Abstract
Synthetic pyrethroid insecticides were introduced into widespread use for the control of insect pests and disease vectors more than three decades ago. In addition to their value in controlling agricultural pests, pyrethroids are at the forefront of efforts to combat malaria and other mosquito-borne diseases and are also common ingredients of household insecticide and companion animal ectoparasite control products. The abundance and variety of pyrethroid uses contribute to the risk of exposure and adverse effects in the general population. The insecticidal actions of pyrethroids depend on their ability to bind to and disrupt voltage-gated sodium channels of insect nerves. Sodium channels are also important targets for the neurotoxic effects of pyrethroids in mammals but other targets, particularly voltage-gated calcium and chloride channels, have been implicated as alternative or secondary sites of action for a subset of pyrethroids. This review summarizes information published during the past decade on the action of pyrethroids on voltage-gated sodium channels as well as on voltage-gated calcium and chloride channels and provides a critical re-evaluation of the role of these three targets in pyrethroid neurotoxicity based on this information.
Keywords: pyrethroid, insecticide, neurotoxicity, voltage-gated sodium channel, voltage-gated calcium channel, voltage-gated chloride channel
Introduction
Current status of pyrethroid insecticides
Synthetic pyrethroid insecticides were introduced into widespread use for the control of insect pests and disease vectors more than three decades ago. By 2002, their use had grown to represent 18% of the dollar value of the world insecticide market (Pickett 2004). In addition to their value in controlling agricultural pests, pyrethroids are at the forefront of efforts to combat malaria and other mosquito-borne diseases despite the threat of pyrethroid resistance in vector populations (Ranson et al. 2011). Pyrethroids are also common ingredients of household insecticide and companion animal ectoparasite control products, whose unregulated use in the home environment increases the risk of exposure and adverse effects in the general population (Power and Sudakin 2007; Ostrea Jr. et al. 2009; Naeher et al. 2010).
The registrations of pyrethroid insecticide products in the United States are subject to regulatory review in response to the mandates of the Food Quality Protection Act (FQPA) of 1996. This act requires the United States Environmental Protection Agency to undertake cumulative risk assessments for groups of pesticides that are considered to have a “common mechanism of toxicity.” Knowledge of the molecular events that underlie the neurotoxic actions of pyrethroids is directly relevant to the determination of whether this large and important class of insecticides constitutes a single “common mechanism” group or multiple groups for the purposes of cumulative risk assessment. A second regulatory consideration introduced in the FQPA is the default presumption of the greater sensitivity of fetuses and neonates to the deleterious effects of pesticides. Knowledge of the differential sensitivity of fetal, neonatal and adult targets for the neurotoxic actions of pyrethroids offers the ability to improve the precision of risk assessments by addressing this default presumption at the pharmacodynamic level.
Pyrethroid neurotoxicity: two syndromes, multiple mechanisms?
The understanding of pyrethroid neurotoxicity is complicated by the existence of two distinct intoxication syndromes in mammals that are associated with different structural subgroups of this insecticide class. Verschoyle and Barnes (Verschoyle and Barnes 1972) provided the first systematic description of the signs of pyrethroid intoxication in rats following oral and intravenous dosing and noted the same syndrome of intoxication for pyrethrins, bioallethrin and resmethrin by either route of administration. This syndrome included hypersensitivity and aggression followed by stimulus-induced bouts of general tremor, convulsive twitching, coma, and death. The principal difference observed between oral and intravenous dosing was the speed of onset of intoxication. The publication of the discovery of deltamethrin (Elliott et al. 1974), the first pyrethroid containing the α-cyano-3-phenoxybenzyl moiety, was accompanied by a brief report describing the acute toxicity of deltamethrin to rats (Barnes and Verschoyle 1974). This report noted that the signs of deltamethrin intoxication following either oral or intravenous administration, which involved salivation without lacrimation followed by jerking leg movements and progressive writhing convulsions (choreoathetosis), were distinctly different than those reported by these authors for other pyrethroids (Verschoyle and Barnes 1972).
A subsequent study (Verschoyle and Aldridge 1980) described both the acute toxicity and the signs of intoxication of 36 pyrethroids following intravenous administration, thereby establishing a taxonomy of pyrethroid intoxication in mammals that persists to the present. Of the 18 esters of various primary alcohols examined in this study, 15 compounds produced signs of intoxication corresponding to those first described for pyrethrins and pyrethroids (Verschoyle and Barnes 1972), which was designated the T (tremor) syndrome, whereas the remaining three compounds did not produce any detectable signs of intoxication at the highest doses tested. By contrast, among the 17 esters of α-cyano-3-phenoxybenzyl alcohol examined 12 produced signs of intoxication like those first described for deltamethrin (Barnes and Verschoyle 1974), which was designated the CS (choreoathetosis with salivation) syndrome, whereas 4 produced the T syndrome of intoxication. One α-cyano-3-phenoxybenzyl ester and one compound in which the α-cyano group was replaced by an α-ethynyl group were found to produce elements of both syndromes (tremor with salivation). This classification of the signs of pyrethroid intoxication into two principal syndromes was confirmed in studies of the intracerebral toxicity of 29 pyrethroids to mice (Lawrence and Casida 1982).
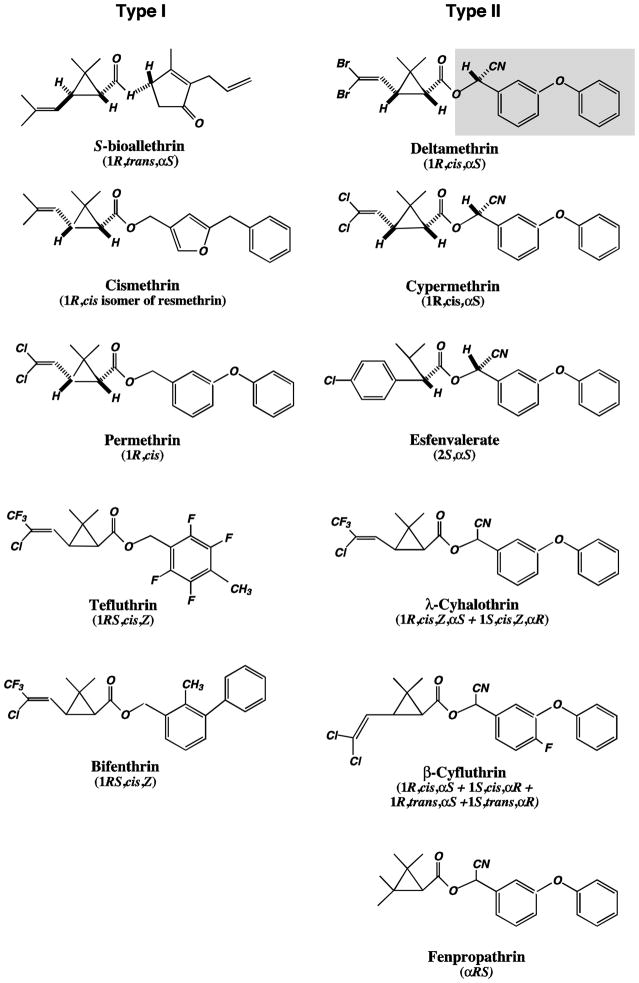
An alternative nomenclature (Type I and Type II) was proposed for subgroups of pyrethroids based on both their syndromes of intoxication (Lawrence and Casida 1982) and their chemical structures, signs of poisoning in insects, and actions on insect nerve preparations (Gammon et al. 1981). Type II pyrethroids contain the α-cyano-3-phenoxybenzyl moiety (e.g., cypermethrin, deltamethrin and fenvalerate) whereas Type I compounds comprise a wide structural variety of compounds lacking the α-cyano-3-phenoxybenzyl group (e.g., pyrethrin I, resmethrin and permethrin) (Fig. 1). The Type I/Type II nomenclature has been widely adopted in the literature and is often used in a manner parallel to the T/CS nomenclature, so that Type I compounds are generally considered to produce the T syndrome of intoxication and Type II compounds are considered to produce the CS syndrome (Lawrence and Casida 1982). However, the correspondence between the Type I/II (structural) and T/CS (symptomatic) taxonomies is not perfect, because some compounds that are Type II structures produce either the T syndrome of intoxication or signs of intoxication that exhibit elements of both the T and CS syndromes (Soderlund et al. 2002). For the purposes of this review, references to Type I and Type II compounds denote the structural classes only without reference to the signs of intoxication.
Figure 1.
Structures of Type I and Type II pyrethroids. Compounds are illustrated either as single isomers or as isomer mixtures with the isomeric compositions shown. The α-cyano-3-phenoxybenzyl moiety that defines the Type II structural class is shaded in the structure of deltamethrin.
Although the initial descriptions of the T and CS syndromes suggested that the signs of intoxication were independent of the route of administration (Verschoyle and Barnes 1972; Verschoyle and Aldridge 1980), it is not clear from these studies that this generalization extends to all pyrethroids or to all routes of administration. For example, comparison of the results of regulatory neurotoxicity studies for nine pyrethroids (reviewed in Soderlund et al. 2002) suggested that the behavioral signs of intoxication following oral exposure were not well correlated with the signs characteristic of the T and CS syndromes following intravenous dosing. The relevance of the T/CS classification to intoxication following oral exposure was recently re-examined in a controlled behavioral neurotoxicity study of 12 pyrethroids (Weiner et al. 2009). This study employed a modified functional observational battery, based on those developed for regulatory behavioral neurotoxicity studies (Allen 2010), that was optimized to facilitate distinction of the T and CS syndromes. The results of this study confirmed that the T/CS classification of intoxication syndromes was also evident following oral administration, but the magnitude and pattern of response traits varied between compounds in each symptomatic group. This study also confirmed that some pyrethroids exhibit intermediate signs of intoxication that contain elements of both the T and CS syndromes.
The insecticidal actions of pyrethroids depend on their ability to bind to and disrupt voltage-gated sodium channels of insect nerves (Soderlund 2005), and there is widespread agreement that voltage-gated sodium channels are also important targets for the neurotoxic effects of pyrethroids in mammals (Soderlund 2010b). Nevertheless, the marked distinction in the signs of intoxication characteristic of the T and CS syndromes has raised persistent questions about the role of alternative or secondary targets for a subset of compounds, particularly Type II structures that produce the CS syndrome. A previous review (Soderlund et al. 2002) provided a critical assessment of the likely toxicological significance of pyrethroid action on putative alternative targets and concluded that actions of pyrethroids on voltage-gated calcium and chloride channels could conceivably contribute to the neurotoxicity of some pyrethroids. Some additional support for this conclusion was recently provided by a principal component analysis of the pyrethroid neurotoxicity to rats in vivo and actions on voltage-gated sodium, calcium and chloride channels in vitro (Breckenridge et al. 2009), which segregated the tested compounds into two distinct mechanistic classes that correspond to the Type I and Type II structural groups.
