Abstract
In a predominantly low-income population-based longitudinal sample of 1,292 children followed from birth, higher level of salivary cortisol assessed at ages 7, 15, and 24 months was uniquely associated with lower executive function ability and to a lesser extent IQ at age 3 years. Measures of positive and negative aspects of parenting and household risk were also uniquely related to both executive functions and IQ. The effect of positive parenting on executive functions was partially mediated through cortisol. Typical or resting level of cortisol was increased in African American relative to White participants. In combination with positive and negative parenting and household risk, cortisol mediated effects of income-to-need, maternal education, and African American ethnicity on child cognitive ability.
The effect of early experience on cognitive development (Ramey & Ramey, 1998) and on the development of the physiological response to stress (Gunnar & Quevedo, 2007) is well established. It is also well established that the physiological response to stress, as indicated by levels of neuroendocrine hormones, glucocorticoids and catecholamines, is related to distinct aspects of cognition, including declarative memory as well as executive functions (Arnsten, 2000; Diamond, Campbell, Park, Halonen, & Zoladz, 2007). No studies to our knowledge, however, have directly examined the extent to which the effects of early experience on stress physiology may mediate well-known effects of early experience on cognitive development.
Relations of early experience to stress physiology and to executive functions are of particular interest in that executive functions are cognitive abilities associated with prefrontal cortex (PFC), including working memory, inhibitory control, and attention shifting or flexibility, that enable the organization of information in goal-directed activities. Executive functions contribute substantially to the self-regulation of behavior (Carlson, Mandell, & Williams, 2004; Hughes & Ensor, 2007) and are central to early academic achievement (Blair & Razza, 2007). They are also a primary aspect of cognitive disability in a range of psychological disorders in children and adults (Zelazo & Muller, 2002).
In humans the link between stress hormone levels and executive functions has been demonstrated in naturalistic studies with preschool children (Blair, Granger, & Razza, 2005; Davis, Bruce, & Gunnar, 2002) and in pharmacological manipulations with adults (Alexander, Hillier, Smith, Tivarus, & Beversdorf, 2007; Lupien, Gillin, & Hauger, 1999). The association between executive functions and stress physiology in part reflects the fact that stress hormone levels modulate synaptic activity in the neural circuitry of PFC that underlies executive functions (Arnsten & Li, 2005; Mizoguchi, Ishige, Takeda, Aburada, & Tabira, 2004). Although by no means the only brain area and cognitive ability affected by stress hormones, PFC and executive functions are sensitive indicators of the effects of stress on development (Cerqueira, Mailliet, Almeida, Jay, & Sousa, 2007). Importantly, under conditions of ongoing or persistent stress, basal levels or set points of physiological stress response systems are altered either upward or downward, a phenomenon referred to as allostasis (McEwen, 2000). Stress physiology, including the hypothalamic-pituitary-adrenal (HPA) axis, is highly influenced by social interaction (Dickerson & Kemeny, 2004; Gunnar & Donzella, 2002) and conditions that are threatening, unpredictable, and lacking in support are associated with elevated levels of glucocorticoid hormones, namely cortisol. When stressful conditions are chronic or persistent, stress response systems are said to be under high allostatic load and adapt to the environment with over or under activation to an extent that impedes flexible regulation of stress physiology (McEwen, 1998, 2000), such as that associated with the self-regulation of behavior and executive functions (Ramos & Arnsten, 2007).
Poverty and child development
The environment of poverty is stressful for children and to date has been associated with increased levels of stress hormones (Evans, 2003). It is important to note that severe disruption of caregiving has been shown to result in under rather than over activation of stress response systems, as seen in altered diurnal variation in cortisol in children experiencing early caregiving adversity (Tarullo & Gunnar, 2006). In the far from optimal but essentially adequate (i.e., not extreme adversity) conditions of poverty, however, increases in stress physiology are typically observed (Lupien, King, Meaney, & McEwen, 200) and likely represent a pathway through which poverty affects child development (Repetti, Taylor, & Seaman, 2004). Recent quasi-experimental evidence of the relation of poverty to stress physiology in a Mexican sample indicated lower cortisol levels in preschool children in homes in participating in a conditional cash transfer program relative to a matched comparison group (Fernald & Gunnar, 2009).
An important question for child development research concerns sources of stress in children’s lives. Given that the HPA axis is under strong social regulation, parenting may act as a primary pathway through which poverty affects stress physiology in children. Research in animal models indicates early caregiving to be a primary influence on the development of the HPA axis (Champagne et al., 2008; Liu, Diorio, Day, Francis, & Meaney, 2000) and to adaptively shape offspring development to meet an expected environment (Cameron, Champagne, Parent, Fish, Ozaki-Kuroda, & Meaney, 2005). In humans, early sensitive parenting is associated with a well regulated stress physiology (Gunnar & Quevedo, 2007) and with higher level of general cognitive (Lugo-Gil & Tamis-Lemonda, 2008) and emotion regulation ability (Sroufe, 1996). Parenting is a primary mechanism through which poverty affects child development (Brody, Murry, Kim, & Brown, 2002; Gershoff, Aber, Raver, & Lennon, 2007; McLoyd, 1998) and likely mediates an association between poverty and elevated stress in young children. Such a mediating model for the association between poverty and the physiological response to stress, however, does not preclude the possibility that high quality parenting in the context of poverty might moderate effects of environmental stressors on children. As well, it does not indicate specific mechanisms through which caregiving is related to stress reactivity and regulation early in development (Tang, Akers, Reeb, Romeo, & McEwen, 2006).
Positive and negative dimensions of parenting
When examining parenting quality as a potential mediator for the effects of poverty on child stress physiology and cognitive development, it is important to consider both positive and negative dimensions of the construct. Although highly related, positive and negative dimensions of parenting can be expected to have distinct effects on child development. A positive, responsive and emotionally supportive parent provides an interactive environment for young children to engage in reciprocal verbal and nonverbal exchanges that are stimulating and rewarding (Tamis-LeMonda, Bornstein, & Baumwell, 2001). In contrast, negative and intrusive parenting focuses on the degree to which parents intrude on children’s interests and behaviors above and beyond the developmental or safety needs of the child and undermine autonomy and nascent attempts at self-regulation (Ispa, Fine, Halgunseth, Harper, Robinson, Boyce, Brooks-Gunn, & Brady-Smith, 2004). It is likely that both positive and negative aspects of parenting uniquely influence the development of stress physiology and early cognitive ability.
