Abstract
Background
African Americans (AA) have a higher prevalence of left ventricular hypertrophy than whites. Several population-based studies have reported an inverse association between adiponectin and left ventricular mass (LVM). However, the relationship between adiponectin levels and LVM has yet to be defined in AA. The Jackson Heart Study (JHS) cohort provides an opportunity to test the hypothesis that the inverse association between adiponectin and LVM may be modified by risk factors common among AA.
Methods and Results
The study population included 2,649 AA JHS participants; mean age, 51 ± 12 years, 63% women, 51% obese, 54% with hypertension and 16% with diabetes. Multiple linear and spline regression was used to assess the association adjusting for demographic, clinical and behavioral covariates. Among all the participants, there was a statistically significant but modest inverse association between adiponectin and left ventricular mass index (LVMI). Hypertension and insulin resistance emerged as statistically significant effect modifiers of this relationship. The inverse association present among the normotensive participants was explained by obesity measures such as the body mass index. Among participants with both hypertension and insulin resistance there was a significant direct association between adiponectin and LVMI after multivariable adjustment (β = 1.55, p = 0.04; per one standard deviation increments in the adiponectin log-value).
Conclusions
The association between serum adiponectin and LVM among AA in the JHS cohort was dependent on hypertension and insulin resistance status. Normotensive AA exhibited an inverse adiponectin – LVM association, whereas participants with hypertension and insulin resistance had a direct association.
Keywords: adiponectin, biomarkers, epidemiology, left ventricular mass, obesity
Obese individuals, particularly those with visceral fat accumulation, have reduced plasma levels of adiponectin.[1, 2] The associations of adiponectin with cardiac risk factors, such as hypertension, type 2 diabetes and obesity, have been described.[3, 4] In mice, adiponectin inhibits hypertrophic signaling in the myocardium.[5] Several studies have reported an inverse association between adiponectin and left ventricular mass (LVM).[6–12] There are however very few large, community-based studies with adequate adjustment for potential confounders to elucidate this apparent inverse relationship between adiponectin and LVM in greater detail.[13–15] Although African Americans (AA) have a higher prevalence of obesity and left ventricular hypertrophy, the relationship between adiponectin and LVM in this population has yet to be explored. The availability of serum adiponectin measurements on more than 4,000 AA participants in the Jackson Heart Study (JHS) allowed us to quantify the association between serum adiponectin and echocardiography-measured LVM in AA enrolled in the JHS, a large community-based cohort. We queried whether an inverse association adiponectin – LVM is present and whether this association is modified by selected covariates such as hypertension, obesity and insulin resistance known to be particularly prevalent among AA and associated with LVM.
Methods
Study Population
JHS is a single-site, prospective cohort study of the risk factors and causes of cardiovascular disease in adult AA. A probability sample of 5,301 AA, aged 21 – 84 years, residing in the three counties surrounding Jackson, MS, was recruited and examined at baseline (2000–2004) by trained and certified technicians according to standardized protocols. Clinic visits and interviews occurred approximately every three years. Annual follow-up interviews and cohort surveillance are ongoing. Details of the study design are published elsewhere.[16, 17]
After exclusion of individuals with prevalent coronary heart disease (n = 375), undetectable adiponectin levels (n = 93), unreliable ultrasound measurements (n = 879) and mitral or aortic regurgitation (n = 1,305), our final study sample included 2,649 participants.
Written consent was obtained from each participant at the inception of the study, and the study protocol was approved by the Institutional Review Boards of the Morehouse School of Medicine and the University of Mississippi Medical Center.
In all participants, the clinic visit included physical examination, anthropometry, survey of medical history and of cardiovascular risk factors and collection of blood and urine for biological variables. We calculated body mass index (BMI, kg/m2) as weight in kilograms divided by height in meters squared. Obesity was defined as BMI ≥ 30, and abdominal obesity as a waist circumference ≥88 cm in women and ≥102 cm in men. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive therapy. Diabetes was defined as fasting plasma glucose ≥126 mg/dL or use of insulin or oral hypoglycemic medications. Smoking status was defined as current smoking versus former and never smoking (collapsed). Alcohol drinking was defined as regular drinking in the past 12 months (yes vs. no). A physical activity score was composed with a Baecke-derived questionnaire and used as a continuous variable.
Participants were further subjected to a standardized 2D echocardiographic examination. Left ventricular mass was calculated using the American Society of Echocardiography corrected formula by Devereux.[18] LVM was indexed to height raised to the power 2.7 (LVMI = LVM/height2.7) in order to normalize heart size to body size.