Scope of this review
The most recent comprehensive review of pyrethroid neurotoxicity covered literature published up to 2001 (Soderlund et al. 2002). In the decade since that review significant new information has been published on the action of pyrethroids on voltage-gated sodium channels as well as on voltage-gated calcium and chloride channels, the two putative secondary targets identified as having the greatest likelihood of contributing to pyrethroid neurotoxicity. This review summarizes progress in each of these areas published during the period from 2000 through early 2011 and provides a critical evaluation of the role of these three targets in pyrethroid neurotoxicity based on this information.
Actions on voltage-gated sodium channels
The compelling evidence for effects on voltage-gated sodium channels as the mechanism of insecticidal activity of pyrethroids and the strong conservation of sodium channel structure, function and pharmacology across animal taxa implicate the voltage-gated sodium channels of the CNS as important sites of action in mammals. Surprisingly, there is little new information since the previous review (Soderlund et al. 2002) on the action of pyrethroids on mammalian sodium channels in their native neuronal environment, but there is additional information on the effects of pyrethroids on sodium channels in cardiac tissue. The majority of studies during the past decade have focused on the actions of pyrethroids on sodium channels of defined subunit structure employing heterologous expression systems, principally oocytes of the frog Xenopus laevis. Results of these studies provide new insight into the state dependence of pyrethroid action on sodium channels and isoform and species differences in sodium channel sensitivity. Moreover, ongoing studies of mutations in insect sodium channels that confer pyrethroid resistance have led to the development of a model of the pyrethroid receptor in insects. The following sections summarize recent developments in each of these areas in greater detail.
Actions of pyrethroids on sodium channels in cardiac tissue
Early studies of the physiological actions of the Type II pyrethroid deltamethrin identified a positive inotropic effect on isolated rat and dog hearts and guinea pig atrial muscle preparations that was not shared by the Type I pyrethroid cismethrin (reviewed in Soderlund et al. 2002). More recently, Spencer et al. (Spencer et al. 2001) showed that tefluthrin, (Type I) and both fenpropathrin and α-cypermethrin (Type II) prolonged action potentials and evoked afterdepolarizations in rat and guinea pig cardiac myocytes. Under voltage clamp conditions the time course of the sodium current decay was prolonged due to a delay in channel inactivation. In whole perfused rat hearts tefluthrin altered spontaneous rhythmic contractions, affecting both the amplitude and frequency of contractions. By contrast, tetramethrin (Type I) caused no significant effects in any of these preparations. A second study (Spencer and Sham 2005) investigated the mechanism underlying the tefluthrin-dependent prolongation of action potentials recorded from rat cardiac myocytes under whole-cell current clamp conditions. Approximately one-third of the action potential prolongation was ascribed to the effects of tefluthrin on the kinetics of sodium channel inactivation identified previously, whereas the remainder of the prolongation was caused by sodium influx-stimulated calcium overload due to reversed sodium-calcium exchange. These results provide evidence for a direct effect of pyrethroids on cardiac sodium channels that cause the proarrhythmic effects seen in whole hearts. However, it is not clear whether cardiac effects contribute in any significant way to pyrethroid intoxication in vivo.
State-dependent modification of sodium channels by pyrethroids
Pyrethroids disrupt nerve function by altering the rapid kinetic transitions between conducting (open) and nonconducting (closed or inactivated) states of voltage-gated sodium channels that underlie the generation of nerve action potentials. The majority of studies of pyrethroid actions on sodium channel gating under voltage-clamp conditions have been performed by equilibrating channels with pyrethroids at hyperpolarized membrane potentials and assessing the effects of pyrethroids upon depolarization. This approach is biased toward the detection of closed-state modification, and most of these studies have not considered the contribution of other channel states to pyrethroid action. However, there is evidence from studies with voltage-clamped squid and crayfish axons that modification by some pyrethroids is enhanced by repetitive depolarization, implying that channel opening increases the affinity of the pyrethroid binding site for these compounds (Brown and Narahashi 1992; de Weille et al. 1988; Salgado and Narahashi 1993). More recently, the discovery that modification by cypermethrin and deltamethrin of cloned insect sodium channels expressed in Xenopus laevis oocytes is absolutely dependent on repeated depolarization (Smith et al. 1998; Vais et al. 2000b) has led to the conclusion that these compounds bind preferentially to insect sodium channels in the open conformation. These results have also prompted new investigations of possible state-dependent modification of mammalian sodium channels by pyrethroids. State-dependent effects of pyrethroids on both insect and mammalian sodium channels are summarized in a recent review (Soderlund 2010a).
The first systematic study of the state-dependent actions of pyrethroids on mammalian sodium channels employed rat Nav1.8 channels. Nav1.8 channels are found in the peripheral nervous system, primarily in sensory neurons (Catterall et al. 2005), and are likely to carry the TTX-resistant, pyrethroid-sensitive component of sodium current found in rat dorsal root ganglion neurons (Ginsburg and Narahashi 1993). Whereas actions on peripheral nerve channels such as Nav1.8 are not relevant to the systemic neurotoxic effects of pyrethroids, they may underlie the paresthesia that can occur following dermal exposure to pyrethroids (Soderlund et al. 2002).
Initial studies of Nav1.8 channels in oocytes documented modification in the closed state by cismethrin, cypermethrin, and deltamethrin (Choi and Soderlund 2002, 2004; Smith and Soderlund 2001; Soderlund and Lee 2001). Results obtained with cismethrin (Choi and Soderlund 2002; Soderlund 2010a) exemplify the properties associated with modification in the closed state. Following equilibration at a hyperpolarized membrane potential cismethrin produced currents during a depolarizing pulse that inactivated much more slowly than control currents and also produced a sodium tail current following membrane repolarization. Cismethrin did not alter the voltage dependence of activation of the peak transient sodium current, but the voltage dependence of the persistent component of the current was shifted in the hyperpolarizing direction. Depolarization to potentials that selectively activated only the cismethrin-modified population of channels permitted the direct observation of the slow kinetics of activation and inactivation of these channels (Soderlund and Lee 2001). The production of sodium currents that exhibit altered kinetics and voltage dependence following exposure at hyperpolarized potentials are hallmarks of sodium channel modification by pyrethroids in the closed state.
A subsequent study examined 11 structurally diverse pyrethroids as modifiers of Nav1.8 channels in oocytes (Choi and Soderlund 2006). All 11 compounds produced clearly detectable closed-state modification. Manual subtraction of scaled control sodium currents from currents obtained following pyrethroid exposure permitted reconstruction and kinetic characterization of currents carried only by the pyrethroid-modified channel population. In all cases, the pyrethroid-modified currents activated and inactivated much more slowly than currents carried by unmodified channels and exhibited a pronounced tail current whose decay kinetics varied with pyrethroid structure. This study also evaluated the effects of repetitive depolarization on the extent of pyrethroid modification. Deltamethrin and three other Type II compounds produced significant increases in channel modification following the application of high-frequency trains of depolarizing prepulses. This use-dependent enhancement was restricted to the four compounds that produced the most persistent tail currents associated with the production of persistently open pyrethroid-modified channels.
Studies of rat Nav1.6 sodium channels expressed in oocytes (Tan and Soderlund 2010) also identified differences between pyrethroids in the relative importance of resting and use-dependent channel modification. Resting modification of Nav1.6 channels by the Type II pyrethroid deltamethrin was barely detectable, but modification was increased approximately four-fold by the application of high-frequency depolarizing prepulses. By contrast, modification by S-bioallethrin, a Type I compound, was not affected by repeated depolarization. Tefluthrin, a Type I compound with high mammalian toxicity, exhibited elements found with both S-bioallethrin and deltamethrin: significant modification in the resting state, but also substantial enhancement of modification upon repeated depolarization. The resting and use-dependent effects of deltamethrin and tefluthrin on Nav1.2 channels were similar to those found with Nav1.6 channels (Tan and Soderlund 2010), and tefluthrin also produced a mixture of resting and use-dependent modification in assays with rat Nav1.3 and rat Nav1.7 channels expressed in oocytes (Tan et al. 2008; Tan and Soderlund 2011). The use-dependent enhancement of the modification of these isoforms by tefluthrin contrasts with the lack of use-dependent modification observed in assays of tefluthrin on Nav1.8 channels (Choi and Soderlund 2006).
The conventional interpretation of use-dependent channel modification is that it signifies preferential binding of an agent to the open state of the channel. However, there is little direct evidence of open-channel modification by pyrethroids. So far, the best evidence has been obtained using D. melanogaster Para/TipE sodium channels that have been pharmacologically modified with Anemonia sulcata toxin II (ATX-II) to remove fast inactivation (Vais et al. 2000b). However, allosteric coupling of the binding sites for ATX-II and pyrethroids (Lombet et al. 1988) suggests that the properties of the pyrethroid receptor of channels modified by ATX-II may differ from those of native channels. It would be valuable to expand the exploration of open channel modification by pyrethroids using techniques, such as site-directed mutagenesis, that remove fast channel inactivation without pharmacological modification.
If pyrethroids can modify open sodium channels, it is puzzling that they do not also readily modify inactivated channels. Studies with Para/TipE channels and deltamethrin in oocytes clearly show that a single long depolarization, which favors the production of inactivated channels, does not promote modification (Vais et al. 2000b). Current models of activation and inactivation gating suggest that open and fast-inactivated channels differ in structure only by the occlusion of the channel by the fast inactivation gate, a highly conserved intracellular domain that is thought to act as a “hinged lid” at the intracellular mouth of the channel (Goldin 2003; Ulbricht 2005). The failure of pyrethroids to modify inactivated channels suggests that inactivation prevents access of the pyrethroid to its binding site or that pyrethroid binding and channel inactivation are mutually exclusive processes, but neither of these seems likely. Permanently charged molecules, such as some local anesthetics, are known to require an open channel to reach their binding site in the inner pore region but uncharged lipophilic agents can gain access to their binding site by diffusion through the membrane and do not require the aqueous pathway provided by the open channel (Hille 1977). The physical properties of pyrethroids argue against the need for an aqueous pathway for access to their binding site.
There are three possible explanations for the use-dependent modification of sodium channels by pyrethroids that do not require binding to the open state of the channel. The first, proposed initially to account for the use-dependent enhancement of modification of TTX-resistant sodium channels in rat DRG neurons by deltamethrin (Tabarean and Narahashi 2001), attributes use-dependent effects to the accumulation of persistently open channels that are modified by compounds such as deltamethrin, which produce long-lived open modified states. This mechanism may also account for the use-dependent enhancement of the modification of rat Nav1.8 channels in oocytes by deltamethrin and related compounds, all of which exhibit very slow rates of deactivation (Choi and Soderlund 2006). However, this mechanism does not adequately account for the observations that deltamethrin and cypermethrin produce no detectable first-pulse (i.e., resting) modification of insect sodium channels expressed in oocytes (Smith et al. 1998; Vais et al. 2000b) and that deltamethrin produces barely-detectable resting modification of rat Nav1.2 and Nav1.6 channels in the same system (Tan and Soderlund 2010).
