focal adhesions. Also, Dyn2 interaction with membrane-bending F-BAR proteins (Itoh et al., 2005) and actin-regulating Spin90/WISH (Kim et al., 2006; Kim et al., 2005) appear to synergistically coordinate actin-dependent tubulation and scission of the plasma membrane.
Although the precise mechanism of Dyn2 action in migration remains to be determined, our data suggest that phosphorylation by Src plays an important regulatory role. Overexpression of Dyn2 in pancreatic cancer cells enhanced cell migration and invasion while overexpression of a phospho-deficient version of Dyn2, Dyn2Y(231,597)F, was unable to enhance these processes (Fig. 2, 4 b-f, 5 a-c and g). Further, the expression of the phospho-mutant of Dyn2 was insufficient to enhance the Dyn2-mediated dissemination and distant metastases of pancreatic cancer cells in vivo (Figs. 6-7). These data, together with previous reports showing that Src phosphorylation of Dyn2 regulates receptor endocytosis, Golgi morphology and focal adhesion turnover (Cao et al., 2010; Shajahan et al., 2004; Wang et al.; Weller et al., 2010) reveal an essential regulatory role for Src in Dyn2 cellular functions. Src-mediated tyrosine phosphorylation of dynamin downstream of EGFR stimulates the GTPase activity and oligomerization of dynamin (Ahn et al., 2002; Ahn et al., 1999). Importantly, both EGFR (Bloomston et al., 2006; Dancer et al., 2007; Thybusch-Bernhardt et al., 2001) and Src (Hakam et al., 2003; Lutz et al., 1998; Morton et al., 2010) are over-expressed in pancreatic cancer, and inhibition of Src activity is sufficient to attenuate the spread of pancreatic cancer metastasis in a mice (Morton et al., 2010).
Thus, from the combined findings reported here, Dyn2 appears to play an important role in cell migration and subsequently in the invasive process exhibited by these tumors. We have not observed any alterations in cell growth by the established Dyn2-expressing cell lines either in vitro or in vivo suggesting that the dissemination process is a reflection of metastatic activity. These observations implicate the dynamins as a new family of oncogenic proteins that could provide useful therapeutic targets. Indeed, a recent study has shown that the dynamin inhibitory compound MiTMAB preferentially attenuates the growth of multiple cancer cell types, but not fibroblasts, in culture (Joshi et al., 2010). This selectivity for neoplastic cell growth is noteworthy and further supports the premise of dynamin involvement in the neoplastic process.
Materials and Methods
Reagents
The polyclonal Dyn2 and pan-dynamin MC63 antibodies have previously been described (Cao et al., 1998; Henley et al., 1998). Monoclonal cortactin antibody, 4f11, was from Upstate Biotechnology (Lake Placid, NY). Mouse Diclonal anti-GFP antibody was from Roche Applied Science (Indianapolis, IN). Polyclonal anti-actin antibody, TRITC labeled phalloidin and 4′,6-diamidino-2-phenylindole (DAPI) were from Sigma (St. Louis, MO). Dynamin inhibitory compounds Dynasore and MiTMAB, from Calbiochem (Cat # 324410 and 324411), were resuspended in dimethyl sulfoxide (DMSO) to 25 mM stock concentrations and stored at −20°C until use. Non-targeting siRNA#1 (Cat # D-001810-01) and Dyn2 siRNA GACAUGAUCCUGCAGUUCA (D2-02; Cat # D-004007-02;) from Dharmacon were used for knockdown experiments with Invitrogen Lipofectamine RNAiMax Reagent (Cat # 13778-075) according to manufacturer’s protocol, except that three time the recommended siRNA and RNAiMAX was used for BxPC-3 cells. Cells were used after 3 days of siRNA treatment.
cDNA constructs
The cDNA constructs encoding WT Dyn2-GFP and Dyn2Y(231/597)F-GFP were previously described (Cao et al., 2010; Jones et al., 1998).
Cell lines
Pancreatic epithelial tumor cell lines, from American Type Culture Collection (ATCC, Manassas, VA), were grown in a 5% CO2/95% air incubator at 37°C. Panc04.03 cells (ATCC CRL-2555) and BxPC-3 cells (ATCC CRL-1687) were maintained in RPMI-1640 Medium and supplemented with 10% fetal bovine serum (Sigma), 100U/ml penicillin and 100g/ml streptomycin.