Parenting, stress physiology, and cognitive ability in children
Although relations between early care and cognitive development are well established, relatively little is known about relations between stress physiology and cognitive ability in early childhood. The primary goal of this study is to examine the longitudinal relation of what can be considered a resting or basal level of salivary cortisol measured across the child’s first two years to cognitive ability at age 3. Cortisol levels follow a pronounced circadian rhythm and are affected by various aspects of children’s experiences. However, by collecting saliva samples from children in the same way in a carefully planned data collection, usually by the same data collector at generally near the same time of day over the child’s first two years and statistically controlling for time of day in our analysis we are able to estimate a reasonably stable component of each child’s level of cortisol. No prior studies of which we are aware have examined longitudinal relations between cortisol and cognition in early childhood. Given that cognitive ability is associated with both early parenting behavior and aspects of stress physiology, it may be that some of the effect of parenting on cognition is mediated through the effect of parenting on stress physiology. Therefore, a second goal of this study is to examine the relation of early parenting to cortisol and cognitive ability, particularly executive functions but also intelligence, and to determine whether cortisol mediates some of the effect of parenting on cognitive ability. Although executive functions are largely distinct from general intelligence (Blair, 2006), the executive function of working memory has been shown to be highly related to general mental ability in a number of studies with adults (Friedman, Miyake, Corley, Young, Defries, & Hewitt, 2006). The relation between executive functions and intelligence is not well studied in children but available evidence indicates that the development of working memory ability underlies the development of the aspect of intelligence referred to as fluid intelligence (Fry & Hale, 1996; Kail, 2007), which is important for reasoning ability and the processing of novel information and is itself highly related to general intelligence (Carroll, 1993).
Finally, given well documented effects of poverty on child cognitive development (Bradley & Corwyn, 2001), a third goal of this analysis is to examine whehter effects of poverty on child cognitive ability are mediated through positive and negative parenting and child cortisol. We expect that positive and negative aspects of parenting are mediators of the effect of poverty, as indicated by income-to-need and maternal education, on child outcomes. Parenting, however, is only one possible route through which poverty affects stress physiology and cognitive ability. Therefore, we include measures of household crowding or density (number of persons/number of rooms) and data collectors’ ratings of the safety and noise level of the home and the area around the home to determine the extent to which these characteristics of the home may account for relations among poverty, stress physiology, and child cognitive ability.
Furthermore, most if not all prior studies of poverty and stress physiology have been conducted with white low-income samples. We addressed this aspect of the literature by examining the relation of poverty to cortisol and child development in a sample of African American as well as white participants in two geographically distinct regions of high poverty in the U.S. Prior analyses conducted with longitudinal samples have demonstrated that risk processes in the context of poverty work similarly in African American and white families (Conger, Wallace, Sun, Simon, McLoyd, & Brody, 2002; Raver, Gershoff, & Aber, 2007). Given the overrepresentation of African American families in deep and persistent poverty in the U.S. (McLoyd, 1998), however, we expect that African American ethnicity in this sample will serve as a marker for a number of unmeasured aspects of risk associated with socioeconomic disadvantage and social inequality.
In sum, we examine the extent to which cortisol and parenting measured in infancy and toddlerhood account for relations of poverty indicators to child cognitive ability at age 3 years. A prior analysis of the sample reported on here demonstrated that high level of positive but not negative parenting in infancy is associated with lower basal levels of cortisol and greater cortisol reactivity to emotional arousal at age 7 and 15 months (Blair et al., 2008). In that prior analysis we also found that typical or resting level of cortisol was increased for African American children, likely reflecting conditions of increased risk associated with deep poverty. This report expands on these findings to include measurement of cortisol at at child age 24 months. The focus in this report, however, is on typical level for cortisol adjusted for time of day of saliva collection over the child’s first two years rather than cortisol reactivity at each time point. We expected that positive parenting would be associated with lower level of cortisol, adjusted for time of day of saliva collection, and negative parenting would be associated with higher level of cortisol. Further, we expected that cortisol level would account for significant variance in both executive functions and IQ and partially mediate effects of positive and negative parenting on both aspects of cognitive ability. Additionally, given established relations between cortisol and executive functions, we expected that the relation of cortisol to executive functions would be greater than that for IQ. Finally, we expected that effects of parenting and cortisol levels on executive functions would mediate effects of poverty on cognitive ability.
Method
Participants
Recruitment
Complex sampling procedures were used to recruit a representative sample of 1,292 families in two regions of the U.S. at the time that mothers gave birth to a child. Low-income families in both regions and African American families in one region were over-sampled. African-American families were not over-sampled in the second region as the target communities were 95+% Caucasian. Further details on the sampling plan and recruitment procedures are available in Burchinal, Vernon-Feagans, Cox and the FLP Investigators (2008b). Based on the mothers’ ethnic status, the sample was 58% Caucasian and 42% African American and 66.6% of the sample had an income-to-need ratio less than 200% of poverty. Just over half of the mothers were not married (51.9%) at the time the study began and the majority (88.8%) of single mothers had never been married.
Procedures
Families were seen in home visits at child ages of approximately 7, 15, 24, and 36 months. At all time points except 15 months, families were seen in two separate visits. All home visits for data collection were two or more hours in duration. During visits for data collection conducted at 7, 15, and 24 months, mothers completed questionnaires concerning family demographics, income, and child temperament, and engaged in a free play interaction (at 7 and 15 months) and an interactive puzzle completion task (at 24 months) with their child that was recorded with digital video for 10 minutes. During the free play interaction mothers were given a standard set of toys and instructed to play with the child as they normally would if they had a little free time during the day. During the puzzle completion task, children were presented with 3 consecutive board puzzles that increased in difficulty. Mothers were instructed to interact and help their children with the puzzles as they saw necessary.
Near the conclusion of the home visit for data collection at 7, 15, and 24 months (usually the second visit at 7 months, usually the first visit at 24 months), at which time the data collectors had been in the home for at least one hour, children were presented with emotion challenge tasks designed to elicit emotional responding, including a mask presentation, barrier task, and arm restraint at 7 months, and a toy removal and mask presentation at 15 and 24 months. All procedures have been previously validated (Stifter & Braungart, 1995). To assess basal levels of cortisol and cortisol response to the emotion arousal, unstimulated whole saliva was collected using either cotton or hydrocellulose absorbent material and expressing sample into 2 ml cryogenic storage vials using a needleless syringe (cotton) or by centrifugation (hydrocellulose). Two prior studies have indicated no differences in cortisol concentrations associated with the two collection techniques (Granger, Kivlighan, Fortunato, Harmon, Hibel, Schwartz & Whembolua, 2007; Harmon, Granger, Hibel & Rumyantseva, 2007). Saliva was collected at baseline prior to the administration of the emotion challenge procedures and at 20 and 40 minutes post peak emotional arousal following exposure to the procedures. For this analysis, only the baseline cortisol measures adjusted for time of day of collection were used.
The characteristics of the sample, repeated interview schedule, length of each interview protocol (2–4 hours), and age of the infants required that in-home assessments were scheduled when families were available. Therefore, time of the day of the interview and saliva collection varied. Mean time of day of saliva sample collection was 13:04 hours (SD = 2.88) at age 7 months, 13:45 hours (SD = 2.94) at 15 months, and was 13:33 hours (SD = 3.20) at 24 months. Time of day of sample collection was moderately correlated between time points, r7,15 = .23, r15,24 = .21. Collection of saliva always occurred near the end of the home visit for data collection. After collection, samples were immediately placed on ice, transported to interviewers homes and frozen (−20 °C). They were stored frozen until batched and shipped on dry-ice overnight to the Behavioral Endocrinology Laboratory at Penn State. Samples were then stored frozen at−80 ° C until assay. On the day of testing, samples were brought to room temperature, centrifuged at 3,000 RPM for 15 minutes, and the clear top-phase of the sample was pipetted into appropriate test wells by robot (Genesis, Tecan).