A second mechanism of use-dependent modification could involve preferential binding to an intermediate channel state that is transiently present during the activation gating process. This explanation, which requires a voltage-dependent change in channel conformation, but not an open channel, to promote binding, could account for the failure of pyrethroids that exhibit use dependence to modify inactivated channels. An analogous mechanism, involving binding to transitional nonconducting states, contributes to sodium channel block by lidocaine and benzocaine (Wang et al. 2004). Finally, a recent study of the action of permethrin and tetramethrin on bee antennal olfactory receptor neurons suggests that the apparent use-dependent enhancement of channel modification observed in this system may be due to the progressive recruitment or unmasking of a pool of electrically silent channels as the result of pyrethroid modification (Kadala et al. 2011).
One provocative hypothesis suggested by the apparent differences in the importance of use-dependent modification among pyrethroids is that the binding site for pyrethroids exists in at least two conformations, one associated with the resting channel and one associated with a channel state (presumably, but not necessarily, open) achieved via channel activation, that have different structure-activity relationships. For example, the modification of rat Nav1.6 sodium channels by S-bioallethrin, deltamethrin and tefluthrin (Tan and Soderlund 2010) appears to proceed either exclusively via the resting state (S-bioallethrin), predominantly if not exclusively via the activation-dependent state (deltamethrin), or via both states with the activation-dependent state exhibiting higher affinity (tefluthrin). This hypothesis is directly relevant to current efforts to model the structure of the sodium channel pyrethroid receptor, which are discussed in a subsequent section of this review.
Differences in pyrethroid sensitivity between rat sodium channel isoforms
Most of the available information on the actions of pyrethroids on mammalian sodium channels was obtained using neuronal tissue preparations, which are now known to express multiple sodium channel isoforms. As a result, the action of pyrethroids has not been correlated with the expression of identified sodium channel isoforms in these tissues. However, a limited number of physiological studies suggest that sodium channel isoforms expressed in various mammalian tissues exhibit differential sensitivity to pyrethroids. The clearest evidence of differential sensitivity to pyrethroids between sodium channel isoforms is found in the responses of the TTX-sensitive and TTX-resistant sodium channel populations in dorsal root ganglion (DRG) neurons to pyrethroids. The TTX-resistant current in these cells is much more sensitive than the TTX-sensitive current to allethrin (Ginsburg and Narahashi 1993), tetramethrin (Tatebayashi and Narahashi 1994; Song et al. 1996) and deltamethrin (Tabarean and Narahashi 1998).
Pore-forming α subunits of voltage-gated sodium channels in mammals are encoded by a family of nine genes (Catterall et al. 2005). These channels, designated Nav1.1 – Nav1.9, are differentially distributed in excitable cells and exhibit unique functional and pharmacological properties. The Nav1.1, Nav1.2, Nav1.3 and Nav1.6 isoforms are expressed in the CNS (Goldin 2001) and represent potential targets for the systemic neurotoxic actions of pyrethroids. In most cases, sodium channel α subunits are coexpressed with either one or two auxiliary β subunits that modulate channel gating and kinetics and regulate channel expression (Meadows and Isom 2005). In mammals, there are four sodium channel β subunits (designated β1 – β4). Typically, a single neuron expresses multiple sodium channel α and β subunits and therefore may contain several distinct heteromultimeric complexes.
Overlapping patterns of sodium channel α subunit expression in the CNS (Felts et al. 1997; Whitaker et al. 2001) limit the utility of native neuronal preparations to identify isoform-dependent differences in pharmacology. This limitation can be overcome by the heterologous expression of cloned individual sodium channel isoforms in the unfertilized oocytes of the frog Xenopus laevis or in transfected mammalian cell lines. The available information on the sensitivity of individual mammalian sodium channel isoforms to pyrethroids is based primarily on expression studies using the Xenopus oocyte system.
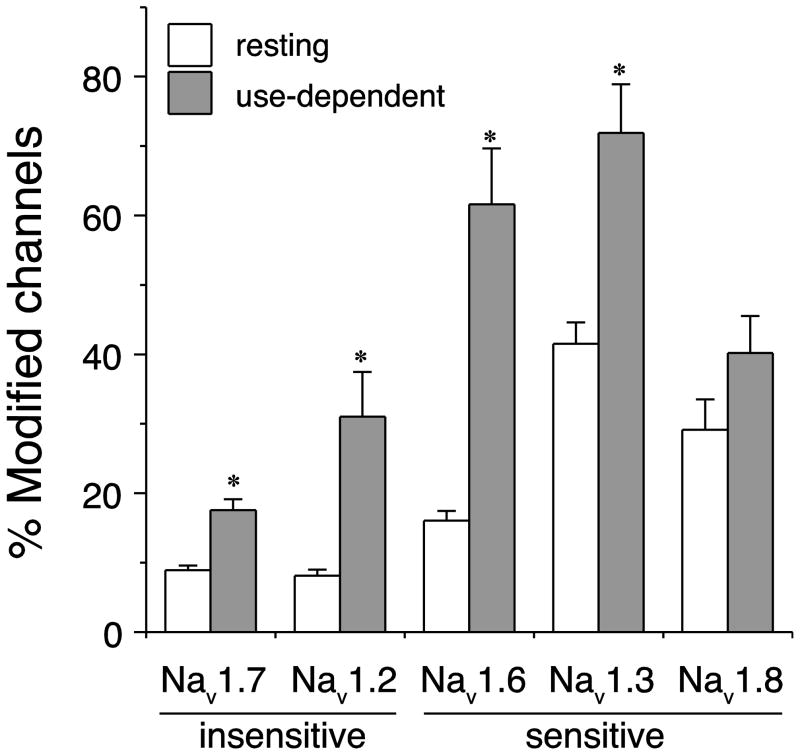
The first studies of an individual mammalian sodium channel isoform employed the rat Nav1.2 and Nav1.8 α subunit isoforms expressed in oocytes. Rat Nav1.2 sodium channels, which are abundantly expressed in the adult CNS, exhibit very low sensitivity to modification by deltamethrin and other pyrethroids (Smith and Soderlund 1998; Vais et al. 2000a). By contrast rat Nav1.8 channels, which are resistant to block by TTX and restricted in distribution to the peripheral nervous system, are sensitive to modification by a wide structural variety of pyrethroids (Choi and Soderlund 2006; Smith and Soderlund 2001; Soderlund and Lee 2001). It is likely that Nav1.8 channels carry the TTX-resistant, pyrethroid-sensitive current recorded from DRG neurons (Rush et al. 2007). More recent studies confirmed the relative insensitivity of rat Nav1.2 channels but showed that the rat Nav1.3 and Nav1.6 exhibit pyrethroid sensitivity equal to or greater than that of the Nav1.8 isoform (Meacham et al. 2008; Tan and Soderlund 2009, 2010). The relative sensitivity of these isoforms to modification by tefluthrin is summarized in Fig. 2. The difference in sensitivity of Nav1.2 and Nav1.6 channels to tefluthrin shown in Fig. 2 corresponds to a difference in potency of approximately 16-fold (resting) and 18-fold (use-dependent) at the EC10 level based on partial concentration-effect curves (Tan and Soderlund 2010). Thus, the difference in the tefluthrin sensitivity between the least sensitive (Nav1.7) and most sensitive (Nav1.3) isoforms in Fig. 2 is likely to exceed 20-fold.
Figure 2.
Comparison of the resting (0 prepulses) and use-dependent (after 100 prepulses) modification of three pyrethroid-sensitive and two pyrethroid-insensitive rat sodium channel isoforms expressed in Xenopus oocytes (Choi and Soderlund 2006; Tan and Soderlund 2009, 2010, 2011). Asterisks indicate significant use-dependent enhancement of channel modification (paired t-tests, P < 0.05).
Species differences in pyrethroid sensitivity
There is now evidence that the generally lower sensitivity of mammalian sodium channel isoforms compared to insect channels contributes to the favorable selective toxicity of pyrethroids. Direct comparisons of the pyrethroid sensitivity of insect and mammalian sodium channel isoforms in the common expression environment of the Xenopus oocyte showed that insect channels are much more sensitive to pyrethroids (Vais et al. 2001). This comparison is exaggerated somewhat by the use of a pyrethroid-insensitive rat sodium channel isoform (Nav1.2) as the basis for comparison, but more sensitive rat isoforms such as Nav1.3, Nav1.6 or Nav1.8 are also at least an order of magnitude less sensitive to pyrethroid modification than insect channels (Smith et al. 1997; Soderlund and Lee 2001). An important component of this species difference in sensitivity has been mapped to a single amino acid polymorphism in the highly conserved intracellular linker sequence between the S4 and S5 transmembrane segments in sodium channel homology domain II. All pyrethroid-sensitive insect channels contain a methionine at this position (M918 of the house fly Vssc1 sequence), but all mammalian channels, irrespective of their relative pyrethroid sensitivity, contain an isoleucine. Replacement of this isoleucine with methionine in rat Nav1.2 (Vais et al. 2000a), Nav1.4 (Wang et al. 2001) or Nav1.8 (Soderlund and Lee 2001) channels significantly increased the sensitivity of these channels, whether expressed in oocytes or mammalian cells and whether assessed as resting or use-dependent modification. As described in subsequent sections of this review, M918 is also an important site of mutations that cause pyrethroid resistance in insects and is implicated as a key component of a postulated pyrethroid receptor on insect sodium channels.
The uncertain fidelity of rodent models in predicting intoxication risks in humans remains a central problem for both experimental and regulatory toxicology. Sodium channel α subunit genes are highly conserved, so that orthologous subunits in rats and humans are >95% identical at the level of amino acid sequence (Goldin 2001). However, this degree of sequence conservation still results in as many as 100 amino acid sequence differences between orthologous channel proteins and could conceivably produce species differences in functional and pharmacological properties. Heterologous expression systems, such as the Xenopus oocyte system, offer the opportunity to compare directly the properties and pyrethroid sensitivities of orthologous rat and human sodium channels. The first study of this type compared the sensitivities of orthologous rat and human Nav1.3 sodium channels (Tan and Soderlund 2009). Surprisingly, human Nav1.3 channels were significantly less sensitive to modification by the pyrethroid insecticide tefluthrin than rat Nav1.3 channels.