Lipid variables, fasting plasma glucose and fasting insulin were measured using standard laboratory techniques. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as [insulin (microunits per milliliter) × fasting blood glucose (millimoles per liter)]/22.5. Insulin resistance status was defined as a HOMA-IR in the highest quartile of its distribution.[19, 20]
Adiponectin measurement
Venous blood samples were withdrawn from each subject at baseline examination after more than 8 hours of fasting as described elsewhere.[16] Serum concentration of adiponectin was measured as total adiponectin at the JHS central laboratory in Minneapolis, MN, by an ELISA system (R&D Systems; Minneapolis, MN) using baseline serum specimens stored at −80°C until assayed. The inter-assay coefficient of variation was 8.8%. No biological degradation has been described using stored specimens, indicating a high validity for our measurements.[21]
Statistical Analyses
Because the distributions of adiponectin and HOMA-IR were skewed, log-transformed values were used for the analyses to approximately normalize the distribution.
The cross-sectional association between adiponectin and LVMI was assessed with Spearman correlation coefficients and multiple linear regression. Using full-models, we assessed the individual effect measure modification for a series of covariates (age, sex, hypertension, obesity and abdominal obesity, diabetes and insulin resistance status) known to be associated with both adiponectin [22] and left ventricular mass.[23, 24] Models including hypertension and insulin resistance were used to also test the three-way interaction between adiponectin and hypertension, insulin resistance on LVMI. These models were constructed by including within the models adjusted for age and sex the three-variable interaction term adiponectin by hypertension by insulin resistance as well as all the two-variable interaction terms adiponectin by hypertension and adiponectin by insulin resistance. The multivariable regression full models were adjusted for age, sex, BMI, alcohol drinking, triglycerides, high-density lipoprotein cholesterol (HDL-cholesterol), hypertension status and HOMA-IR. We also modeled continuous LVM as a nonlinear predictor of adiponectin concentrations using generalized additive model (GAM). In GAM, we used penalized splines smoothing function to fit a curve that describes the relationship between adiponectin and LVM without assuming a linear relationship. For all the analyses, the nominal p-value was set at 0.05 for the main and the interactive effects.
Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, 2005).
Additional Analyses
Because hypertension medication directly influences blood pressure values, a direct determinant of LVM, we also run the same analyses after excluding 1,118 subjects who had been on medical treatment for hypertension. We also assessed the effects of the use of medications that inhibit the renin-angiotensin-aldosterone system (such as angiotensin-converting enzyme inhibitors, ACE inhibitors, and angiotensin receptor blockers, ARB) and of statins, all reported to increase adiponectin levels.
Results
The overall characteristics of the JHS study sample (mean age ± SD, 51 ± 12 years; 63% women) are as follows. BMI had a mean (standard deviation) of 31.3 (6.9) kg/m2. Obesity had a prevalence of 51% and that of abdominal obesity 60.5%. Systolic blood pressure (SBP) ranged from 73 to 210 mm Hg, and the mean was 124 mm Hg. Hypertension was present in 54% of participants. The prevalence of diabetes was 15.7%. Serum adiponectin level ranged from 0.4 to 41.4 μg/mL, and the mean was 5.1 μg/mL. LVM ranged from 66.4 g to 379.9 g, and the mean was 144.0 g. The characteristics of the participants across adiponectin quartiles are presented in Table 1. Age, sex, BMI, alcohol drinking, hypertension status, HDL-cholesterol, triglycerides and HOMA-IR emerged as the main variables that varied statistically significant across quartiles.
Table 1.
Baseline characteristics (mean ± SD or percentage) of the participants across adiponectin quartiles (N = 2,649)
| Quartile 1 (N = 663) | Quartile 2 (N = 662) | Quartile 3 (N = 662) | Quartile 4 (N = 662) | P-Value§ | |
|---|---|---|---|---|---|