Stable cell lines
BxPC-3 cells were electroporated in the presence of expression plasmids pEGFP-N1 (Clontech, Mountain View, CA) encoding WT Dyn2, Dyn2Y(231/597)F or empty vector as previously described (Cao et al., 1998). Subsequently the cells were grown for 10-15 days before resistant clones were isolated, clonal lines were expanded and expression was confirmed by fluorescence microscopy and western blot. Growth medium for BxPC-3 stable cells was supplemented with 250 mg/L G418 (Invitrogen).
Microscopy
Cells were fixed in formaldehyde and processed for immunofluorecence as described previously (Cao et al., 1998). Fluorescent micrographs were acquired with a Zeiss Axio Observer D.1 epifluorescence microscope (Carl Zeiss) equipped with a Hamamatsu Orca II camera (Hamamatsu Photonics, Hamamatsu City, Japan), and analyzed with IVision software (BioVision).
Western Blot
Western blot analysis was performed as described (Cao et al., 1998) with minor modification. Cellular protein was harvested in RIPA buffer supplemented with EDTA-free Complete protease inhibitor cocktail (Roche Applied Science) and the protein concentration was determined using Pierce BCA Protein Assay Kit (Pierce Chemical Co.) using BSA as a standard. Cellular proteins separated by continuous SDS-PAGE were transferred to polyvinyldifluoride membranes which were subsequently blocked in PBS (150 mM NaCl, 10 mM NaH2PO4, pH 7.2) containing 5% milk and reacted with appropriate primary antibodies, washed in PBS, and incubated with secondary antibodies conjugated to HRP. Immunoreactive bands were detected with SuperSignal West Pico Chemiluminescent Substrate (Pierce) using autoradiography film (Denville Scientific, Inc., Denville, NJ).
Live-time imaging and kymography
BxPC-3 stable cells plated at low density were grown overnight on imaging dishes in growth medium containing 10% fetal bovine serum. Images of live cells were taken every 15 sec for 10 min with the microscope system described above using a 40x objective. Kymography of membrane protrusion was performed using the ImageJ (NIH) Kymography macro (http://www.embl.de/eamnet/html/downloads.html). Briefly, IVision movies were converted into .tiff stacks and then into ImageJ movies. Subsequently, ImageJ was used to select a line along advancing lamellipodia in each cell. The line of interested was processed through the kymography macro to generate kymograph images that were then copied into Photoshop (Adobe Systems Incorporated, San Jose, CA, USA) for measurement. Lamellipodium protrusion speed was calculated from the distance and time that the leading lamellipodial membrane traveled during advancing phases of membrane protrusion.
Cell migration and invasion assays
Wound Healing Assay
Untreated Panc04.03 cells or cells treated 48 hrs with siRNAs were plated to confluence on gridded coverslips (Bellco Biotechnology, Vineland, NJ), serum starved (0.2% FBS) for 16 hrs and wounded with a plastic pipette tip. After washing thoroughly, wounds were stimulated with low serum media containing 50 ng/mL EGF or carrier, supplemented with DMSO, Dynasore or MiTMAB. Phase contrast images of the wound area were acquired 1 and 5 hrs after stimulation with a 10x objective using the grid pattern to locate the exact same area of the wound at different times. Resulting images were compiled in Adobe Photoshop. Migration distance was determined by measuring the difference in wound-edge location in four regions of every image. Average migration speed was calculated from the average distance traveled and the time elapsed between images.
Individual cell motility Assay
BxPC-3 stable cell lines were plated in imaging dishes containing gridded coverslips in growth media (10% FBS). Four hrs after plating, images were captured hourly for 11 hrs using a 10x objective. The resultant images of each selected grid were aligned using the grid pattern as reference and compiled into movies. The motion paths of individual cells within each grid were generated with IVision software by tracking the center of area of each cell in the field in each frame acquired. Total distance, the sum of the distances that each cell traveled between all frames of the 11 hour movies, and net distance, the distance traveled by each cell from the first to last frame, were measured using IVision. Cells that migrated out of the field of view during the experiment were eliminated from the analysis. For individual cell motility assays using dynamin inhibitors, the drugs or carrier were added after the first image was acquired.