Model of the sodium channel pyrethroid receptor
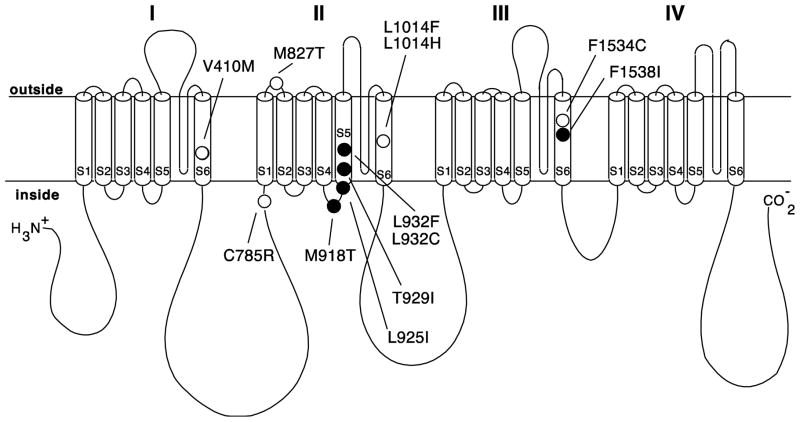
The covalent labeling of the sodium channel α subunit protein by a pyrethroid photoaffinity ligand (Trainer et al. 1997) and the functional modification of channels expressed only from cloned α subunits (Smith and Soderlund 1998, 2001; Trainer et al. 1997) provide unambiguous evidence that the pyrethroid binding site is intrinsic to the α subunit protein. Point mutations in insect sodium channel α subunit genes that are associated with pyrethroid resistance provide additional insight into the sodium channel domains that are important for pyrethroid action and may therefore be involved in pyrethroid binding. Comparisons of partial or complete coding sequences of the para-orthologous sodium channel genes from pyrethroid-sensitive and pyrethroid-resistant strains of various arthropod species identified more than 25 unique amino acid substitutions that are associated with pyrethroid resistance (Soderlund 2005; Dong 2007; Soderlund 2008). Studies employing site-directed mutagenesis and expression in oocytes have confirmed that 12 point mutations (at 10 sequence positions) modify the sensitivity of insect sodium channels to pyrethroids (Fig. 3), whereas the remaining mutations remain uncharacterized with respect to function. The majority of resistance-associated mutations are found in or adjacent to the intracellular linkers between transmembrane helices S4 and S5 (designated L4–5) or within transmembrane helices S5 and S6. Models of the structure of sodium channels and related voltage-gated potassium channels place the L4–5, S5 and S6 segments of each homology domain in close proximity to each other at or near the inner mouth of the channel pore, with the S6 helices involved in activation gating of the channel (Lipkind and Fozzard 2000; Shrivastava et al. 2004; Zhao et al. 2004). The existence of multiple pyrethroid resistance mutations in these regions suggests that the pyrethroid receptor may be located close to the inner mouth of the channel. In particular, the clustering of confirmed sites of resistance mutations in the L4–5, S5 and S6 segments of homology domain II (see Fig. 3) led to models postulating a pyrethroid receptor formed primarily by residues in these segments of homology domain II with some contribution from the S6 helices and associated regions of homology domains I and III (Lee and Soderlund 2001; Vais et al. 2003; Tan et al. 2005).
Figure 3.
Sodium channel gene mutations associated with knockdown resistance shown in relation to the extended transmembrane structure of a voltage-sensitive sodium channel α subunit. Circles denote mutated amino acids known to reduce the pyrethroid sensitivity of insect channels expressed in Xenopus oocytes. Mutations are labeled according to the sequence positions of the corresponding residues of the most abundant splice variant of the house fly Vssc1 sodium channel. Filled circles identify mutations considered to form the pyrethroid receptor of the Vssc1 sodium channel (O’Reilly et al. 2006; Du et al. 2009).
Recently, information on resistance mutations has been incorporated into a new high-resolution structural model of the pyrethroid receptor of the house fly Vssc1 sodium channel (O’Reilly et al. 2006; Usherwood et al. 2007; Du et al. 2009). The open configuration of the pore region of the Vssc1 channel, formed by the L4–5, S5 and S6 segments of homology domains I–IV, was modeled based on the crystal structures of homologous voltage-gated potassium channels. Computer-aided docking of pyrethroid ligands and the predicted impact of resistance mutations in these domains on ligand docking yielded a putative pyrethroid receptor that includes four resistance-associated residues in the L4–5 and S5 segments of homology domain II and one resistance-associated residue in the S6 segment of homology domain III (see Fig. 3). Additional site-directed mutagenesis studies in these regions identify residues, other than those involved in pyrethroid resistance, that contribute to the proposed pyrethroid receptor (Du et al. 2009; Usherwood et al. 2007).
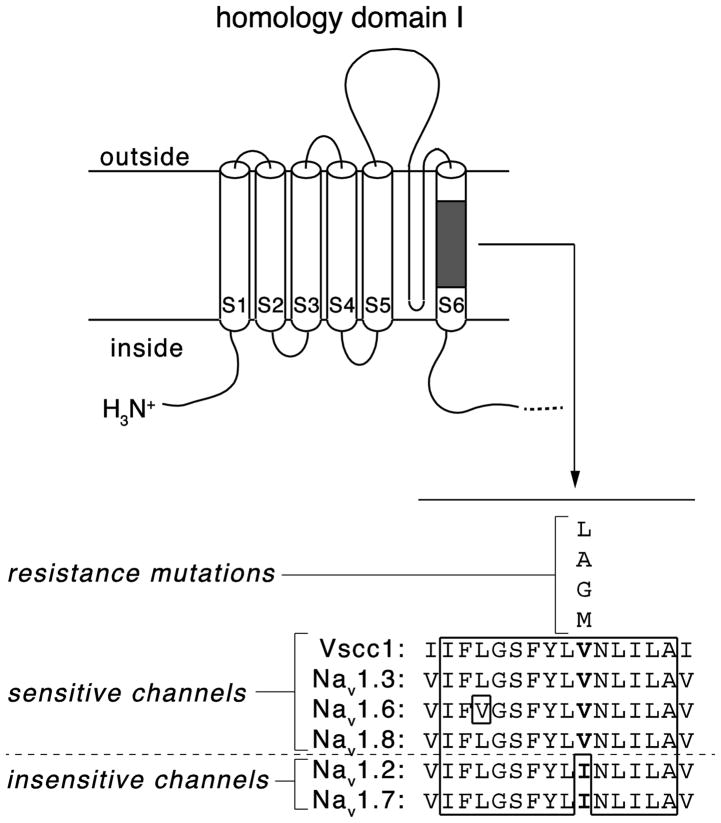
Amino acid residues in mammalian sodium channels that align with the putative elements of the pyrethroid receptor are absolutely conserved across all nine mammalian sodium channel isoforms. Therefore this model provides little insight into the molecular basis for the differential sensitivity of mammalian sodium channel isoforms to pyrethroids. However, alignments of the amino acid sequences of four pyrethroid-sensitive channels (house fly Vssc1 and rat Nav1.3, Nav1.6 and Nav1.8) and two insensitive channels (rat Nav1.2 and Nav1.7) identified a single polymorphic site located in an otherwise highly-conserved sequence context in the S6 segment of homology domain I (Tan and Soderlund 2011). The three sensitive rat sequences share a valine at this position, whereas the two insensitive sequences have an isoleucine residue (Fig. 4). This residue, V410 in the house fly Vssc1 sequence, is the site of multiple amino acid substitutions associated with pyrethroid resistance (Soderlund 2005; Dong 2007; Soderlund 2008), one of which (V410M; see Fig. 3) has been shown to alter the pyrethroid sensitivity of Vssc1 channels expressed in Xenopus oocytes (Lee and Soderlund 2001). If the current model of the pyrethroid receptor is correct then amino acid sequence differences at V410 and other locations distant from the proposed receptor must alter pyrethroid sensitivity indirectly by affecting either channel gating or allosteric modulation of the receptor itself.
Figure 4.
Identification of a sodium channel amino acid sequence polymorphism associated with differential pyrethroid sensitivity. Top: diagram of sodium channel homology domain I showing the location of a conserved block of amino acid sequence in transmembrane segment 6. Bottom: Aligned amino acid sequences in transmembrane segment 6 from four pyrethroid-sensitive channels (house fly Vssc1, rat Nav1.3, rat Nav1.6 and rat Nav1.8) and two pyrethroid-insensitive channels (rat Nav1.2 and rat Nav1.7). Residues enclosed in the solid line are identical in all six sequences. Polymorphic residues aligning with V410 of Vssc1 are shown in boldface type; substitutions at the valine residue aligning with V410 of Vssc1 that are associated with pyrethroid resistance in insects are shown above the aligned sequences.
Finally, neither the current receptor model nor information on other domains and residues that affect pyrethroid action explain the difference in pyrethroid sensitivity between the rat and human orthologs of the Nav1.3 channel. These two sequences differ at only 54 of 1951 amino acid residues (Tan and Soderlund 2009), and none of these differences align with elements of the proposed pyrethroid receptor or any other residue identified in resistant insects as a putative determinant of pyrethroid sensitivity. Therefore, additional structural determinants of pyrethroid sensitivity remain to be identified.
Actions on voltage-gated calcium channels
Direct effects of pyrethroids on voltage-gated calcium channels have been explored as a possible explanation for the enhanced neurotransmitter release in the CNS that accompanies the CS syndrome of intoxication (Soderlund et al. 2002). A subsequent comprehensive and critical review summarized the evidence for the action of pyrethroids on voltage-gated calcium channels based on literature published up to 2003 (Shafer and Meyer 2004). Since that review, additional information has been obtained using two experimental approaches: biochemical studies of neurotransmitter release and calcium uptake using presynaptic nerve terminals isolated from brain and cultured brain neurons; and, electrophysiological studies of native calcium currents in various cell types or of calcium currents carried by channels of defined subunit structure expressed in Xenopus oocytes or HEK (human embryonic kidney) cells. Results of these studies are summarized in the following sections.
Biochemical studies
A number of studies conducted prior to 2000 examined the effects of pyrethroids on spontaneous and evoked neurotransmitter release using mammalian brain presynaptic nerve terminals (synaptosomes) or brain slice preparations (reviewed in: Shafer and Meyer 2004; Soderlund et al. 2002). These studies yielded conflicting evidence regarding the mechanism of pyrethroid-induced or -enhanced neurotransmitter release. In some cases, release was completely inhibited by TTX and therefore ascribed to an action on voltage-gated sodium channels, but in other cases pyrethroid-dependent release was partially insensitive to TTX, correlated with calcium uptake, and attributed to a direct effect of pyrethroids on voltage-gated calcium channels.