| Age, years | 48.7±11.7 | 50.7±12.0 | 51.9±12.4 | 53.9±12.9 | < 0.001 |
| Women, % | 43.4 | 59.2 | 69.6 | 81.4 | < 0.001 |
| BMI, kg/m2 | 32.1±6.4 | 31.9±6.7 | 31.3 (6.9) | 29.9±7.2 | < 0.001 |
| Waist circumference, cm | 102.9±14.5 | 100.9±14.5 | 98.8±16.0 | 93.7±15.5 | < 0.001 |
| Obesity*, % | 58.1 | 56.0 | 49.6 | 42.8 | < 0.001 |
| Abdominal obesity†, % | 62.6 | 63.9 | 61.8 | 53.6 | 0.001 |
| Systolic blood pressure, mm Hg | 123.2±15.5 | 123.4±17.0 | 124.1±17.5 | 124.8±17.7 | 0.29 |
| Diastolic blood pressure, mm Hg | 79.9±9.9 | 78.6±10.3 | 78.3±10.1 | 77.5±10.6 | < 0.001 |
| Hypertension, % | 53.4 | 53.9 | 53.9 | 55.0 | 0.56 |
| Total cholesterol, mg/dL | 197.2±39.0 | 197.2±41.6 | 197.4±37.8 | 199.3±40.6 | 0.74 |
| Triglycerides, mg/dL | 126.2±83.8 | 103.6±81.0 | 100.3±99.9 | 85.6±66.9 | < 0.001 |
| HDL-cholesterol, mg/dL | 44.6±11.1 | 49.1±11.2 | 53.2±12.8 | 60.4±15.6 | < 0.001 |
| Fasting plasma glucose, mg/dL | 104.8±36.2 | 98.0±26.6 | 96.3±27.8 | 94.3±27.7 | < 0.001 |
| HOMA-IR | 4.6±2.6 | 3.7±2.0 | 3.4±1.9 | 2.6±1.4 | < 0.001 |
| Type II Diabetes, % | 19.0 | 16.2 | 13.7 | 15.1 | < 0.001 |
| Current Smokers, % | 14.3 | 12.6 | 12.0 | 11.6 | 0.13 |
| Alcohol drinking‡, % yes | 53.4 | 50.5 | 49.9 | 47.0 | 0.02 |
| Physical activity score | 8.9±2.5 | 8.8±2.5 | 8.7±2.5 | 8.6±2.6 | 0.06 |
| Adiponectin, μg/mL | 1.8 (0.5) | 3.3 (0.4) | 5.2 (0.7) | 10.2 (4.4) | < 0.001 |
| Left ventricular mass, g | 152.0±40.4 | 145.2±36.2 | 142.9±41.7 | 138.1±39.6 | < 0.001 |
| LVMI, g/m2.7 | 35.1±8.1 | 34.8±8.0 | 34.6±9.2 | 34.8±9.6 | 0.82 |
Body Mass Index equal or above 30 kg/m2;
Defined as waist circumference equal or above 88 cm in women and 102 cm in men;
Alcohol drinking in the past 12 months (yes vs. no);
Differences in mean values were tested by 1-way ANOVA. Difference in the proportions was tested by t-test.
In accordance with previous observations, serum adiponectin levels had a positive and statistically significant correlation with HDL-cholesterol (Spearman r = 0.43) and a negative correlation with HOMA-IR (r = − 0.36), triglycerides (r = − 0.29), waist circumference (r = − 0.24) and BMI (r = − 0.15); all p-values < 0.0001. There was no statistically significant correlation between adiponectin and systolic blood pressure (r = 0.02; p-value = 0.21). In the overall sample, we observed a statistically significant negative correlation between adiponectin levels and LVM (r = − 0.19; p-value = 0.0001) and between adiponectin and LVMI (r = − 0.04; p-value = 0.047).
Several effect modifiers emerged as statistically significant: hypertension (p = 0.03), insulin resistance (p = 0.01) and hypertension in conjunction with insulin resistance (p = 0.04). Sex and obesity did not appear to affect the adiponectin – LVMI relationship as effect modifiers. Based on the finding that hypertension and insulin resistance were significant effect modifiers, the subsequent analyses were performed based on sub-stratification by these variables. Among the normotensive participants (N = 1,206), the significant inverse association adiponectin – LVMI present in the crude model appeared mediated by measures of obesity such as BMI and by insulin resistance (Table 2). It is notable that there was no significant association between adiponectin and LVMI among participants with hypertension, and that this association became statistically significant when adjusted for BMI or insulin resistance (Table 2). The variance explained in the fully-adjusted models was 22%.
Table 2.
The relationship of left ventricular mass index with adiponectin* according to hypertension status
| Without Hypertension N = 1,206 |
With Hypertension N = 1,418 |
|||
|---|---|---|---|---|
| β | P-value | β | P-value | |
| Crude (unadjusted) | −0.92|| | 0.001 | 0.47 | 0.17 |
| Age-adjusted | −2.08 | 0.07 | −0.94 | 0.59 |
| Sex-adjusted | −1.08 | 0.0004 | −0.002 | 0.99 |
| BMI-adjusted | −0.29 | 0.28 | 0.73 | 0.02 |
| HOMA-IR-adjusted† | −0.50 | 0.11 | 0.98 | 0.02 |
| Fully-adjusted‡ | −0.69 | 0.04 | 0.43 | 0.36 |
Per 1 standard deviation increment in the log values;
Adjusted for HOMA-IR;
Adjusted for age, sex, BMI, alcohol drinking in the past 12 months (yes vs. no), HDL-cholesterol, triglycerides and HOMA-IR (log-transformed).