Transwell chambers
Polycarbonate membranes (#PFA8, Neuro Probe, Inc) containing 8 μm pores were coated with 0.01% gelatin and assembled into Blind Well Chambers (#BW200L, Neuro Probe, Inc) that contained growth medium (RPMI-1640 supplemented with 10% FBS) in the bottom chamber. Subsequently, BxPC-3 stable cell lines were trypsinized (1x trypsin-EDTA, 0.5% Trypsin, 1 mM 4Na-EDTA; GIBCO), resuspended in low serum media (0.2% FBS) and counted with a hemocytometer. 2×105 cells were plated into the top chamber. 40 min after plating, the top chamber was supplemented with or without DMSO or dynamin inhibitors. Four hrs after plating, the filters were fixed with formaldehyde, stained with TRITC phalloidin and DAPI, and imaged on a Zeiss 510 confocal microscope (Carl Zeiss, Inc). The number of nuclei on the top and bottom of the filter were counted and the result was expressed as a percentage of cells that migrated through the filter.
Histology and Tissue MicroArray
Histological samples containing normal and PDAC tissue from 9 de-identified patients or benign, PDAC and metastasis tissue from 39 de-identified patients were obtained from the Mayo Clinic SPORE in Pancreatic Cancer tissue core, were stained with the Dyn2 antibody by Tissue and Cell Molecular Analysis (TACMA) facility (Mayo Clinic, MN) and relative Dyn2 expression was compared to normal or benign tissue. Tissue MicroArrays representing 85 patients, described previously (Muders et al., 2006), were stained with Dyn2 antibody by TACMA, staged using International Union Against Cancer guidelines, graded according to WHO, and Dyn2 expression was scored semi-quantitatively by a pathologist (L.Z.) with respect to intensity (scale: 0-3). Normal ducts within the TMAs generally exhibited an intensity score of 1, therefore ductal epithelium or PDAC within the TMAs with intensity scores of 2-3 were characterized as over-expressed.
Orthotopic implantation, Xenogen Imaging and autopsy
BxPC-3 stable clones were transduced with lentivirus encoding cDNAs for expression of FRα, firefly luciferase (Fluc) as previously reported (Hasegawa et al., 2006). Orthotopic implantation of 1×106 clonal BxPC-3 cells into 5-6 week old female athymic BALB/c mice (nu/nu) obtained from Harlan was performed similar to (Alves et al., 2001). General anesthesia and analgesics were performed by intraperitoneal (IP) injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) followed by IP injection of buprenorphine (0.1 mg/kg). Two weeks after implantation, tumor cell spreading was visualized in anesthetized mice injected with 200 μl of 15 mg/mL D-Luciferin (Gold Biotechnology) using a Xenogen IVIS 200 imaging system. The resulting luminescent images were imported into IVision to analyze tumor area and spatial distribution. The IVision auto-segmentation feature was used to automatically identify distinct tumors that were subsequently scored for location. 8 weeks after implantation, mice were euthanized by IP injection of Sleepaway® sodium pentobarbital euthanasia solution (Fort Dodge Animal Health, Fort Dodge, IA) and autopsied for macroscopic tumors. All experiments were done under IACUC approval.
Acknowledgements
The authors are grateful for support from the National Institutes of Health NCI R01-CA104125 (M.A.M), T32-DK007198 (R.D.E.), the Mayo Clinic Center for Cell Signaling in Gastroenterology (NIDDK P30DK084567), the Mayo Clinic SPORE in Pancreatic Cancer (P50 CA 102701), the Lustgarten Foundation for Pancreatic Cancer Research and the Fraternal Order of the Eagles.