At approximately 36 months of age, children were administered tasks to assess executive functions and IQ. Children were seated across from the experimenter at a convenient location in the home. All tasks were administered in a standard order. The executive function tasks were administered at the conclusion of an assessment session in which children also completed a series of tasks with the mother that included a picture book reading task, an empathy task, and a puzzle task. Cumulatively, these tasks took about one hour to complete.
Measures
Executive function was assessed with three tasks modeled on tasks previously used successfully with young children. Full details on the executive function tasks are available in Willoughby, Blair, Wirth, & Greenberg (2010). In the span-like working memory task, children are presented with a line drawing of an animal figure above which is a color dot. Both the animal and color dot are located within the outline of a house. After establishing that the child knows the required colors and animals, the examiner asks the child to name the animal and then to name the color. The examiner then flips a page containing only the outline of the house. The examiner then asks the child which animal was/is/lives in the house. Percent correct responding on one 1-item trial, two 2-item trials, and two 3-item trials was used for analysis.
The Item Selection attention shifting task is modeled on the Flexible Item Selection Task developed by Jacques and Zelazo (2001). In the version of the task developed for flipbook administration, children are first presented with a page on which there are two line drawn items that are identical in terms of shape, size or color. The examiner draws the child’s attention to the dimension along which the items are identical then flips a page which presents the same two items again, to the right of which is a dashed vertical line and a picture of a third item. When presenting the new, third item to the child the examiner states, “See, here is a new picture. The new picture is the same as one of these two pictures. Show me which of these two pictures is the same as this new picture?” Percent correct responding on 14 trials was used for analysis. This task is preceded by a pretest in which children demonstrate knowledge of color, shape, and size.
The Spatial Conflict inhibitory control task is a Simon task similar to that used by Diamond et al. (2007) in which children alternate same-side and opposite side responding to line drawings of a toy car and a toy boat. A picture of the car is placed in front of the child on the right and a picture of the boat is placed in front of the child on the left. The examiner then flips pages on which are printed pictures of the car or the boat in either the same side or the opposite side position. Children are presented with 16 same side trials and then are presented with 16 intermixed same side and opposite side trials. Percent correct responding on opposite side trials was used for analysis.
Children were also administered a go no-go task in which they were asked to selectively withhold responding to a specific stimulus and a Stroop like task which required the inhibition of a prepotent response. Rates of completion on these latter tasks were too low to warrant inclusion. As is standard for executive function measures with children (Zelazo, 2006), children were required to successfully complete pretest trials for all tasks in which they clearly demonstrated knowledge of the rules for the task and the ability to successfully complete the pretest trials as instructed. Children were also required to complete 75% of test trials in a given task in order to receive a score for that task. Of 1,105 children administered the executive function tasks, 764 successfully completed the working memory span task, 795 successfully completed the attention flexibility task, and 866 successfully completed the spatial conflict inhibitory control task. For the go no-go and the Stroop-like tasks, only 465 and 497 children met criteria for completion. All tasks were scored as percent correct responding: working memory, M=.27, SD=.25; attention shifting, M=.52, SD=.24; inhibitory control M=.66, SD=.26. Scores were moderately correlated (r = .22 – .32, p < .001).
Intelligence
The receptive verbal ability and block design subscales of the Wechsler Preschool and Primary Scales of Intelligence (WPPSI; Wechsler, 2002) were used to assess child intelligence at age 36 months.
Income-to-need was calculated as the estimated total household income divided by the federal poverty threshold for 2005 adjusted for number of persons in the home. Income-to-need was highly correlated across time points (r = .80, p < .0001) and averaged at child ages 7, 15, and 24 months to create a single indicator.
Salivary cortisol
All samples were assayed for salivary cortisol using a highly-sensitive enzyme immunoassay US FDA 510k cleared for use as an in vitro diagnostic measure of adrenal function (Salimetrics, State College, PA). The test used 25 μl of saliva for singlet determinations, had a range of sensitivity from .007 to 1.8 μg/dl, and average intra-and inter-assay coefficients of variation of less than 10% and 15%. All samples were assayed in duplicate. The criterion for repeat testing was variation between duplicates greater than 20%, and the average of the duplicates was used in all analyses. The cortisol distributions were subject to log transformation to correct positive skew. Outliers greater than 3 standard deviations from the mean were treated as missing (n = 15, 16, and 17 at 7, 15, and 24 months.) Time of day of saliva collection was significantly related to cortisol level at each time point, r = −.25, −.19, −.32, all p < .01 at 7, 15, and 24 months. We also examined child temperature, time since eating, time since sleeping, and use of medications (e.g., acetaminophen) as influences on child cortisol levels at 7 and 24 months (data not available at 15 months.) Small significant relations of time since eating and time since sleeping with cortisol at 7 months were accounted for by adjustment for time of day of saliva collection.
Parenting
Mother-child interactions in the free play at 7 and 15 months and in the structured interaction at 24 months were coded to assess levels of mothers’ sensitivity, detachment, intrusiveness, positive regard, negative regard, and animation in interacting with the child (Cox, Paley, Burchinal, & Payne, 1999; NICHD ECCRN, 1999). Ratings for each code were made on a 1–5 scale at 7 and 15 months and a 1–7 scale at 24 months, with one being not at all characteristic and five (or seven) being highly characteristic. Factor analyses conducted with an oblique rotation (i.e., Promax) at each time point indicated distinct positive and negative dimensions of parenting. Maternal positive parenting included five maternal characteristics: sensitivity, detachment (reverse-scored), positive regard (e.g., positive feelings expressed toward child), animation (level of energy), and stimulation for development (appropriate level of scaffolding of activities with child). Maternal negative parenting included two maternal characteristics: intrusiveness and negative regard (level of harsh, negative feelings expressed toward child). Inter-rater reliability was determined by calculating the intra-class correlation (ICC) for ratings made by two coders to approximately 30% of the tapes randomly drawn at the infant and toddler assessments. ICCs were .85 – .91 for positive parenting and .72 –.86 across 7, 15, and 24 month assessments.
Household risk characteristics
Information on the number of persons residing in the home was obtained from the primary caregiver in response to a structured questionnaire. Information on the number of rooms in the home and safety and noise level of the home and neighborhood were obtained from data collector ratings completed at the conclusion of data collection in the home at 7 and 24 months. Density was calculated by dividing the number of persons in the home by the number of rooms. Safety and noise level ratings were combined to create an overall rating ranging from 1 (very unsafe/very noisy) to 4 (very safe/quiet).