To further define the interaction of hypertension and insulin resistance on the adiponectin – LVMI relationship, the entire sample was sub-stratified by both variables, as shown in Table 3. The inverse association between adiponectin and LVM that was found among participants without hypertension or insulin resistance (N = 998) was explained by BMI (Table 3). Among those with hypertension and insulin resistance (N = 331), the association was a statistically significant direct association that persisted after adjustment for age, sex, BMI, HDL-cholesterol, triglycerides and alcohol drinking; β = 1.55, p = 0.04 (Table 3). The equivalent raw values of the log-transformed beta coefficients (obtained by exponentiation of the initial values) are in the order of 5 to 13, thus of a very large magnitude. The Table 1 of the online supplemental material presents the beta coefficients for LVMI across adiponectin quartiles using raw values of adiponectin levels in order to assure an easier interpretation of the regression models results. This table confirms a non-linear complex trend in the association between adiponectin and LVMI.
Table 3.
The relationship of left ventricular mass index with adiponectin* according to hypertension and insulin resistance (IR) status
| Without Hypertension | With Hypertension | |||||||
|---|---|---|---|---|---|---|---|---|
| Without IR N = 998 |
With IR N = 208 |
Without IR N = 1,087 |
With IR N = 331 |
|||||
| β | P-value | β | P-value | β | P-value | β | P-value | |
| Crude (unadjusted) | −0.54 | 0.08 | −0.24 | 0.78 | 0.17 | 0.67 | 2.61 | 0.0008 |
| Age-adjusted | −0.66 | 0.03 | −0.51 | 0.56 | −0.08 | 0.84 | 2.40 | 0.003 |
| Sex-adjusted | −0.62 | 0.06 | −0.50 | 0.58 | −0.31 | 0.47 | 2.12 | 0.01 |
| BMI-adjusted | −0.17 | 0.56 | −0.49 | 0.54 | 0.32 | 0.40 | 1.97 | 0.004 |
| Cholesterol-adjusted† | −0.41 | 0.25 | −1.01 | 0.29 | 0.18 | 0.69 | 2.59 | 0.002 |
| Fully-adjusted‡ | −0.20 | 0.56 | −1.57 | 0.08 | −0.22 | 0.63 | 1.55 | 0.04 |
Per 1 standard deviation increment in the log values;
Adjusted for HDL-cholesterol and triglycerides;
Adjusted for age, sex, BMI, alcohol drinking in the last 12 months (yes vs. no), HDL-cholesterol and triglycerides.
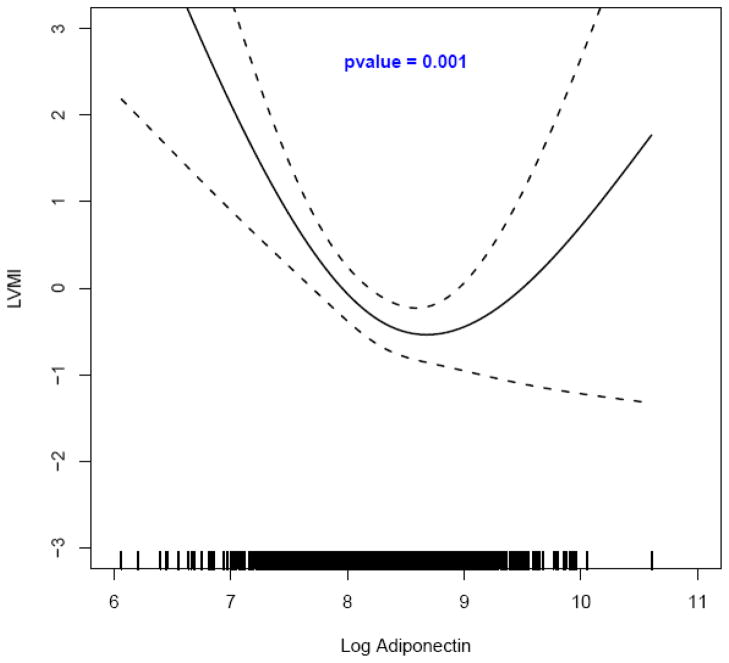
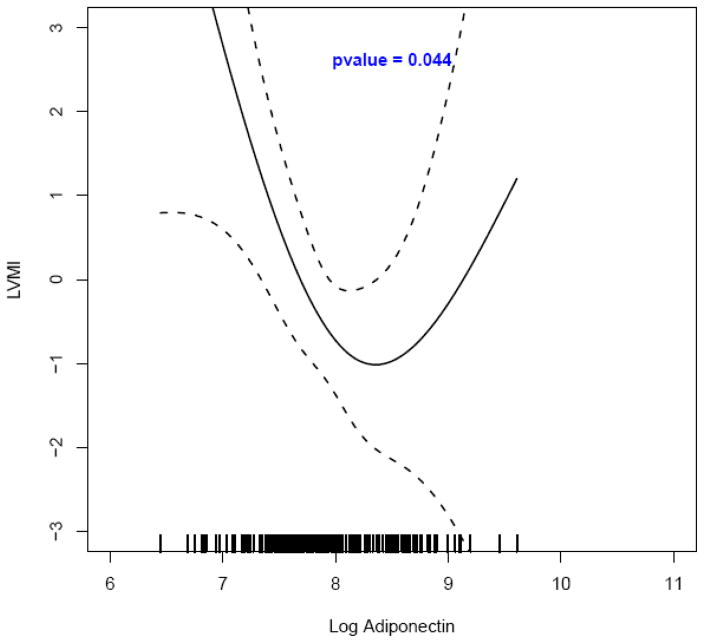
Using spline regression with multivariable adjustment, a significant curvilinear bidirectional relationship was observed between adiponectin and LVMI among normotensive participants (p = 0.001, N = 1,206; Figure 1). The spline regression analysis among hypertensives with insulin resistance exhibited the same curvilinear adiponectin – LVMI relationship (p = 0.04, N = 331; Figure 2). Among participants with hypertension a direct relationship between adiponectin and LVMI emerged although not statistically significant (Figure 1 of the online supplemental material). Curvilinear bidirectional adiponectin – LVMI relationships also emerged among those without hypertension and without insulin resistance (N = 998), among those without hypertension and with insulin resistance (N = 208), as well as among those with hypertension and without insulin resistance (N = 1,087), relationships that reached statistical significance in the second mentioned group (Figures 2, 3 and 4 of the online supplemental material).