Recent studies (Symington et al. 2007b; Symington et al. 2008) provide a more comprehensive picture of the action of pyrethroids on both calcium uptake and depolarization-evoked neurotransmitter release in rat brain synaptosome preparations. These studies employed 11 commercially-available pyrethroids that included members of both the Type I and Type II structural classes and compounds previously characterized as producing either the T or CS syndromes of intoxication. Five of the six Type II compounds (cyfluthrin, cyhalothrin, cypermethrin, deltamethrin, and esfenvalerate) and permethrin, a Type I compound, were potent enhancers of both calcium uptake and neurotransmitter release. For deltamethrin, both of these effects were inhibited by ω-conotoxin MVIIC, a selective blocker of N-type calcium channels. The remaining five compounds either were much less potent in both assays (cismethrin and bifenthrin) or enhanced glutamate release without a corresponding enhancement of calcium influx (bioallethrin, fenpropathrin and tefluthrin). Since these experiments were performed in the presence of TTX to eliminate effects on voltage-gated sodium channels, the results provide evidence that at least some pyrethroids directly enhance depolarization-evoked calcium uptake and neurotransmitter release in isolated presynaptic terminals from brain. Use of this system to characterize the joint action of pairs of pyrethroids identified either additive or antagonistic interactions in assays of calcium uptake but found additive, antagonistic or synergistic interactions in neurotransmitter release assays (Symington et al. 2011). The existence of synergistic effects on neurotransmitter release in the absence of corresponding synergistic effects on calcium uptake was interpreted as evidence for a third mechanism, other than actions at sodium and calcium channels, contributing to the effects of a subset of pyrethroids on neurotransmitter release.
Assays of pyrethroid-induced calcium uptake into cultured mouse brain neocortical neurons (Cao et al. 2011) failed to confirm a direct action of pyrethroids on voltage-gated calcium channels. In this system, nine of the eleven pyrethroids examined (encompassing both Type I and Type II structures) produced concentration-dependent increases in intracellular calcium that were completely inhibited by TTX, thereby implying a secondary effect on calcium influx as a result of activation of voltage-gated sodium channels. Further studies with tefluthrin and deltamethrin using specific blockers of calcium influx pathways provided evidence that secondary calcium influx was mediated by N-methyl-D-aspartate receptors (presumably as a result of depolarization-induced glutamate release), L-type calcium channels, and the sodium/calcium exchanger operating in a reversed mode.
Electrophysiological studies
Electrophysiological studies of native calcium currents published prior to 2000 (reviewed in: Shafer and Meyer 2004; Soderlund et al. 2002) found that tetramethrin (Type I) selectively blocked T-type calcium currents with either much less effect or no effect on the L-type currents in the same cells, whereas deltamethrin and fenvalerate (Type II) had no effect on either current. More recent studies with neurosecretory cells showed that tefluthrin (Type I) produced partial inhibition of L-type calcium currents at concentrations that also caused extensive modification of the sodium currents in these cells (Wu et al. 2009). In PC12 cells, allethrin (Type I) caused a net increase in the composite whole-cell calcium current. Dissection of this current using subtype-specific inhibitors showed that allethrin increased the amplitude and altered the voltage dependence of ω-conotoxin GVIA-insensitive currents, carried predominantly by L-type calcium channels, but decreased the amplitude of nimodipine-insensitive currents carried predominantly by N-type channels. The latter study corroborates earlier evidence for differential modulation of calcium channel subtypes by Type I pyrethroids and provides evidence for the activation of native calcium channels by pyrethroids, an effect not seen in previous electrophysiological studies.
An alternative to studies with native calcium channels involves assays of the effects of pyrethroids on single calcium channel isoforms expressed either in HEK cells or Xenopus oocytes. Hildebrand et al. (2004) showed that allethrin was equally effective as a blocker of T-, L- or P-/Q-type calcium channel isoforms expressed in HEK cells. For all three channel types, block was accompanied by an increase in the rate of channel inactivation and a shift of the voltage-dependence of inactivation to more negative potentials. In addition, block of L- and P-/Q-type channels was enhanced by repeated stimulation, whereas block of T-type channels was not effected. Similarly, deltamethrin caused the partial block of an N-type calcium channel isoform expressed in Xenopus oocytes (Symington and Clark 2005). In contrast to calcium channels expressed in HEK cells, pyrethroid block of N-type channels in oocytes was accompanied by a slight hyperpolarizing shift in the voltage dependence of activation and a slowing of channel activation and inactivation. Insertion of the T422E mutation in the rat Cav2.2 (N-type) calcium channel reversed the effect of deltamethrin, which caused a significant increase in the peak transient calcium current in the mutated channel (Symington et al. 2007a). The T422E mutation mimics complete phosphorylation of threonine-422, suggesting that deltamethrin may differentially affect up- and down-regulated calcium channels. Subsequent studies using an activator of protein kinase C to increase the extent of channel phosphorylation in oocytes confirmed the enhancement of the Cav2.2 calcium current by deltamethrin following protein kinase C activation (Alves et al. 2010).
Actions on chloride channels
Chloride channels gated by transmembrane potential changes or cell volume changes rather than by small-molecule ligands (such as γ-aminobutyric acid or glycine) are involved in the stabilization of membrane resting potential, transepithelial transport, and cell volume regulation in virtually all cell types (Jentsch et al. 2001). Many voltage-dependent chloride currents are carried by members of the CLC class of chloride channels (Jentsch et al. 2001; Maritzen et al. 2006), but the molecular entities responsible for other chloride currents such as ICl,swell, an important regulator of cell volume homeostasis, are not known (Sardini 2006).
The involvement of voltage-gated chloride channels as secondary targets for Type II pyrethroids producing the CS intoxication syndrome was originally proposed based on the blocking effects of deltamethrin (Type II) but not cismethrin (Type I) on chloride conductance in rat muscle and voltage-gated chloride channels in neuroblastoma cells and on the modification of the signs of deltamethrin (CS) intoxication syndrome by agents that act as activators of voltage-gated chloride channels (reviewed in Soderlund et al. 2002). Studies published subsequent to that review provide information on the action of a wider variety of Type I and Type II structures on voltage-gated chloride channels as well as new information on the selective effects of some pyrethroids on multiple anion conductances in cardiac tissue.
Voltage-gated chloride channels in neuroblastoma cells
The hypothesis that selective block of voltage-gated chloride channels by Type II pyrethroids contributes to the CS intoxication syndrome was based on the use cismethrin and deltamethrin as model Type I and Type II compounds, respectively (reviewed in Soderlund et al. 2002). A subsequent study (Burr and Ray 2004) evaluated structure-activity relationships for chloride channel block in N1E-115 neuroblastoma cells by 14 pyrethroids that produce either the T or CS syndromes. The results of this study failed to confirm a strong correlation between chloride channel block and the CS intoxication syndrome. Specifically esfenvalerate and λ-cyhalothrin, Type II structures that produce the CS intoxication syndrome, were without effect on chloride channels whereas bioallethrin, a Type I structure that produces the T syndrome, blocked chloride channels. The authors concluded that differences between the action of Type I and Type II compounds on chloride channels cannot, in themselves, account for the differences between the two pyrethroid intoxication syndromes.
Anion conductances in cardiac tissue
Recent studies provide evidence for divergent effects of pyrethroids on multiple distinct anion conductances in cardiac tissue. Borg et al. (2002) showed that a novel anionic background current (IAB) in guinea pig ventricular myocytes was selectively activated by tefluthrin. Both the functional and pharmacological properties of this current distinguished it from chloride conductances gated by voltage, volume change or cAMP. By contrast, three Type I (allethrin, tefluthrin and tetramethrin) and two Type II (cyfluthrin and fenpropathrin) pyrethroids inhibited volume-sensitive iodide efflux from HeLa cells, presumably through an effect on the unknown carrier of ICl,swell (Culliford et al. 2004). However, bifenthrin and cypermethrin were ineffective as inhibitors of volume sensitive iodide efflux in this system. Further, only tetramethrin inhibited volume-sensitive taurine efflux from HeLa cells, thereby implying that different volume-regulated channels mediate inorganic and organic anion transport.
A subsequent study compared directly the effects of four pyrethroids (α-cypermethrin, fenpropathrin, tefluthrin and tetramethrin) on IAB, ICl,swell and the cyclic AMP-dependent chloride current (ICl,cAMP) in guinea pig cardiac myocytes (Borg et al. 2007). Among the four pyrethroids tested α-cypermethrin, fenpropathrin and tefluthrin activated IAB whereas only fenpropathrin significantly inhibited ICl,swell and none of the four compounds affected ICl, cAMP. These data show that pyrethroids distinguish among three distinct anion currents in cardiac myocytes. It is interesting a single pyrethroid (fenpropathrin) can act either as an activator or inhibitor or have no effect depending on the current examined.
Studies of human cardiac myocytes identified an additional background anion current, termed IANION, that differed in ionic selectivity from IAB in the guinea pig heart and also was not activated by tefluthrin (Li et al. 2007). The molecular basis of this current and its relationship to IAB and other chloride currents in cardiac tissue remains to be clarified.
Significance of ion channel effects
Voltage-gated sodium channels
This review summarizes four areas of recent progress in understanding the action of pyrethroids on sodium channels, each of which provides new insight into the molecular basis for the neurotoxic actions of these compounds. First, use-dependent channel modification is now recognized as an important component of the interaction between at least some pyrethroids and some channel isoforms. Use-dependent modification is critical to the action of deltamethrin and related Type II compounds on insect sodium channels and many mammalian isoforms. The conventional interpretation of use-dependent effects is that the open state of the channel exhibits higher affinity than the resting state; open-channel modification has been inferred for insect (para/TipE) sodium channels expressed in oocytes, but enhanced binding to the open state (as opposed to the accumulation of persistently open channels, recruitment of silent channels, or binding to a transitional nonconducting state) has not been demonstrated unambiguously, particularly for mammalian channels. The emphasis on use-dependent modification, especially with insect channels, has tended to obscure the fact that for some pyrethroid – channel combinations modification occurs extensively, and even exclusively, via the closed or resting state. If use-dependent modification reflects binding to the open channel, then a necessary conclusion from these studies is that the pyrethroid receptor exists in two conformations (open and closed) that exhibit distinct structure-activity relationships for pyrethroid modification. The emphasis to date on the use of a relatively small number of pyrethroids as representatives of the larger class limits our understanding of these two structure-activity relationships and the extent to which they overlap.