Data analysis
Total sample size recruited at study entry was 1,292 with 1,204 children seen at age 7 months, 1,169 at 15 months, 1,144 at 24 months, and 1,123 at 36 months. To assess possible differential attrition in the sample at each time point we examined a number of variables for which we had complete information collected at child age of approximately 2 months. Few variables indicated differences between families who were present and those who were missing at each time point. Complete information on missing data is available from the first author upon request. To avoid bias in estimates associated with listwise deletion we used full information maximum likelihood estimation for all analyses. Structural equation models were estimated using Mplus 5.1 and tests of mediation were conducted using MacKinnon’s (2008) conceptualization of mediation in which indirect effects involve Sobel tests in order to evaluate the statistical significance of the product of coefficients linking the focal to the outcome variable through the mediating variable.
Results
Preliminary analysis
Table 1 presents means and standard deviations and Table 2 correlation among the variables in the analysis. The measure of executive function is the mean percent correct responding on the operation span, spatial conflict, and dimensional set shifting tasks. IQ is the full scale estimate derived from the WPPSI Block Design and Vocabulary subtests. These are presented for descriptive purposes here and are examined as latent variables below. Log transformed cortisol measures are adjusted for time of day. The table indicates that both executive function and IQ at 36 months have small negative correlations with cortisol at 7, 15, and 24 months. The measures of parenting at child ages 7, 15, and 24 months, particularly positive parenting, have small correlations with cortisol at most time points. Positive and negative parenting are moderately correlated with executive function and IQ. Maternal education, family income, and household density and safety are moderately correlated with parenting and with the cognitive measures. Executive function and IQ are moderately correlated.
Table 1.
Descriptive Statistics f or Variables in the Analysis
| N | Mean | SD | Range | |
|---|---|---|---|---|
| Cortisol 7mos (log μg/dl) | 1106 | −1.88 | .69 | −3.9 – .22 |
| Cortisol 15mos (log μg/dl) | 991 | −1.99 | .76 | −4.3 – .49 |
| Cortisol 24mos (log μg/dl) | 939 | −2.08 | .73 | −4.4 – .25 |
| Positive parenting 7mos | 1141 | 2.90 | .79 | 1.0 – 4.8 |
| Positive parenting 15mos | 1100 | 2.79 | .80 | 1.0 – 5.0 |
| Positive parenting 24mos | 1055 | 2.89 | .81 | 1.0 – 4.8 |
| Negative parenting 7mos | 1141 | 2.41 | .77 | 1.0 – 5.0 |
| Negative parenting 15mos | 1100 | 2.27 | .69 | 1.0 – 5.0 |
| Negative parenting 24mos | 1055 | 2.43 | .87 | 1.0 – 5.0 |
| Executive functions 36mos | 950 | .49 | .21 | .00 – 1.0 |
| IQ 36mos | 1046 | 93.64 | 16.5 | 45 – 142 |
| Income-to-need | 1236 | 1.76 | 1.5 | .00 – 16.5 |
| Maternal education | 1123 | 12.97 | 2.00 | 7 – 20 |
| Household density 7mos | 1152 | 1.55 | .62 | .67 – 5.0 |
| Household density 24mos | 1098 | 1.51 | .58 | .50 – 5.5 |
| Household safety 7mos | 1177 | 3.00 | .58 | 1.0 – 4.0 |
| Household safety 24mos | 1105 | 3.00 | .49 | 1.0 – 4.0 |
Table 2.
Correlation Among Observed Variables
| C7 | C15 | C24 | P7 | P15 | P24 | N7 | N15 | N24 | EF | IQ | INR | Ed | D7 | D24 | S7 | S24 | AA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cort 7 | 1.00 | |||||||||||||||||
| Cort 15 | .03 | 1.00 | ||||||||||||||||
| Cort 24 | .09 | .11 | 1.00 | |||||||||||||||
| Positive7 | −.11 | −.07 | −.08 | 1.00 | ||||||||||||||
| Positive15 | −.10 | −.10 | −.03 | .65 | 1.00 | |||||||||||||
| Positive24 | −.10 | −.13 | −.09 | .56 | .63 | 1.00 | ||||||||||||
| Negative7 | .07 | .03 | .09 | −.19 | −.19 | −.23 | 1.00 | |||||||||||
| Negative15 | .07 | .02 | .01 | −.22 | −.31 | −.31 | .41 | 1.00 | ||||||||||
| Negative24 | .06 | .09 | .09 | −.27 | −.36 | −.53 | .35 | .41 | 1.00 | |||||||||
| EF | −.09 | −.11 | −.12 | .29 | .30 | .33 | −.21 | −.21 | −.25 | 1.00 | ||||||||
| IQ | −.07 | −.09 | −.08 | .32 | .36 | .43 | −.21 | −.25 | −.36 | .46 | 1.00 | |||||||
| Income | −.05 | −.03 | −.04 | .35 | .43 | .42 | −.28 | −.29 | −.31 | .28 | .38 | 1.00 | ||||||
| Mat ed | −.03 | −.10 | −.04 | .40 | .44 | .46 | −.24 | −.29 | −.32 | .27 | .37 | .58 | 1.00 | |||||
| Dense7 | .03 | .11 | .03 | −.21 | −.24 | −.27 | .15 | .18 | .19 | −.22 | −.28 | −.38 | −.33 | 1.00 | ||||
| Dense24 | .01 | .03 | .01 | −.19 | −.25 | −.22 | .16 | .20 | .16 | −.18 | −.24 | −.36 | −.33 | .51 | 1.00 | |||
| Safety7 | −.03 | −.08 | −.05 | .21 | .25 | .28 | −.16 | −.12 | −.17 | .11 | .23 | .37 | .34 | −.21 | −.16 | 1.00 | ||
| Safety24 | −.02 | −.08 | −.06 | .16 | .22 | .22 | −.09 | −.13 | −.20 | .11 | .20 | .35 | .29 | −.15 | −.16 | .42 | 1.00 | |
| AA vs W | .16 | .10 | .13 | −.36 | −.34 | −.37 | .35 | .27 | .34 | −.35 | −.37 | −.38 | −.24 | .28 | .25 | −.20 | −.12 | 1.00 |
correlations larger than .10 are significant at p < .01 level
Notable in the table is the high level of risk associated with African American ethnicity in this sample. On every indicator examined, African American children and families fare worse than do white participants. Levels of cortisol are significantly higher at each time point and child executive function and IQ significantly lower. Ratings of positive parenting are significantly lower and negative parenting significantly higher. Income-to-need and maternal education are significantly lower for African Americans and African American families’ homes are significantly more crowded and rated as less safe than are the homes of white participants.
Structural equation modeling
To examine the relation of cognitive ability at age 3 years to child cortisol, observed parenting, and household risk, we used structural equation modeling. We modeled executive function using a single latent variable with the working memory, inhibitory control, and attentional set shifting tasks as indicators. Similarly, IQ was modeled using a single latent variable with the Block Design and Vocabulary subtests as indicators. The latent cortisol and positive and negative parenting variables were indicated by measures at 7, 15, and 24 months. All cortisol measures were adjusted for time of day. The latent household risk variable was indicated by household density and combined noise and safety ratings at 7 and 24 months. We included observed variables for mean income-to-need, maternal education measured, African American ethnicity, child sex and age at the 3 year assessment (M = 37.05, SD = 1.8).