Figure 1. The relationship between adiponectin* and left ventricular mass index† by spline regression modeling‡ among participants without hypertension (N = 1,206).
*Adiponectin was log-transformed from units expressed as μg/mL (in order to have logarithmic values above the unit);
†Multivariable-adjusted values (residuals) of left ventricular mass index (LVMI);
‡ Adjustment was performed for age, sex, BMI, alcohol drinking, HDL-cholesterol, triglycerides and HOMA-IR (log-transformed).
Figure 2. The relationship between adiponectin* and left ventricular mass index† by spline regression modeling‡ among participants with hypertension and insulin resistance (N = 331).
*Adiponectin was log-transformed from units expressed as μg/mL (in order to have logarithmic values above the unit);
†Multivariable-adjusted values (residuals) of left ventricular mass index (LVMI);
‡ Adjustment was performed for age, sex, BMI, alcohol drinking, HDL-cholesterol and triglycerides.
Similar results were obtained among participants without medication for hypertension (N = 1,531). Specifically, the beta coefficients changed their sign from β = −0.78 (p = 0.02) among participants without hypertension and without insulin resistance (age- and sex-adjusted model) to β = 3.32 (p = 0.008) among those with both hypertension and insulin resistance (fully-adjusted model). Moreover, in the assessment of the potential confounding effects of the use of medication that inhibits the renin-angiotensin-aldosterone system and of statins we did not find among hypertensives any statistical significant effect on the adiponectin – LVMI relationship (the online data supplement). Similarly, we did not observe significant differences in the mean level of adiponectin among hypertensives compared with normotensives either with or without insulin resistance (data not shown).
The association between adiponectin and LVMI according to hypertension, diabetes and abdominal obesity stratified by age (an effect modifier when dichotomized using the median age of 50 years as the cutpoint) and sex is presented in the Table 2 of the online supplemental material and in the Table 3 of the online supplemental material. Among participants without hypertension, the inverse association became non-significant after adjustment for age, BMI or insulin resistance in both men and women, but was maintained among younger participants.
Discussion
Principal findings
In accordance with previous studies, we observed a modest negative correlation between adiponectin and left ventricular mass index in the overall JHS community-based sample of African Americans. Hypertension and insulin resistance emerged as the major effect modifiers of the adiponectin – LVMI relationship. A statistically significant inverse association was evident among normotensives in the crude model, but this association was attenuated in multivariate models by the effects of adiposity and insulin resistance. Intriguing is the observation of a statistically significant direct association between adiponectin and LVMI among those with both hypertension and insulin resistance that was not hypothesized a priori.
In the context of previous studies
The prevalence of cardiovascular disease and diabetes is greater in African Americans (AA) than in whites.[25] Several studies such as CARDIA[26, 27], HyperGen[28] and Dallas Heart Study[29] have shown that AA have an increased left ventricular mass and an increased prevalence of concentric left ventricular hypertrophy (LVH) in comparison with whites. In epidemiological investigations, cardiovascular risk factors such as diabetes, hypertension, smoking and obesity have been shown to be associated with increased LVMI.[30–32]
The role of adiponectin in relation to cardiac mass and function has attracted some recent attention. Studies performed in small study samples have reported inverse association between adiponectin and LVM, but the study participants for these investigations were mainly from hospital-based or convenience samples or involved patients with preexisting disease, such as type 2 diabetes, hypertension or obesity.[6–12] Only three large, community-based studies with adequate adjustment for potential confounders are available.[13–15] A significant inverse association between adiponectin and ECG-measured LVH in apparently healthy individuals with normal and high blood pressure was observed in 2,839 Japanese male workers who were not taking medications for hypertension.[13] In two community-based samples of Swedish elderly, adiponectin concentrations were inversely associated with ejection fraction in men but not with LVMI after adjustment for potential confounders.[14] In 2,615 participants from the Framingham Offspring Study, adiponectin concentrations were inversely related to LVM but not to cardiac structure and function markers in linear regression models that adjusted for clinical correlates.[15] To our knowledge, the current study is among the first to examine the adiponectin – LVM relationship in AA, a population group at high-risk for left ventricular hypertrophy.