Bibliography
- Ahn S, Kim J, Lucaveche CL, Reedy MC, Luttrell LM, Lefkowitz RJ, et al. Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J Biol Chem. 2002;277:26642–51. doi: 10.1074/jbc.M201499200. [DOI] [PubMed] [Google Scholar]
- Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274:1185–8. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- Alves F, Contag S, Missbach M, Kaspareit J, Nebendahl K, Borchers U, et al. An orthotopic model of ductal adenocarcinoma of the pancreas in severe combined immunodeficient mice representing all steps of the metastatic cascade. Pancreas. 2001;23:227–35. doi: 10.1097/00006676-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23:74–9. doi: 10.1159/000093497. [DOI] [PubMed] [Google Scholar]
- Bouvet M, Yang M, Nardin S, Wang X, Jiang P, Baranov E, et al. Chronologically-specific metastatic targeting of human pancreatic tumors in orthotopic models. Clin Exp Metastasis. 2000;18:213–8. doi: 10.1023/a:1006767405609. [DOI] [PubMed] [Google Scholar]
- Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–85. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Cao H, Chen J, Krueger EW, McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the “constitutive” endocytosis of transferrin. Mol Cell Biol. 2010;30:781–92. doi: 10.1128/MCB.00330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao WT, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 2009;583:1337–43. doi: 10.1016/j.febslet.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer J, Takei H, Ro JY, Lowery-Nordberg M. Coexpression of EGFR and HER-2 in pancreatic ductal adenocarcinoma: a comparative study using immunohistochemistry correlated with gene amplification by fluorescencent in situ hybridization. Oncol Rep. 2007;18:151–5. [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27:6970–80. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–47. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–90. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114–30. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–43. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Gomez TS, Hamann MJ, McCarney S, Savoy DN, Lubking CM, Heldebrant MP, et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat Immunol. 2005;6:261–70. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- Hakam A, Fang Q, Karl R, Coppola D. Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci. 2003;48:1972–8. doi: 10.1023/a:1026122421369. [DOI] [PubMed] [Google Scholar]
- Hansen CG, Nichols BJ. Molecular mechanisms of clathrin-independent endocytosis. J Cell Sci. 2009;122:1713–21. doi: 10.1242/jcs.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Chelala C, Bhakta V, Chaplin T, Caulee K, Baril P, et al. Genome-wide DNA copy number analysis in pancreatic cancer using high-density single nucleotide polymorphism arrays. Oncogene. 2008;27:1951–60. doi: 10.1038/sj.onc.1210832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Nakamura T, Harvey M, Ikeda Y, Oberg A, Figini M, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12:6170–8. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TA, Odell LR, Quan A, Abagyan R, Ferguson G, Robinson PJ, et al. Long chain amines and long chain ammonium salts as novel inhibitors of dynamin GTPase activity. Bioorg Med Chem Lett. 2004;14:3275–8. doi: 10.1016/j.bmcl.2004.03.096. [DOI] [PubMed] [Google Scholar]
- Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Jones SM, Howell KE, Henley JR, Cao H, McNiven MA. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science. 1998;279:573–7. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- Joshi S, Perera S, Gilbert J, Smith CM, Mariana A, Gordon CP, et al. The dynamin inhibitors MiTMAB and OcTMAB induce cytokinesis failure and inhibit cell proliferation in human cancer cells. Mol Cancer Ther. 2010;9:1995–2006. doi: 10.1158/1535-7163.MCT-10-0161. [DOI] [PubMed] [Google Scholar]
- Kessels MM, Engqvist-Goldstein AE, Drubin DG, Qualmann B. Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J Cell Biol. 2001;153:351–66. doi: 10.1083/jcb.153.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Kim SH, Lim CS, Choi KY, Park CS, Sung BH, et al. Interaction of SPIN90 with the Arp2/3 complex mediates lamellipodia and actin comet tail formation. J Biol Chem. 2006;281:617–25. doi: 10.1074/jbc.M504450200. [DOI] [PubMed] [Google Scholar]
- Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–95. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim S, Lee S, Kim SH, Park ZY, Song WK, et al. Interaction of SPIN90 with dynamin I and its participation in synaptic vesicle endocytosis. J Neurosci. 2005;25:9515–23. doi: 10.1523/JNEUROSCI.1643-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruchten AE, McNiven MA. Dynamin as a mover and pincher during cell migration and invasion. J Cell Sci. 2006;119:1683–90. doi: 10.1242/jcs.02963. [DOI] [PubMed] [Google Scholar]