The significance of use-dependent modification is directly related to a second area of recent progress, the development of a structural model of the pyrethroid receptor on the house fly sodium channel. This model of the open state of the channel, predicted to be the high-affinity binding site for compounds that act in a use-dependent manner, provides a wealth of testable hypotheses about the role of different amino acid residues in the receptor region in pyrethroid binding. For example, one elaboration of this model suggests that pyrethroids and batrachotoxin bind to residues lying on opposite faces of the domain IIIS6 helix (Du et al. 2009), a spatial relationship congruent with the known allosteric coupling of the pyrethroid and batrachotoxin binding sites (Soderlund et al. 2002). So far, efforts to model the structure of the pyrethroid receptor have focused exclusively on the open state of the sodium channel. In light of the evidence for closed-state modification by some pyrethroids, it would be of interest to generate a comparable model of the Vssc1 sodium channel in the resting state. The available data from studies with insect channels expressed in oocytes suggest that the pyrethroid receptor of resting channels should accommodate compounds such as cismethrin and permethrin, which are capable of modifying closed channels, but exclude compounds such as deltamethrin and cypermethrin, which require activation for channel modification. A multi-state model would also be useful in understanding the differential state-specific modification of mammalian sodium channel isoforms by various pyrethroids.
A third area of recent progress involves the identification of a subset of mammalian sodium channel isoforms that are particularly sensitive to pyrethroid modification. The greater sensitivity of the rat Nav1.3 and Nav1.6 channels to pyrethroids may be particularly important to pyrethroid intoxication given their patterns of developmental and anatomical expression. The Nav1.3 isoform is preferentially expressed in the embryonic and early postnatal rodent CNS (Felts et al. 1997) and may therefore be an important target for developmental neurotoxic effects attributed to pyrethroids (Shafer et al. 2005). The Nav1.6 isoform is also expressed in the embryonic CNS (Felts et al. 1997), but it also is the most abundantly-expressed sodium channel α subunit in the adult brain (Auld et al. 1988) where it is preferentially expressed in regions of brain axons associated with action potential initiation (Hu et al. 2009). As such, the Nav1.6 isoform is not only a candidate target for developmental neurotoxic effects but also is a likely target for the central neurotoxic effects of pyrethroids in adult animals.
The fourth area of recent progress is the use of heterologous expression systems to examine directly the relative pyrethroid sensitivity of rat and human sodium channel orthologs. The unanticipated discovery of the substantial difference in pyrethroid sensitivity between rat and human Nav1.3 sodium channels, despite their extensive amino acid sequence conservation, resulted from the first comparative study of species differences in pyrethroid sensitivity. These results have important implications for understanding the value and limitations of toxicological studies in rats as the basis for assessing risk to humans. The Nav1.3 sodium channel isoform is abundantly expressed in the embryonic and neonatal CNS of rats but is much less abundant than the Nav1.1, Nav1.2 and Nav1.6 isoforms in the CNS of adult rats (Felts et al. 1997; Shah et al. 2001). The Nav1.3 isoform is also highly expressed in the embryonic human CNS (Thimmapaya et al. 2005) but, unlike in rats, is strongly expressed in some regions of the adult CNS (Thimmapaya et al. 2005; Whitaker et al. 2000; Whitaker et al. 2001). The high pyrethroid sensitivity of sodium channels formed from the rat Nav1.3 isoform suggests that this isoform might be a significant target for developmental neurotoxic effects of pyrethroids in rats (Meacham et al. 2008). The lower pyrethroid sensitivity of human Nav1.3 channels raises the possibility that the rat model may overestimate the sensitivity of the developing human CNS to pyrethroids.
There are two inherent shortcomings that may limit the ultimate validity of these studies. First, the majority of studies have relied on defined sodium channel complexes expressed in Xenopus oocytes. It will be important in the future to determine whether the pyrethroid pharmacology of channels defined in the oocyte system is conserved for channels in other cellular expression systems that better reflect the cellular environment of neurons. For insect channels there is currently no viable alternative to Xenopus oocytes for functional expression, so any comparative studies of insect and mammalian channels must be restricted to that system. Second, effects on sodium channels have been characterized exclusively at the level of isolated sodium currents under voltage clamp conditions. It remains important to correlate the effects on isolated sodium currents with electrical excitability of cells to assess the functional consequences of pyrethroid intoxication.
It is likely that most if not all of the acute neurotoxic effects of pyrethroids result from actions on voltage-gated sodium channels. Among the structural subclasses of pyrethroids, qualitative differences in sodium channel modification are generally correlated with the production of different intoxication syndromes (Soderlund et al. 2002). Most Type I compounds, which induce burst discharges in invertebrate neurons following a single stimulus and rapidly-decaying sodium tail currents under voltage clamp conditions, produce the T syndrome of intoxication in rodents. Most Type II compounds, which cause use-dependent nerve block resulting from a degradation of the resting membrane potential in invertebrate neurons and long-lived sodium tail currents under voltage-clamp conditions, produce the CS syndrome of intoxication. A small number of compounds from both the Type I and Type II structural classes produce intermediate effects on neuronal excitability and tail current decay and also produce signs of intoxication that include elements of both the T and CS syndromes. Recent voltage clamp studies with insect and mammalian channels of defined subunit structure identify molecular determinants of differential sensitivity between mammalian isoforms and between mammalian and insect channels but are otherwise completely consistent with the results of prior experiments with native neurons. The possibility that different groups of pyrethroids may bind preferentially to either resting or open sodium channels suggests another way in which qualitatively different types of sodium channel modification can arise from molecular interaction with a single target. It remains to be determined whether state-dependent binding is strongly correlated with the Type I/Type II structural classes of pyrethroids and the toxicological outcomes identified as the T and CS intoxication syndromes.
Voltage-gated calcium channels
Research published during the past decade provides additional evidence for the ability of pyrethroids to modify the function of voltage-gated calcium channels both in their native environment and in heterologous expression systems. However, these results have not resolved longstanding inconsistencies regarding the nature and significance of these effects. Recent studies using synaptosomes show direct effects of pyrethroids on depolarization-evoked calcium influx and neurotransmitter release that are independent of actions on sodium channels in the same preparation. However, studies with cultured neurons show that pyrethroid-dependent calcium influx is a secondary consequence of actions on sodium channels. It remains unclear whether pyrethroid actions on calcium channels in synaptosomal assays are meaningful under circumstances in which direct effects of sodium channels in the same preparations are not blocked.
Recent studies also provide additional evidence for the direct effects of pyrethroids on isolated calcium currents under voltage clamp conditions. The most commonly observed effect of pyrethroids in these assays is inhibition of calcium channels, which is not consistent with the hypothesis that direct effects on nerve terminal calcium channels enhance calcium uptake and neurotransmitter release. However, two recent findings provide some evidence for calcium channel activation. First, in PC12 cells pyrethroids selectively activate a current attributed to L-type channels but block the current attributed to N-type channels in the same cell type. Second, studies with Cav2.2, an N-type calcium channel isoform, in Xenopus oocytes indicate that pyrethroid modification may result either in inhibition or activation depending on the phosphorylation state of the channel.
Despite the results of these new studies, the toxicological significance of effects of pyrethroids on calcium channels remains unresolved. There is no clear connection between the effects observed on calcium channels in vitro and pyrethroid intoxication, either at the level of the intact nerve or the whole animal. Moreover, there are no effects of pyrethroids, either in vitro or in vivo, that cannot plausibly be connected to actions on sodium channels. Thus, whereas effects on calcium channels may contribute in some way to the action of at least some pyrethroids, there is no basis at present to identify effects on calcium channels as an essential mechanism of pyrethroid intoxication.
Chloride channels
The two principal findings reported during the past decade have significantly altered both our understanding of the actions of pyrethroids on chloride channels and the toxicological significance of those actions. First, the expanded evaluation of pyrethroids as inhibitors of neuronal voltage-gated chloride channels failed to confirm an obligatory involvement of chloride channel inhibition in the CS intoxication syndrome. This result highlights the hazard of limiting experimental investigations to individual compounds (such as cismethrin and deltamethrin) as representatives of groups of compounds and then extrapolating the results of such studies to the entire group. Despite the lack of evidence that chloride channel inhibition is a component of the CS syndrome, actions at this target may still contribute to the neurotoxic actions of some pyrethroids.
Second, the evidence for the selective modulation of cardiac chloride conductances by pyrethroids identifies an entirely new set of actions of these insecticides on chloride channels. These effects include both activation and inhibition of distinct chloride conductances, in at least one instance by the same pyrethroid. The selective effects of pyrethroids on chloride transport mechanisms suggests that they may be valuable probes for the pharmacological dissection of these processes, but there is no evidence at present that any of these effects on cardiac chloride conductances play a role in pyrethroid intoxication.
Conclusions
In the three decades since the introduction of the first compounds with sufficient photostability for agricultural use, synthetic pyrethroids have become important pest management tools in agriculture, public health, and a variety of household applications. Pyrethroids are neurotoxic not only to insects but also to mammals. For most pyrethroids, acute toxicity is low to moderate and is limited by inefficient absorption by some routes and by rapid metabolic detoxication. However, the intrinsic toxicity of pyrethroids, which is observed upon direct administration to the CNS, is significant. Structural subclasses of pyrethroids produce two distinct syndromes of acute intoxication, which have complicated efforts to forge causal connections between effects on neuronal target sites in vitro and the physiological consequences of poisoning in vivo.
The insecticidal properties of pyrethroids derive from their ability to alter the function of voltage-gated sodium channels in insect neuronal membranes, thereby disrupting electrical signaling in the nervous system. Although the discovery and development of pyrethroids involved the optimization of insecticidal activity using whole insects, optimization for high intrinsic activity on insect sodium channels was implicit throughout this process. The structure, function and pharmacology of voltage-gated sodium channels are highly conserved between insects and mammals. Therefore, it is not surprising that pyrethroids also alter the function of mammalian sodium channels. For the Type I and Type II structural subclasses of pyrethroids, qualitative differences in sodium channel modification are generally correlated with the production of different intoxication syndromes, suggesting that actions on sodium channels are sufficient to account for the acute toxicity of this insecticide class.
There is broad agreement that voltage-gated sodium channels are the primary target sites for the neurotoxic actions of pyrethroids, but there is less agreement regarding the identity and toxicological significance of other targets that may be involved in pyrethroid intoxication in mammals. The relevance of alternative or secondary targets has been typically proposed as a means of accounting for the unique intoxication signs caused by the majority of Type II pyrethroids. Pyrethroids also are known to affect a variety of voltage- and ligand-gated ion channels, but some of these effects were subsequently shown to have little relevance to intoxication (Soderlund et al. 2002). Among these alternative targets, only voltage-gated calcium and chloride channels appeared to have potential relevance to pyrethroid intoxication. Research during the past decade has provided new information on the action of pyrethroids on calcium channels but has failed to identify effects that are essential to explain intoxication. During the same period, research on the action of pyrethroids on neuronal voltage-gated chloride channels has provided strong evidence against an obligatory role for these targets in the CS syndrome of intoxication. Thus, the potential significance of secondary targets in pyrethroid intoxication remains to be established.