Potential mechanisms
A growing body of evidence suggests that a decrease in adiponectin plasma levels plays an important part in the pathogenesis of many comorbid conditions such as insulin resistance, diabetes and atherosclerosis.[3, 33, 34] Experimental studies have demonstrated that decreased plasma adiponectin levels can predispose to left ventricular hypertrophy. Similarly, increased adiponectin expression can attenuate LVH induced by pathological stimuli.[5] Adiponectin may directly inhibit hypertrophic signaling in the myocardium by activating adenosine monophosphate-activated kinase (AMPK), which activates eukaryotic elongation factor-2 kinase and the inhibitor of cardiac myocyte protein synthesis.[35] Adiponectin protects the ischemic heart from injury through the activation of independent pathways involving both AMPK-mediated anti-apoptotic actions and COX-2-mediated anti-inflammatory actions.[22] These mechanistic studies of the anti-hypertrophic properties of adiponectin are consistent with the finding of an inverse association between adiponectin and LVMI.
Although we observed an inverse adiponectin – LVMI relationship among normotensive African-Americans, we are intrigued by the ‘paradoxical’ observation of a direct adiponectin – LVMI relationship among hypertensives with insulin resistance. The mechanistic basis of this finding remains to be further defined. Although the primary determinants of adiponectin levels are related to adipose tissue, it is important to note that adiponectin is also expressed in both cardiac and skeletal muscle under certain circumstances. Increased levels of adiponectin have been described in patients with both systolic and diastolic forms of heart failure.[36, 37] Moreover, increased adiponectin expression has been noted in the context of a high salt diet and increased activation of the renin-angiotensin-aldosterone system.[38–42] We speculate that the positive adiponectin – LVM relationship reflects a common set of determinants – high salt diet, activation of the renin-angiotensin-aldosterone system and hypertension. These findings suggest that the prognostic value of adiponectin levels may be contextual and depend on factors involved in blood pressure regulation in addition to metabolic factors such as adiposity.
In addition, recent studies have documented that adiponectin exerts an anti-apoptotic effect that is mediated by elevated sphingosine-1-phosphate levels.[43] These findings suggest that adiponectin could contribute to the expansion of the total number of cells in the heart and increase LVM in the cardiotoxic context of hypertension and insulin resistance. Similarly, there is evidence that certain anti-hypertrophic pathways induced by adiponectin can become down-regulated in certain contexts characterized by a state of ‘adiponectin resistance’.[44, 45] Taken together, these studies are consistent with the possibility that the nature of the adiponectin – LVM relationship may be context-specific. Future studies are needed to define the implications of both direct and inverse associations between adiponectin and LVM.
Strengths and limitations
Strengths of the present study include the largest community-based cohort of exclusively African Americans with a wide range of biological, behavioral and demographic attributes with strict quality control methods. Some limitations should be acknowledged. As this is a cross-sectional study, causality cannot be elucidated nor can it be determined if LVM is related to the longitudinal tracking of adiponectin concentrations. Second, measures of different multimeric forms of serum adiponectin were not available for the present study. This could be of importance, since some studies suggest a difference in biological activity between different isoforms of adiponectin with regard to metabolic abnormalities[46] and ventricular mass.[10]
Conclusion
The major finding of the present study was an inverse relationship between serum adiponectin and LVMI in normotensive and non-insulin resistant participants. This association became non-significant after multivariable adjustment that included obesity measures. On the contrary, serum adiponectin was directly associated with LVMI in participants with hypertension and insulin resistance. This suggests that plasma adiponectin levels may have different prognostic value related to LVM depending on the metabolic context and the underlying risk factors.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the JHS study for their valuable contributions. A full list of participating JHS investigators and institutions can be found at http://jhs.jsums.edu/jhsinfo/Directory/tabid/55/Default.aspx.
Sources of Funding
The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (N01-HC-95170), University of Mississippi Medical Center (N01-HC-95171), and Tougaloo College (N01-HC-95172) NIH contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Center on Minority Health and Health Disparities (NCMHD) with additional support from NHLBI contract HL076784 and the National Institute of Aging (AG028321).