Measurement model
A measurement model with correlations among all latent indicators and observed variables fit the data well χ2 (174) = 467.0, p = .0001, CFI = .95, RMSEA = .036. Correlations are presented in Table 3 and loadings of observed variables on latent indicators are reported in Table 4. Inspection of parameter estimates indicated that all of the factor loadings were statistically significant and in the expected direction and that all of the latent variances were statistically significant. All correlations between latent variables were large and significant (all p < .001.) Correlation between EF and IQ latent variables was very high (φ = .92), a finding consistent with numerous studies examining latent variable correlations for these constructs. The executive function latent variable was moderately correlated with the latent positive (φ = .59) and negative (φ = −.59) parenting variables as was IQ (φ = .61 and −.61). Both executive function and IQ were correlated with the household risk latent variable (φ = −.44 and = − .53, respectively). Executive function and IQ were negatively related to the cortisol latent variable, with a larger relation for executive function (φ = −.56) than for IQ (φ = − .37). Cortisol was negatively related to positive parenting (φ = −.47), and positively related to negative parenting (φ = .37) and household risk (φ = .26).
Table 3.
Correlation Among Latent and Observed Variables
| CORT | POS | NEG | RISK | EF | IQ | Income | Mat ed | AA | |
|---|---|---|---|---|---|---|---|---|---|
| CORT | 1.00 | ||||||||
| POS | −.42 | 1.00 | |||||||
| NEG | .37 | −.60 | 1.00 | ||||||
| RISK | .26 | −.54 | .48 | 1.00 | |||||
| EF | −.56 | .59 | −.59 | −.44 | 1.00 | ||||
| IQ | −.37 | .61 | −.61 | −.53 | .92 | 1.00 | |||
| Income | −.15 | .53 | −.50 | −.64 | .42 | .46 | 1.00 | ||
| Mat ed | −.18 | .57 | −.48 | −.57 | .41 | .46 | .57 | 1.00 | |
| AA | .48 | −.46 | .55 | .39 | −.52 | −.45 | −.38 | −.24 | 1.00 |
all correlations significant at p < .05 level or greater
Table 4.
Loadings of Observed Variables on Latent Indicators
| Latent Indicator | Observed Variable | Unstandardized Coefficient | Standardized Coefficient |
|---|---|---|---|
| Executive Functions | Attention Shifting | 1.00 | .68 |
| Working Memory | .84 | .55 | |
| Inhibitory Control | 1.17 | .46 | |
| IQ | Vocabulary | 1.00 | .74 |
| Block Design | .68 | .61 | |
| Cortisol | 7 months | 1.00 | .30 |
| 15 months | .97 | .26 | |
| 24 months | .93 | .27 | |
| Positive Parenting | 7 months | 1.00 | .68 |
| 15 months | 1.11 | .76 | |
| 24 months | 1.22 | .82 | |
| Negative Parenting | 7 months | 1.00 | .53 |
| 15 months | .89 | .53 | |
| 24 months | 1.43 | .67 | |
| Household Riska | 7 monthsb | 1.00 | .62 |
| 7 monthsc | −.83 | −.55 | |
| 24 monthsb | .89 | .59 | |
| 24 monthsc | −.61 | −.48 |
all coefficients significant at p < .0001
household risk included measures of
density and ratings of
home safety at 7 and 24 months
Structural model
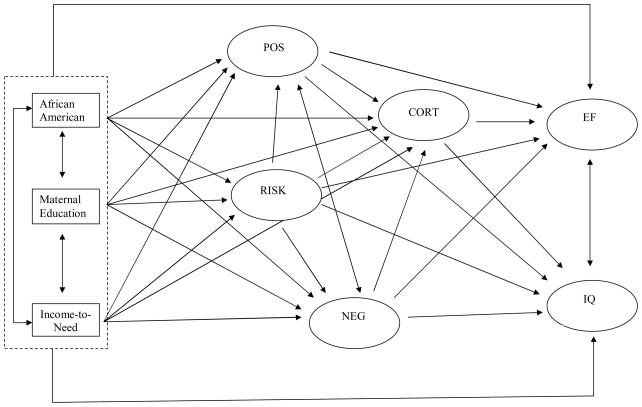
Figure 1 presents the hypothesized structural model. In this model we examined direct effects of poverty indicators, income-to-need, maternal education, and African American ethnicity on all latent variables (direct paths of each poverty indicator to executive function and IQ are indicated jointly in the figure for clarity of presentation.) We also examined indirect effects of poverty indicators on executive function and IQ through household risk, positive and negative parenting, and cortisol to determine the extent to which these variables mediate effects of poverty on child cognitive outcomes. As well, indirect effects of household risk through positive and negative parenting and cortisol were examined. Finally, to examine the extent to which cortisol mediates effects of parenting on child cognitive outcomes, we examined indirect effects of positive and negative parenting on executive function and IQ through cortisol.
Figure 1.
Hypothesized model relating poverty indicators, household risk, positive and negative parenting, and cortisol to cognitive outcomes at age 3 years.
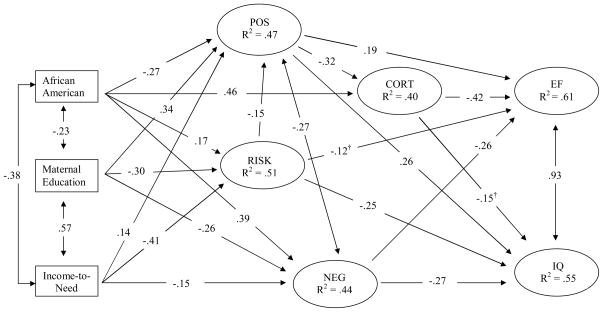
The observed structural model is in Figure 2. This model fit the data well, χ2 (188) = 474.4, p = .0001, CFI = .94, RMSEA = .035, SRMR = .031. All effects are reported as standardized coefficients. Direct effects were observed in which executive function was negatively predicted by cortisol, β = −.42, p < .0001, while the relation of cortisol to IQ was marginal, β = −.15, p = .06. Both executive function and IQ were predicted by negative parenting (β = −.26, p = .003, β = −.27, p < .0001, respectively), positive parenting, (β = .19, p = .04, β = .26, p < .0001), and by household risk (marginally for executive function, β = −.12, p = .07, significantly for IQ, β = −.25, p < .0001). Neither executive function nor IQ was directly predicted by observed variables maternal education, income-to-need ratio, African American ethnicity, or age at assessment.
Figure 2.
Observed model relating poverty indicators, household risk, positive and negative parenting, and cortisol to cognitive outcomes at age 3 years. All paths presented as standardized effects. All paths p < .05 except † p < .10
Does cortisol mediate the effects of parenting on child cognitive ability?