Acknowledgments
Research that was conducted in the author’s laboratory and the preparation of this review were supported by grants from National Institute of Environmental Health Sciences, National Institutes of Health (R01-ES08962 and R01-ES013686) and the Pyrethroid Working Group, a consortium of firms (Bayer CropScience, DuPont Crop Protection, FMC Corporation, Syngenta Crop Protection Inc., and Valent Corporation) that market pyrethroid-based insecticide products in the United States. The contents of this paper are solely the responsibility of the author and do not necessarily represent the official views of any of the sponsors.
References
- Allen SL. Regulatory aspects of neurotoxicity assessment. In: Krieger RI, editor. Hayes’ Handbook of Pesticide Toxicology. 3. Vol. 1. Elsevier; New York: 2010. pp. 587–602. [Google Scholar]
- Alves A-M, Symington SB, Lee SH, Clark JM. PKC-dependent phosphorylations modify the action of deltamethrin on rat brain N-type (Cav2.2) voltage-sensitive calcium channel. Pestic Biochem Physiol. 2010;97:101–108. [Google Scholar]
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel α subunit with novel gating properties. Neuron. 1988;1:449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Verschoyle RD. Toxicity of new pyrethroid insecticide. Nature. 1974;248:711. doi: 10.1038/248711a0. [DOI] [PubMed] [Google Scholar]
- Borg JJ, Hancox JC, Spencer CI, Kozlowski RZ. Tefluthrin modulates a novel anionic background conductance (IAB) in guinea-pig ventricular myocytes. Biochem Biophys Res Commun. 2002;292:208–215. doi: 10.1006/bbrc.2002.6631. [DOI] [PubMed] [Google Scholar]
- Borg JJ, Hancox JC, Zhang H, Spencer CI, Li H, Kozlowski RZ. Differential pharmacology of the cardiac anionic background current IAB. Eur J Pharmacol. 2007;569:163–170. doi: 10.1016/j.ejphar.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Breckenridge CB, Holden L, Sturgess N, Weiner M, Sheets L, Sargent D, Soderlund DM, Choi J-S, Symington S, Clark JM, Burr S, Ray D. Evidence for a separate mechanism of toxicity for the Type I and Type II pyrethroid insecticides. Neurotoxicology. 2009;30:S17–S31. doi: 10.1016/j.neuro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Brown LD, Narahashi T. Modulation of nerve membrane sodium channel activation by deltamethrin. Brain Res. 1992;584:71–76. doi: 10.1016/0006-8993(92)90879-e. [DOI] [PubMed] [Google Scholar]
- Burr SE, Ray DE. Structure-activity and interaction effects of 14 different pyrethroids on voltage-gated chloride ion channels. Toxicol Sci. 2004;77:341–346. doi: 10.1093/toxsci/kfh027. [DOI] [PubMed] [Google Scholar]
- Cao Z, Shafer TJ, Murray TF. Mechanisms of pyrethroid insecticide-induced stimulation of calcium influx into neocortical neurons. J Pharmacol Exp Ther. 2011;336:197–205. doi: 10.1124/jpet.110.171850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Choi J-S, Soderlund DM. Actions of cismethrin, deltamethrin and cyclosporin A on Nav1.8 sodium channels expressed in Xenopus oocytes. Soc Neurosci Abst. 2002;28:743.720. doi: 10.1016/j.neulet.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Choi J-S, Soderlund DM. Cylcosporin A and deltamethrin block the downregulation of Na v1.8 sodium channels expressed in Xenopus oocytes. Neurosci Lett. 2004;367:389–393. doi: 10.1016/j.neulet.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Choi J-S, Soderlund DM. Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Nav1.8 sodium channels expressed in Xenopus oocytes. Toxicol Appl Pharmacol. 2006;211:233–244. doi: 10.1016/j.taap.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Culliford SJ, Borg JJ, O’Brien MJ, Kozlowski RZ. Differential effects of pyrethroids on volume-sensitive anion and organic osmolyte pathways. Clinical and Experimental Pharmacology and Physiology. 2004;31:134–144. doi: 10.1111/j.1440-1681.2004.03965.x. [DOI] [PubMed] [Google Scholar]
- de Weille JR, Vijverberg HPM, Narahashi T. Interactions of pyrethroids and octylguanidine with sodium channels of squid giant axons. Brain Res. 1988;445:1–11. doi: 10.1016/0006-8993(88)91067-0. [DOI] [PubMed] [Google Scholar]
- Dong K. Insect sodium channels and insecticide resistance. Invert Neurosci. 2007;7:17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Lee J-E, Nomura Y, Zhang T, Zhorov B, Dong K. Identification of a cluster of residues in transmembrane 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides. Biochem J. 2009;419:377–385. doi: 10.1042/BJ20082082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M, Farnham AW, Janes NF, Needham PH, Pulman DA. Synthetic insecticide with a new order of activity. Nature. 1974;248:710–711. doi: 10.1038/248710a0. [DOI] [PubMed] [Google Scholar]
- Felts PA, Yokoyama S, Dib-Hajj S, Black JA, Waxman SG. Sodium channel α-subunit mRNAs I, II, III, NaG, Na6 and hNE (PN1): different expression patters in developing rat nervous system. Mol Brain Res. 1997;45:71–82. doi: 10.1016/s0169-328x(96)00241-0. [DOI] [PubMed] [Google Scholar]
- Gammon DW, Brown MA, Casida JE. Two classes of pyrethroid action in the cockroach. Pestic Biochem Physiol. 1981;15:181–191. [Google Scholar]
- Ginsburg KS, Narahashi T. Differential sensitivity of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels to the insecticide allethrin in rat dorsal root ganglion neurons. Brain Res. 1993;627:239–248. doi: 10.1016/0006-8993(93)90326-i. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Mechanisms of sodium channel inactivation. Curr Opin Neurobiol. 2003;13:284–290. doi: 10.1016/s0959-4388(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Hildebrand ME, McRory JE, Snutch TP, Stea A. Mammalian voltage-gated calcium channels are potently blocked by the pyrethroid insecticide allethrin. J Pharmacol Exp Ther. 2004;308:805–813. doi: 10.1124/jpet.103.058792. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor interaction. J Gen Physiol. 1977;67:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Yang M, Hou H, Shu Y. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nature Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Stein VFW, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2001;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Kadala A, Charreton M, Jakob I, Le Conte Y, Collet C. A use-dependent sodium current modification induced by type I pyrethroid insecticides in honeybee antennal olfactory neurons. Neurotoxicology. 2011;32:320–330. doi: 10.1016/j.neuro.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Lawrence LJ, Casida JE. Pyrethroid toxicology: mouse intracerebral structure-toxicity relationships. Pestic Biochem Physiol. 1982;18:9–14. [Google Scholar]
- Lee SH, Soderlund DM. The V410M mutation associated with pyrethroid resistance in Heliothis virescens reduces the pyrethroid sensitivity of house fly sodium channels expressed in Xenopus oocytes. Insect Biochem Mol Biol. 2001;31:19–29. doi: 10.1016/s0965-1748(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang H, Hancox JC, Kozlowski RZ. An outwardly rectifying anionic background current in atrial myocytes from the human heart. Biochem Biophys Res Commun. 2007;359:765–770. doi: 10.1016/j.bbrc.2007.05.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. KcsA crystal structure as framework for a molecular model of the Na+ channel pore. Biochemistry. 2000;39:8161–8170. doi: 10.1021/bi000486w. [DOI] [PubMed] [Google Scholar]
- Lombet A, Mourre C, Lazdunski M. Interactions of insecticides of the pyrethroid family with specific binding sites on the voltage-dependent sodium channel from mammalian brain. Brain Res. 1988;459:44–53. doi: 10.1016/0006-8993(88)90284-3. [DOI] [PubMed] [Google Scholar]
- Maritzen T, Blanz J, Jentsch T. Physiological functions of the CLC chloride transport proteins. Adv Mol Cell Biol. 2006;38:9–57. [Google Scholar]
- Meacham CA, Brodfuehrer PD, Watkins JA, Shafer TJ. Developmentally-regulated sodium channel subunits are differentially sensitive to α-cyano containing pyrethroids. Toxicol Appl Pharmacol. 2008:273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc Res. 2005;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Naeher LP, Tulve NS, Egeghy PP, Barr DB, Adetona O, Fortmann RC, Needham LL, Bozeman E, Hilliard A, Sheldon LS. Organophosphorus and pyrethroid insecticide urinary metabolite concentrations in young children living in a southeastern United States city. Sci Total Environ. 2010;408:1145–1153. doi: 10.1016/j.scitotenv.2009.10.022. [DOI] [PubMed] [Google Scholar]
- O’Reilly AO, Khambay BPS, Williamson MS, Field LM, Wallace BA, Davies TGE. Modelling insecticide binding sites at the voltage-gated sodium channel. Biochem J. 2006;396:255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrea EM, Jr, Bielawski DM, Poecion NC, Jr, Corrion M, Villaneuva-Uy E, Bernardo RC, Jin Y, Janisse JL, Ager JW. Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair, cord blood and meconium) matrices to detect fetal exposure of environmental pesticides. Environ Res. 2009;109:116–122. doi: 10.1016/j.envres.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett JA. New opportunities in neuroscience, but a great danger that some may be lost. In: Beadle DJ, Mellor IR, Usherwood PNR, editors. Neurotox ’03: Neurotoxicological targets from functional genomics and proteomics. Society of Chemical Industry; London: 2004. pp. 1–10. [Google Scholar]
- Power LE, Sudakin DL. Pyrethrin and pyrethroid exposures in the United States: a longitudinal analysis of incidents reported to poison centers. J Med Toxicol. 2007;3:94–99. doi: 10.1007/BF03160917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheles mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their role in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado VL, Narahashi T. Immobilization of sodium channel gating charge in crayfish giant axons by the insecticide fenvalerate. Mol Pharmacol. 1993;43:626–634. [PubMed] [Google Scholar]
- Sardini A. Cell volume homeostasis: the role of volume-sensitive chloride channels. Adv Mol Cell Biol. 2006;38:199–214. [Google Scholar]