This study was partially supported by PHS Award UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources (NCRR) to the first author (A.B.) who was also supported by the NIH grant UH1 HL073461 provided by the National Heart, Lung and Blood Institute and by the NIH grants U54 RR026137 and P20 RR11104 from NCRR.
Footnotes
Disclosures
None
References
- 1.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 2.Yatagai T, Nagasaka S, Taniguchi A, Fukushima M, Nakamura T, Kuroe A, Nakai Y, Ishibashi S. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52:1274–1278. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 4.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 5.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong SJ, Park CG, Seo HS, Oh DJ, Ro YM. Associations among plasma adiponectin, hypertension, left ventricular diastolic function and left ventricular mass index. Blood Press. 2004;13:236–242. doi: 10.1080/08037050410021397. [DOI] [PubMed] [Google Scholar]
- 7.Top C, Sahan B, Onde ME. The relationship between left ventricular mass index and insulin sensitivity, postprandial glycaemia, and fasting serum triglyceride and adiponection levels in patients with type 2 diabetes. J Int Med Res. 2007;35:909–916. doi: 10.1177/147323000703500621. [DOI] [PubMed] [Google Scholar]
- 8.Ybarra J, Pou JM, Planas F, Ballesta-Lopez C, Echevarne F, Romeo JH, Navarro-Lopez F. Correlation between insulin resistance surrogates and echocardiographic findings in asymptomatic patients with morbid obesity: a cross-sectional study. Endocr Pract. 2007;13:590–600. doi: 10.4158/EP.13.6.590. [DOI] [PubMed] [Google Scholar]
- 9.Ebinc H, Ebinc FA, Ozkurt ZN, Dogru MT, Tulmac M, Yilmaz M, Caglayan O. Impact of adiponectin on left ventricular mass index in non-complicated obese subjects. Endocr J. 2008;55:523–528. doi: 10.1507/endocrj.k07e-098. [DOI] [PubMed] [Google Scholar]
- 10.Kozakova M, Muscelli E, Flyvbjerg A, Frystyk J, Morizzo C, Palombo C, Ferrannini E. Adiponectin and left ventricular structure and function in healthy adults. J Clin Endocrinol Metab. 2008;93:2811–2818. doi: 10.1210/jc.2007-2580. [DOI] [PubMed] [Google Scholar]
- 11.Paakko T, Ukkola O, Ikaheimo M, Kesaniemi YA. Plasma adiponectin levels are associated with left ventricular hypertrophy in a random sample of middle-aged subjects. Ann Med. 2010;42:131–137. doi: 10.3109/07853890903449827. [DOI] [PubMed] [Google Scholar]
- 12.Ybarra J, Resmini E, Planas F, Navarro-Lopez F, Webb S, Pou JM, Santos A, Ballesta-Lopez C. Relationship between adiponectin and left atrium size in uncomplicated obese patients: adiponectin, a link between fat and heart. Obes Surg. 2009;19:1324–1332. doi: 10.1007/s11695-009-9924-5. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuhashi H, Yatsuya H, Tamakoshi K, Matsushita K, Otsuka R, Wada K, Sugiura K, Takefuji S, Hotta Y, Kondo T, Murohara T, Toyoshima H. Adiponectin level and left ventricular hypertrophy in Japanese men. Hypertension. 2007;49:1448–1454. doi: 10.1161/HYPERTENSIONAHA.106.079509. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson S, Lind L, Zethelius B, Venge P, Flyvbjerg A, Soderberg S, Ingelsson E. Adiponectin and cardiac geometry and function in elderly: results from two community-based cohort studies. Eur J Endocrinol. 2010;162:543–550. doi: 10.1530/EJE-09-1006. [DOI] [PubMed] [Google Scholar]
- 15.McManus DD, Lyass A, Ingelsson E, Massaro JM, Meigs JB, Aragam J, Benjamin EJ, Vasan RS. Relations of Circulating Resistin and Adiponectin and Cardiac Structure and Function: The Framingham Offspring Study. Obesity (Silver Spring) 2011 Feb 24; doi: 10.1038/oby.2011.32. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6-4–17. [PubMed] [Google Scholar]
- 17.Sempos CT, Bild DE, Manolio TA. Overview of the Jackson Heart Study: a study of cardiovascular diseases in African American men and women. Am J Med Sci. 1999;317:142–146. doi: 10.1097/00000441-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 19.Wedick NM, Mayer-Davis EJ, Wingard DL, Addy CL, Barrett-Connor E. Insulin resistance precedes weight loss in adults without diabetes: the Rancho Bernardo Study. Am J Epidemiol. 2001;153:1199–1205. doi: 10.1093/aje/153.12.1199. [DOI] [PubMed] [Google Scholar]
- 20.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr. 2007;85:910–918. doi: 10.1093/ajcn/85.3.910. [DOI] [PubMed] [Google Scholar]