In the model in Figure 2, we also tested for indirect effects of latent and observed variables, summarized in Table 5. Examination of direct effects of positive and negative parenting and household risk latent variables on the cortisol latent variable indicated that cortisol was inversely related to positive parenting, β = −.32, p = .008, but was unrelated to negative parenting and to household risk. The relation between positive parenting and cortisol resulted in a significant indirect effect of positive parenting on executive function through cortisol, β = .13, p = .03. The test of the indirect effect of positive parenting on IQ through cortisol was not significant, β = .05, p = .12.
Table 5.
Standardized Indirect Effects
| EF | IQ | |
|---|---|---|
| Positive parenting → Cortisol | .13 | ns |
| African American → Cortisol | −.19 | ns |
| African American → Negative Parenting | −.10 | −.10 |
| African American → Household Risk | ns | −.04 |
| African American → Positive Parenting | −.05 | −.07 |
| African American → Positive Parenting → Cortisol | −.04 | ns |
| Income-to-Need → Negative Parenting | .04 | .04 |
| Income-to-Need → Household Risk | ns | .10 |
| Income-to-Need → Positive Parenting | ns | .03 |
| Income-to-Need → Positive Parenting → Cortisol | ns | ns |
| Maternal Education → Negative Parenting | .07 | .07 |
| Maternal Education → Household Risk | ns | .08 |
| Maternal Education → Positive Parenting | .07 | .06 |
| Maternal Education → Positive Parenting → Cortisol | .05 | ns |
| Household Risk → Positive Parenting | ns | −.04 |
| Household Risk → Positive parenting → Cortisol | ns | ns |
all coefficients significant at p < .05 level or greater
Do cortisol and parenting variables mediate the effects of poverty including income-toneed, maternal education, household risk, and African American ethnicity on child cognitive ability?
Analysis of indirect effects indicated that income-to-need was related to executive function through negative parenting, β = .04, p = .02, but not through positive parenting or through the path including positive parenting and cortisol. In contrast, maternal education was indirectly related to executive function through negative parenting, β = .07, p = .004, and also through positive parenting, β = .07, p = .03, and through the path including positive parenting and cortisol, β = .05, p = .04. Income-to-need and maternal education were both indirectly related to IQ through positive parenting, β = .03, p = .03 and β = .06, p = .008, negative parenting, β = .04, p = .01 and β = .07, p = .002, and household risk, β = .10, p < .0001 and β = .08, p < .0001, respectively. Indirect effects of African American ethnicity on executive function were observed through negative parenting, β = −.10, p = .006, positive parenting, β = −.05, p = .05, and through the path including positive parenting and cortisol, β = −.04, p = .04. Similarly, indirect effects of African American ethnicity on IQ were observed through negative parenting, β = −.10, p < .0001, positive parenting, β = −.07, p < .0001, and also through household risk, β = −.04, p = .001. Cortisol was higher in African American children, β = .46, p < .0001. Cortisol was unrelated to income-to-need and maternal education. Higher level of cortisol in African American relative to White participants in this sample resulted in an indirect effect of African American ethnicity on executive function through cortisol, β = −.19, p < .0001. This indirect effect was not present for IQ.
Discussion
In this analysis, level of salivary cortisol measured at child ages 7, 15, and 24 months and adjusted for time of day of saliva sample collection was significantly higher in children in poverty and shown to partially mediate effects of poverty and parenting on child cognitive abilities. These associations are consistent with well defined neurobiological models linking early experience with the development of the HPA axis component of the stress response system (Meaney & Szyf, 2005) and linking stress and stress hormones with cognition, particularly executive functions (Arnsten, 2000; Lupien, Maheu, Tu, Fiocco, & Shramek, 2007). The novel contributions of this analysis are in demonstrating 1) that relations between the glucocorticoid hormone cortisol and cognitive ability are present in early childhood, 2) that the effect of parenting on cortisol is associated with positive rather than negative aspects of parenting behavior, and 3) that cortisol and both positive and negative parenting mediate associations between the conditions of poverty and child cognitive ability at age 3 years.
Findings from this analysis extend the study of poverty, stress physiology, and executive functions to early childhood and provide increased specificity in the identification of relations among variables. As noted in the introduction, several studies have demonstrated that poverty is associated with increased levels in stress physiology indicators in children (Evans, 2003; Fernald & Gunnar, 2009; Lupien, King, Meaney, & McEwen, 2001). Only one prior study, however, with an adolescent sample, has reported effects linking chronic poverty, assessed in terms of income-to-need, with both stress physiology and cognitive ability (Evans & Schamberg, 2009). No previous study of which we are aware has examined multiple aspects of poverty in a sample in early childhood with the goal of linking poverty with stress physiology, parenting, and cognitive development. The analysis presented here examined both positive and negative aspects of parenting and found that positive parenting was reduced in lower income homes and inversely related to cortisol level over the child’s first two years. Findings emphasizing the relation of positive parental behavior to child stress physiology are consistent with data from animal models indicating it is the absence of nurturing behavior rather than high levels of negative parenting that may be most relevant to development (Meaney, 2001). It is not clear in the present study, however, whether positive maternal behavior is affecting stress physiology through a tactile and kinesthetic nurturing process or whether other aspects of parenting behavior, such as structuring of opportunities and appropriate levels of stimulation are the operative mechanisms, or even perhaps if positive parenting behavior is a marker for other aspects of early experience important for the development of stress physiology such as exposure to novelty (Tang et al., 2006) and types of experience that promote flexible regulation of stress physiology (e.g., Parker, Buckmaster, Sundlass, Schatzberg, & Lyons, 2006).
Increased cortisol levels in African American children
No studies to our knowledge have previously examined poverty, parenting, stress physiology, and cognitive ability in children in an ethnically diverse sample. A major finding of this analysis is that the typical level or set point for cortisol is higher in African American children than in white children in infancy and early childhood and that higher cortisol partially mediates an association between African American ethnicity and lower executive function ability at age 3 years. The percentage of African American families in extreme poverty in this sample, as in the U.S. as a whole, is disproportionate. Unfortunately, we were unable to directly test whether the effect for ethnicity is accounted for by poverty give the low representation of higher income African American participants in the sample, a characteristic of the communities from which it was drawn. As such, it may be the case that the finding also reflects possible preexisting differences between African American and white participants in the sample relating to genetic background and to epigenetic processes of development. Unmeasured aspects of risk might include proximal influences of inequality that act directly on stress physiology and cognitive development but also intergenerational influences of social inequality on physiological stress response systems such as have been hypothesized to contribute to persistent racial disparities in birth and health outcomes (Kuzawa & Sweet, 2009; Lu & Halfon, 2003). Such an intergenerational mechanism may be one contributor to long standing disparities in health and educational outcomes in African Americans relative to the U.S. population as a whole (Nisbett, 2009). In combination with poor quality schools and reduced educational and employment opportunities, findings for higher cortisol levels in African American children in this sample may provide one indication of processes through which social inequality perpetuates racial disparities in physical and mental health.