- Krueger EW, Orth JD, Cao H, McNiven MA. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell. 2003;14:1085–96. doi: 10.1091/mbc.E02-08-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, De Camilli P. Dynamin at actin tails. Proc Natl Acad Sci U S A. 2002;99:161–6. doi: 10.1073/pnas.012607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MP, Esser IB, Flossmann-Kast BB, Vogelmann R, Luhrs H, Friess H, et al. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun. 1998;243:503–8. doi: 10.1006/bbrc.1997.8043. [DOI] [PubMed] [Google Scholar]
- Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 2008;582:2102–11. doi: 10.1016/j.febslet.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–50. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- McNiven MA. Dynamin: a molecular motor with pinchase action. Cell. 1998;94:151–4. doi: 10.1016/s0092-8674(00)81414-2. [DOI] [PubMed] [Google Scholar]
- McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol. 2000;151:187–98. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaljevic AL, Michalski CW, Friess H, Kleeff J. Molecular mechanism of pancreatic cancer--understanding proliferation, invasion, and metastasis. Langenbecks Arch Surg. 2010;395:295–308. doi: 10.1007/s00423-010-0622-5. [DOI] [PubMed] [Google Scholar]
- Mooren OL, Kotova TI, Moore AJ, Schafer DA. Dynamin2 GTPase and cortactin remodel actin filaments. J Biol Chem. 2009;284:23995–4005. doi: 10.1074/jbc.M109.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JP, Karim SA, Graham K, Timpson P, Jamieson N, Athineos D, et al. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2010;139:292–303. doi: 10.1053/j.gastro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Muders MH, Dutta SK, Wang L, Lau JS, Bhattacharya R, Smyrk TC, et al. Expression and regulatory role of GAIP-interacting protein GIPC in pancreatic adenocarcinoma. Cancer Res. 2006;66:10264–8. doi: 10.1158/0008-5472.CAN-06-2321. [DOI] [PubMed] [Google Scholar]
- Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2006;103:17955–60. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto J, Grossbard ML, Kozuch P. Metastatic pancreatic cancer 2008: is the glass less empty? Oncologist. 2008;13:562–76. doi: 10.1634/theoncologist.2007-0181. [DOI] [PubMed] [Google Scholar]
- Orth JD, Krueger EW, Cao H, McNiven MA. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc Natl Acad Sci U S A. 2002;99:167–72. doi: 10.1073/pnas.012607899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JD, McNiven MA. Dynamin at the actin-membrane interface. Curr Opin Cell Biol. 2003;15:31–9. doi: 10.1016/s0955-0674(02)00010-8. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–47. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Kessels MM, Kelly RB. Molecular links between endocytosis and the actin cytoskeleton. J Cell Biol. 2000;150:F111–6. doi: 10.1083/jcb.150.5.f111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan A, McGeachie AB, Keating DJ, van Dam EM, Rusak J, Chau N, et al. Myristyl trimethyl ammonium bromide and octadecyl trimethyl ammonium bromide are surface-active small molecule dynamin inhibitors that block endocytosis mediated by dynamin I or dynamin II. Mol Pharmacol. 2007;72:1425–39. doi: 10.1124/mol.107.034207. [DOI] [PubMed] [Google Scholar]
- Schafer DA. Regulating actin dynamics at membranes: a focus on dynamin. Traffic. 2004;5:463–9. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Schlunck G, Damke H, Kiosses WB, Rusk N, Symons MH, Waterman-Storer CM, et al. Modulation of Rac localization and function by dynamin. Mol Biol Cell. 2004;15:256–67. doi: 10.1091/mbc.E03-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:20392–400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- Thybusch-Bernhardt A, Beckmann S, Juhl H. Comparative analysis of the EGF-receptor family in pancreatic cancer: expression of HER-4 correlates with a favourable tumor stage. Int J Surg Investig. 2001;2:393–400. [PubMed] [Google Scholar]
- Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci U S A. 1997;94:377–84. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–9. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cao H, Chen J, McNiven MA. A direct interaction between the large GTPase dynamin-2 and FAK regulates focal adhesion dynamics in response to active Src. Mol Biol Cell. 22:1529–38. doi: 10.1091/mbc.E10-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller SG, Capitani M, Cao H, Micaroni M, Luini A, Sallese M, et al. Src kinase regulates the integrity and function of the Golgi apparatus via activation of dynamin 2. Proc Natl Acad Sci U S A. 2010;107:5863–8. doi: 10.1073/pnas.0915123107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Huang B, Graham M, Raimondi A, Heuser JE, Zhuang X, et al. Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nat Cell Biol. 2010;12:902–8. doi: 10.1038/ncb2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J, Jeong MJ, Cho HJ, Oh ES, Han MY. Dynamin II interacts with syndecan-4, a regulator of focal adhesion and stress-fiber formation. Biochem Biophys Res Commun. 2005;328:424–31. doi: 10.1016/j.bbrc.2004.12.179. [DOI] [PubMed] [Google Scholar]