- 21.Shand B, Elder P, Scott R, Frampton C, Willis J. Biovariability of plasma adiponectin. Clin Chem Lab Med. 2006;44:1264–1268. doi: 10.1515/CCLM.2006.227. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 24.de Simone G, Devereux RB, Roman MJ, Alderman MH, Laragh JH. Relation of obesity and gender to left ventricular hypertrophy in normotensive and hypertensive adults. Hypertension. 1994;23:600–606. doi: 10.1161/01.hyp.23.5.600. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. American Heart Association Statistics Committee and Stroke Statistics Subcommittee: Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 26.Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, Gidding S, Kurosaki T, Wong ND, Manolio TA. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–387. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 27.Lorber R, Gidding SS, Daviglus ML, Colangelo LA, Liu K, Gardin JM. Influence of systolic blood pressure and body mass index on left ventricular structure in healthy African-American and white young adults: the CARDIA study. J Am Coll Cardiol. 2003;41:955–960. doi: 10.1016/s0735-1097(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 28.Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, Province MA, Oberman A, Kitzman DW, Hopkins PN, Liu JE, Devereux RB. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension. 2004;43:1182–1188. doi: 10.1161/01.HYP.0000128738.94190.9f. [DOI] [PubMed] [Google Scholar]
- 29.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 30.de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, Kitzman DW, Hopkins PN, Arnett DK, Devereux RB. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323–331. doi: 10.1097/00004872-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Fox E, Taylor H, Andrew M, Han H, Mohamed E, Garrison R, Skelton T. Body mass index and blood pressure influences on left ventricular mass and geometry in African Americans: The Atherosclerotic Risk In Communities (ARIC) Study. Hypertension. 2004;44:55–60. doi: 10.1161/01.HYP.0000132373.26489.58. [DOI] [PubMed] [Google Scholar]
- 32.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110:267–278. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 35.Hattori Y, Akimoto K, Nishikimi T, Matsuoka H, Kasai K. Activation of AMP-activated protein kinase enhances angiotensin ii-induced proliferation in cardiac fibroblasts. Hypertension. 2006;47:265–270. doi: 10.1161/01.HYP.0000198425.21604.aa. [DOI] [PubMed] [Google Scholar]
- 36.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 37.Haugen E, Furukawa Y, Isic A, Fu M. Increased adiponectin level in parallel with increased NT-pro BNP in patients with severe heart failure in the elderly: A hospital cohort study. Int J Cardiol. 2008;125:216–219. doi: 10.1016/j.ijcard.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Kamari Y, Shimoni N, Koren F, Peleg E, Sharabi Y, Grossman E. High-salt diet increases plasma adiponectin levels independent of blood pressure in hypertensive rats: the role of the renin-angiotensin-aldosterone system. J Hypertens. 2010;28:95–101. doi: 10.1097/HJH.0b013e3283325eee. [DOI] [PubMed] [Google Scholar]
- 39.Lely AT, Krikken JA, Bakker SJ, Boomsma F, Dullaart RP, Wolffenbuttel BH, Navis G. Low dietary sodium and exogenous angiotensin II infusion decrease plasma adiponectin concentrations in healthy men. J Clin Endocrinol Metab. 2007;92:1821–1826. doi: 10.1210/jc.2006-2092. [DOI] [PubMed] [Google Scholar]
- 40.Krikken JA, Dallinga-Thie GM, Navis G, Dullaart RP. Short term dietary sodium restriction decreases HDL cholesterol, apolipoprotein A-I and high molecular weight adiponectin in healthy young men: Relationships with renal hemodynamics and RAAS activation. Nutr Metab Cardiovasc Dis. 2010 Jul 31; doi: 10.1016/j.numecd.2010.03.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaidya A, Forman JP, Underwood PC, Hopkins PN, Williams GH, Pojoga LH, Williams JS. The Influence Of Body-Mass Index And Renin-Angiotensin-Aldosterone System Activity On The Relationship Between 25-Hydroxyvitamin D And Adiponectin In Caucasian Men. Eur J Endocrinol. 2011;164:995–1002. doi: 10.1530/EJE-11-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Springer J, Anker SD, Doehner W. Adiponectin resistance in heart failure and the emerging pattern of metabolic failure in chronic heart failure. Circ Heart Fail. 2010;3:181–182. doi: 10.1161/CIRCHEARTFAILURE.110.945063. [DOI] [PubMed] [Google Scholar]
- 45.Lin HV, Kim JY, Pocai A, Rossetti L, Shapiro L, Scherer PE, Accili D. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes. 2007;56:1969–1976. doi: 10.2337/db07-0127. [DOI] [PubMed] [Google Scholar]
- 46.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.