Biological sensitivity to context
Associations among poverty, cortisol, and cognitive development take on particular meaning in that the mechanisms through which physiological stress affects physical and mental health are well established (McEwen, 2000). Consistent with an understanding of allostasis as adaptation of stress physiology to environmental demands, results linking poverty and early experience with salivary cortisol and cognitive ability in this sample are perhaps best understood within the framework of biological sensitivity to context (Boyce & Ellis, 2005). In the biological sensitivity theory, early experience is understood to shape stress response systems to meet expected environments with consequences for behaviors important for regulating behavior in that environment. In the model, both highly supportive and highly unsupportive environments are understood to lead to elevated stress physiology (Ellis, Essex, & Boyce, 2005). In unsupportive environments, however, this increase would not be well regulated and stress hormones would remain elevated, facilitating reactive and inflexible rather than reflective and flexible forms of behavior and cognition. In supportive and structured environments, however, regulation of stress hormones would occur and facilitate reflective and flexible forms of behavior and cognition, such as executive functions (Blair, 2010).
Given the link between stress hormones and synaptic activity in PFC, it was expected that cortisol would be related to executive functions but less so to IQ. Such a finding is consistent with the research described above linking stress hormones with PFC and linking PFC with executive functions. As with research on adult samples (Kane et al., 2005), however, executive function and IQ latent variables were highly correlated. The close association between the constructs is consistent with theory and research indicating that executive functions are important building blocks for the development of children’s thinking (Zelazo, Muller, Frye, & Marcovitch, 2003) and key contributors to the development of intelligence (Piaget, 1952). A number of prior studies indicate that executive functions are central to the development of fluid intelligence in children (Fry & Hale, 1996; Kail, 2007) and adults (Engle, Tuholski, Lauglin, & Conway, 1999). Findings here are in agreement with these prior studies and indicate the influence of early experience on executive functions as one pathway through which intelligence develops.
Limitations and directions for future research
Questions concerning specific pathways through which early experience might influence stress physiology important for cognitive development and self-regulation highlight key directions for future research as well as key limitations. Although the data examined in this study are longitudinal, they cannot address mechanisms through which poverty and stress response physiology are causally related to cognitive ability in children. Results are consistent with prior studies examining the neurobiology of relations among early experience, the stress response, and cognitive ability. As such, they suggest that one way in which poverty affects children’s development is through increased stress. Only one aspect of stress physiology, salivary cortisol as an indicator of the activity of the HPA axis, however, was measured. Additional indicators of stress physiology over multiple time points, including measures of sympathetic and parasympathetic systems are desirable.
Furthermore, it is important to emphasize that the focus of this study was on the typical or resting level for cortisol measured over the child’s first two years. Saliva samples were collected in the home by data collectors and there may have been some effect of the presence of the data collector on child cortisol. It would be desirable to have typical day saliva samples collected by parents to assess potential effects of the data collection process on child cortisol levels. Given the standardized nature of the data collection protocol, however, and the collection of saliva near the end of the visit after the data collectors had been in the home for more than an hour, it is likely that these effects were present but minimal. For example, work by Fernald and Gunnar (2009) has shown that although there can be some effect of data collectors’ presence in the home on child cortisol levels, effects are similar across children and dissipate within one hour. Cortisol does, however, follow a well defined diurnal pattern. This variation necessitates that cortisol levels be adjusted for time of day of saliva collection. Future studies that collect the cortisol awakening response and multiple samples throughout the day are needed to address questions concerning the ways in which the conditions of poverty are related to diurnal variation in cortisol in early childhood.
Further points related to cortisol concern the dynamic nature of the HPA axis and our focus on a typical level for cortisol over the child’s first two years rather than cortisol reactivity. By combining a methodological approach in which we sampled saliva after being in the home for one hour and a statistical approach using structural equation modeling, we were able to identify what can be considered a general or typical level of cortisol in children over the first two years. This approach, however, in no way obviates that fact that the HPA axis is a dynamic and reactive system and that measures of HPA reactivity provide meaningful information about effects of stress on development. In this analysis, we focused on the typical cortisol level rather than reactivity in order to address hypotheses concerning allostatic load in early childhood and also for purposes of modeling the cortisol data as a single latent variable. Furthermore, by focusing on the typical cortisol level or set point over the child’s first two years, we did not address questions concerning the relative influence of cortisol and parenting variables at 7, 15, and 24 months on child cognitive ability at age 3 years. The conditions of poverty over the child’s first two years tend to be stable in this sample but the impact of cortisol and/or parenting on cognitive ability may be greater at earlier as opposed to later time points in development.
It is also necessary to emphasize that the sample participating in this study is predominantly low-income. It may be that findings are most specific to low-income samples of this type, at least with respect to the distinct effects of positive and negative parenting and household risk on cortisol and cognitive ability. In more advantaged samples or samples in contexts substantially different from those of families participating in this study, sources of stress and effects of family and household variables on cortisol may vary from those reported here. The model relating early stress to alterations in stress sensitive physiological systems and to self-regulation, however, would seem to be highly generalizable. As well, the central role of early caregiving in this process seems very generalizable. As such, studies employing randomized designs are needed to further establish associations among variables and to intervene to promote self-regulation and school readiness among children at risk. In this regard, an exemplary study demonstrated the reestablishment of a more typical diurnal cortisol pattern in 3 to 6 year old children receiving a therapeutic intervention in foster care (Fisher, Stoolmiller, Gunnar, & Burraston, 2007).
The inclusion of measures of stress physiology and executive functions as well as other indicators of self-regulation in randomized intervention studies with low-income samples is an important direction for future research. The longitudinal findings of noted early intervention programs such as Abecedarian (Campbell, Pungello, Miller-Johnson, Burchinal, & Ramey, 2001) and Perry Preschool (Schweinhart et al., 2005) demonstrated that the programs substantially improved life outcomes of program recipients. The overall benefits of these programs in promoting advantageous outcomes such as educational attainment and reducing disadvantageous outcomes such as criminality appear to have resulted to some extent from the promotion of self-regulation in program recipients (Heckman, 2006, 2007). As such, it may be that observed beneficial program outcomes occurred in part through effects on neurobiological systems important for self-regulation including executive functions, as well as aspects of personality and self-perception associated with self-regulation (Knudsen, Heckman, Cameron, & Schonkoff, 2006). Currently a number of early parenting (Landry, Smith, Swank, & Guttentag, 2008) and preschool programs (Bierman, Domitrovich, Nix et al., 2008; Bodrova & Leong, 2007; Raver et al., 2011; Raver, Jones, Li-Grining, Zhai, Metzger, & Solomon, 2009) have demonstrated impressive benefits to child cognitive, social-emotional, and self-regulation abilities using randomized designs. The inclusion of measures of stress physiology and multiple aspects of self-regulation in future evaluations of similar early care and education programs can help to further establish the point that such programs are highly effective at promoting optimal outcomes for children at risk and represent an efficient and cost-effective social policy response to persistent and pervasive threats to healthy child development associated with the conditions of poverty.