- Shafer TJ, Meyer DA. Effects of pyrethroids on voltage-sensitive calcium channels: a critical evaluation of strengths, weaknesses, data needs, and relationship to assessment of cumulative neurotoxicity. Toxicol Appl Pharmacol. 2004;196:303–318. doi: 10.1016/j.taap.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspec. 2005;113:123–136. doi: 10.1289/ehp.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BS, Stevens EB, Pinnock RD, Dixon AK, Lee K. Developmental expression of the novel voltage-gated sodium channel auxiliary subunit 3, in rat CNS. J Physiol. 2001;534:763–776. doi: 10.1111/j.1469-7793.2001.t01-1-00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava IH, Durell SR, Guy HR. A model of voltage gating developed using the KvAP channel crystal structure. Biophys J. 2004;87:2255–2270. doi: 10.1529/biophysj.104.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Ingles PJ, Soderlund DM. Actions of the pyrethroid insecticides cismethrin and cypermethrin on house fly Vssc1 sodium channels expressed in Xenopus oocytes. Arch Insect Biochem Physiol. 1998;38:126–136. doi: 10.1002/(SICI)1520-6327(1998)38:3<126::AID-ARCH3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Lee SH, Ingles PJ, Knipple DC, Soderlund DM. The L1014F point mutation in the house fly Vssc1 sodium channel confers knockdown resistance to pyrethroids. Insect Biochem Mol Biol. 1997;27:807–812. doi: 10.1016/s0965-1748(97)00065-9. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. NeuroToxicology. 1998;19:823–832. [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Potent actions of the pyrethroid insecticides cismethrin and cypermethrin on rat tetrodotoxin-resistant peripheral nerve (SNS/PN3) sodium channels expressed in Xenopus oocytes. Pestic Biochem Physiol. 2001;70:52–61. doi: 10.1002/(SICI)1520-6327(1998)38:3<126::AID-ARCH3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Soderlund DM. Sodium channels. In: Gilbert L, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. Vol. 5. Elsevier; New York: 2005. pp. 1–24. [Google Scholar]
- Soderlund DM. Pyrethroids, knockdown resistance and sodium channels. Pest Manag Sci. 2008;64:610–616. doi: 10.1002/ps.1574. [DOI] [PubMed] [Google Scholar]
- Soderlund DM. State-dependent modification of voltage-gated sodium channels by pyrethroids. Pestic Biochem Physiol. 2010a;97:78–86. doi: 10.1016/j.pestbp.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Toxicology and mode of action of pyrethroid insecticides. In: Krieger RI, editor. Hayes’ Handbook of Pesticide Toxicology. 3. Vol. 2. Elsevier; New York: 2010b. pp. 1665–1686. [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Mechanisms of pyrethroid toxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Lee SH. Point mutations in homology domain II modify the sensitivity of rat Na v1.8 sodium channels to the pyrethroid cismethrin. Neurotoxicology. 2001;22:755–765. doi: 10.1016/s0161-813x(01)00065-1. [DOI] [PubMed] [Google Scholar]
- Song J-H, Nagata K, Tatebayashi H, Narahashi T. Interactions of tetramethrin, fenvalerate and DDT at the sodium channel in rat dorsal root ganglion neurons. Brain Res. 1996;708:29–37. doi: 10.1016/0006-8993(95)01239-7. [DOI] [PubMed] [Google Scholar]
- Spencer CI, Sham JSK. Mechanisms underlying the effects of the pyrethroid tefluthrin on action potential durations in isolated rat ventricular myocytes. J Pharmacol Exp Ther. 2005;315:16–23. doi: 10.1124/jpet.105.084822. [DOI] [PubMed] [Google Scholar]
- Spencer CI, Yuill KH, Borg JJ, Hancox JC, Kozlowski RZ. Action of pyrethroid insecticides on sodium currents, action potentials, and contractile rhythm in isolated mammalian ventricular myocytes and perfused hearts. J Pharmacol Exp Ther. 2001;298:1067–1082. [PubMed] [Google Scholar]
- Symington SB, Clark JM. Action of deltamethrin on N-type (Cav2.2) voltage-sensitive calcium channels in rat brain. Pestic Biochem Physiol. 2005;82:1–15. [Google Scholar]
- Symington SB, Frisbie RK, Clark JM. Characterization of 11 commercial pyrethroids on the functional attributes of rat brain synaptosomes. Pestic Biochem Physiol. 2008;92:61–69. [Google Scholar]
- Symington SB, Frisbie RK, Kim H-J, Clark JM. Mutation of threonine-422 to glutamic acid mmics the phosphorylation state and alters the action of deltamethrin on Cav2.2. Pestic Biochem Physiol. 2007a;88:312–320. [Google Scholar]
- Symington SB, Frisbie RK, Lu KD, Clark JM. Action of cismethrin and deltamethrin on functional attributes of isolated presynaptic nerve terminals from rat brain. Pestic Biochem Physiol. 2007b;87:172–181. [Google Scholar]
- Symington SB, Hodgdon HE, Frisbie RK, Clark JM. Binary mixtures of pyrethroids produce differential effects on Ca2+ influx and glutamate release at isolated presynaptic nerve terminals from rat brain. Pestic Biochem Physiol. 2011;99:131–139. [Google Scholar]
- Tabarean IV, Narahashi T. Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the Type II pyrethroid deltamethrin. J Pharmacol Exp Ther. 1998;284:958–965. [PubMed] [Google Scholar]
- Tabarean IV, Narahashi T. Kinetics of modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by tetramethrin and deltamethrin. J Pharmacol Exp Ther. 2001;299:988–997. [PubMed] [Google Scholar]
- Tan J, Choi J-S, Soderlund DM. Action of pyrethroid insecticides on rat Nav1.6 sodium channels expressed in Xenopus oocytes. The Toxicologist. 2008;97 Abstract 2274. [Google Scholar]
- Tan J, Liu Z, Wang R, Huang ZY, Chen AC, Gurevitz M, Dong K. Identification of amino acid residues in the insect sodium channel critical for pyrethroid binding. Mol Pharmacol. 2005;67:513–522. doi: 10.1124/mol.104.006205. [DOI] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Human and rat Nav1.3 voltage-gated sodium channels differ in inactivation properties and sensitivity to the pyrethroid insecticide tefluthrin. Neurotoxicology. 2009;30:81–89. doi: 10.1016/j.neuro.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Nav1.6 sodium channels. Toxicol Appl Pharmacol. 2010;247:229–237. doi: 10.1016/j.taap.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Action of tefluthrin on rat Nav1.7 voltage-gated sodium channels expressed in Xenopus oocytes. Pestic Biochem Physiol. 2011 doi: 10.1016/j.pestbp.2011.06.001. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- Thimmapaya R, Neelands T, Niforatos W, Davis-Taber RA, Choi W, Putman CB, Kroeger PE, Packer J, Gopalakrishnan M, Faltynek CR, Surowy CS, Scott VE. Distribution and functional characterization of human Nav1.3 splice variants. Eur J Neurosci. 2005;22:1–9. doi: 10.1111/j.1460-9568.2005.04155.x. [DOI] [PubMed] [Google Scholar]
- Trainer VL, McPhee JC, Boutelet-Bochan H, Baker C, Scheuer T, Babin D, Demoute J-P, Guedin D, Catterall WA. High affinity binding of pyrethroids to the α subunit of brain sodium channels. Mol Pharmacol. 1997;51:651–657. doi: 10.1124/mol.51.4.651. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. Sodium channel inactivation: molecular determinants and modulation. Physiol Rev. 2005;85:1271–1301. doi: 10.1152/physrev.00024.2004. [DOI] [PubMed] [Google Scholar]
- Usherwood PNR, Davies TGE, Mellor IR, O’Reilly AO, Peng F, Vais H, Khambay BPS, Field LM, Williamson MS. Mutations in DIIS5 and the DIIS4-5 linker of Drosophila melanogaster sodium channel define binding domains for pyrethroids and DDT. FEBS Lett. 2007;581:5485–5492. doi: 10.1016/j.febslet.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Vais H, Atkinson S, Eldursi N, Devonshire AL, Williamson MS, Usherwood PNR. A single amino acid change makes a rat neuronal sodium channel highly sensitive to pyrethroid insecticides. FEBS Lett. 2000a;470:135–138. doi: 10.1016/s0014-5793(00)01305-3. [DOI] [PubMed] [Google Scholar]
- Vais H, Atkinson S, Pluteanu F, Goodson SJ, Devonshire AL, Williamson MS, Usherwood PNR. Mutations of the para sodium channel of Drosophila melanogaster identify putative binding sites for pyrethroids. Mol Pharmacol. 2003;64:914–922. doi: 10.1124/mol.64.4.914. [DOI] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Devonshire AL, Usherwood PNR. The molecular interactions of pyrethroid insecticides with insect and mammalian sodium channels. Pest Manag Sci. 2001;57:877–888. doi: 10.1002/ps.392. [DOI] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Goodson SJ, Devonshire AL, Warmke JW, Usherwood PNR, Cohen CJ. Activation of Drosophila sodium channels promotes modification by deltamethrin: reductions in affinity caused by knock-down resistance mutations. J Gen Physiol. 2000b;115:305–318. doi: 10.1085/jgp.115.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoyle RD, Aldridge WN. Structure-activity relationships of some pyrethroids in rats. Arch Toxicol. 1980;45:325–329. doi: 10.1007/BF00293813. [DOI] [PubMed] [Google Scholar]
- Verschoyle RD, Barnes JM. Toxicity of natural and synthetic pyrethrins to rats. Pestic Biochem Physiol. 1972;2:308–311. [Google Scholar]
- Wang S-Y, Barile M, Wang GK. A phenylalanine residue at segment D3-S6 in Nav1.4 voltage-gated Na+ channels is critical for pyrethroid action. Mol Pharmacol. 2001;60:620–628. [PubMed] [Google Scholar]
- Wang S-Y, Mitchell J, Moczydlowski E, Wang GK. Block of inactivation-deficient Na+ channels bo local anesthetics in stably transfected mammalian cells: evidence for drug binding along the activation pathway. J Gen Physiol. 2004;124:691–701. doi: 10.1085/jgp.200409128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ML, Nemec M, Sheets L, Sargent D, Breckenridge C. Comparative functional observational battery of twelve commercial pyrethroid insecticides in male rats following acute oral exposure. Neurotoxicology. 2009;30:S1–S16. doi: 10.1016/j.neuro.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Whitaker WRJ, Clare JJ, Powell AJ, Chen YH, Faull RLM, Emson PC. Distribution of voltage-gated sodium channel α-subunit and β-subunit mRNAs in human hippocampal formation, cortex, and cerebellum. J Comp Neurol. 2000;422:123–139. doi: 10.1002/(sici)1096-9861(20000619)422:1<123::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Whitaker WRJ, Faull RLM, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Mol Brain Res. 2001;88:37–53. doi: 10.1016/s0169-328x(00)00289-8. [DOI] [PubMed] [Google Scholar]
- Wu S-N, Wu Y-H, Chen B-S, Liu Y-C. Underlying mechanism of action of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology. 2009;2009:70–77. doi: 10.1016/j.tox.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yarov-Yarovoy V, Scheuer T, Catterall WA. A gating hinge in Na+ channels: a molecular switch for electrical signaling. Neuron. 2004;41:859–865. doi: 10.1016/s0896-6273(04)00116-3. [DOI] [PubMed] [Google Scholar]