Acknowledgments
We would like to thank the many families and research assistants that made this study possible. Support for this research was provided by the National Institute of Child Health and Human Development grants R01 HD51502 and P01 HD39667 with co-funding from the National Institute on Drug Abuse.
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. Journal of Cognitive Neuroscience. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Through the looking glass: Differential noradenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bierman KL, Domitrovich CE, Nix RL, Gest SD, Welsh JA, Greenberg MT, Blair C, Nelson K, Gill S. Promoting academic and social-emotional school readiness: The Head Start REDI Program. Child Development. 2008;79:1802–1817. doi: 10.1111/j.1467-8624.2008.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behavioral and Brain Sciences. 2006;29:109–125. doi: 10.1017/S0140525X06009034. [DOI] [PubMed] [Google Scholar]
- Blair C. Stress and the development of self-regulation in context. Child Development Perspectives. 2010 doi: 10.1111/j.1750-8606.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, Hibel L, Fortunato C, the Family Life Project Investigators Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44:1095–1109. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Razza RP. Cortisol reactivity is positively related to executive function in preschool children attending Head Start. Child Development. 2005;76:554–567. doi: 10.1111/j.1467-8624.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false-belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Bodrova E, Leong DJ. Tools of the mind: The Vygotskian approach to early childhood education. 2. Upper Saddle River NJ; Pearson: 2007. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brody G, Murry V, Kim S, Brown A. Longitudinal pathways to competence and psychological adjustment among African American children living in rural single-parent households. Child Development. 2002;73:1505–1516. doi: 10.1111/1467-8624.00486. [DOI] [PubMed] [Google Scholar]
- Burchinal P, Vernon-Feagans L, Cox M and the Family Life Project Investigators. Cumulative social risk and infant development in rural low-income communities. Parenting: Science and Practice. 2008 doi: 10.1080/15295190701830672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FA, Pungello EP, Miller-Johnson S, Burchinal M, et al. The development of cognitive and academic abilities: Growth curves from an early childhood educational experiment. Developmental Psychology. 2001;37:231–242. doi: 10.1037/0012-1649.37.2.231. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Mandell D, Williams L. Executive function and theory of mind: Stability and prediction from ages 2 to 3. Developmental Psychology. 2004;40:1105–1122. doi: 10.1037/0012-1649.40.6.1105. [DOI] [PubMed] [Google Scholar]
- Cameron N, Champagne F, Parent E, Fish C, Ozaki-Kuroda K, Meaney M. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. New York, NY, US: Cambridge University Press; 1993. [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. Journal of Neuroscience. 2007;27:2781. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, et al. Maternal care and hippocampal plasticity. Journal of Neuroscience. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger R, Wallace L, Sun Y, Simon R, McLoyd V, Brody G. Economic pressure in African American families: A replication and extension of the family stress model. Developmental Psychology. 2002;38:179–193. [PubMed] [Google Scholar]
- Cox M, Paley B, Burchinal M, Payne C. Marital perceptions and interactions across the transition to parenthood. Journal of Marriage and the Family. 1999;61:611–625. [Google Scholar]
- Davis EP, Bruce J, Gunnar MR. The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Developmental Psychobiology. 2002;40:43–56. doi: 10.1002/dev.10012. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity. 2007 doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson, Kemeny Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary–developmental theory. Development and Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences, USA. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LC, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Social Science and Medicine. 2009;68:2180–2189. doi: 10.1016/j.socscimed.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, Defries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychological Science. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Fry AF, Hale S. Processing speed, working memory, and fluid intelligence: Evidence for a developmental cascade. Psychological Science. 1996;7:237–241. [Google Scholar]
- Gershoff ET, Aber JL, Raver CC, Lennon MC. Income is not enough: incorporating material hardship into models of income associations with parenting and child development. Child Development. 2007;78:70–95. doi: 10.1111/j.1467-8624.2007.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua G-L. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology and Behavior. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: A mechanism for later trauma vulnerability. Progress in Brain Research. 2008;167:137–147. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A, Granger DA, Hibel LC, Rumyantseva O. Measuring salivary cortisol in studies of child development: Watch out - what goes in may not come out of commonly used saliva collection devices. Developmental Psychobiology. 2008;49:495–500. doi: 10.1002/dev.20231. [DOI] [PubMed] [Google Scholar]
- Heckman J. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312:1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- Heckman J. The economics, technology, and neuroscience of human capability formation. Proceedings of the National Academy of Sciences, USA. 2007;104:13250–13255. doi: 10.1073/pnas.0701362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Ensor R. Executive function and theory of mind: Predictive relations from ages 2 to 4. Developmental Psychology. 2007;43:1447–1459. doi: 10.1037/0012-1649.43.6.1447. [DOI] [PubMed] [Google Scholar]
- Ispa JM, Fine MA, Halgunseth LC, et al. Maternal intrusiveness, maternal warmth, and mother-toddler relationship outcomes: Variations across low-income ethnic and acculturation groups. Child Development. 2004;75:1613–1631. doi: 10.1111/j.1467-8624.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- Jacques S, Zelazo PD. The Flexible Item Selection Task (FIST): A measure of executive function in preschoolers. Developmental Neuropsychology. 2001;20:573–591. doi: 10.1207/S15326942DN2003_2. [DOI] [PubMed] [Google Scholar]
- Kail RV. Longitudinal evidence that increases in processing speed and working memory enhance children’s reasoning. Psychological Science. 2007;18:312–313. doi: 10.1111/j.1467-9280.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Conway ARA. Working memory capacity and fluid intelligence are strongly related constructs: Comment on Ackerman, Beier, and Boyle (2005) Psychological Bulletin. 2005;131:66–71. doi: 10.1037/0033-2909.131.1.66. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Heckman JJ, Cameron JL, Shonkoff JP. Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proceedings of the National Academy of Science. 2006;103:10155–10162. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. American Journal of Human Biology. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR, Guttentag C. A responsive parenting intervention: the optimal timing across early childhood for impacting maternal behaviors and child outcomes. Developmental Psychology. 2008;44:1335–1353. doi: 10.1037/a0013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal neurogenesis, and cognitive development in rats. Nature Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Maternal and Child Health Journal. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- Lugo-Gil J, Tamis-LeMonda CS. Family resources and parenting quality: links to children’s cognitive development across the first 3 years. Child Development. 2008;79:1065. doi: 10.1111/j.1467-8624.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans. Behavioral Neuroscience. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney M, McEwen M. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McLoyd V. Socioeconomic disadvantage and child development. American Psychologist. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24 doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. Journal of Neuroscience. 2004;24:5492–5499. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Child Health and Human Development (NICHD) Early Childcare Network. The effects of infant childcare on infant-mother attachment security: Results of the NICHD study of early childcare. Child Development. 1999;68:860–879. doi: 10.1111/j.1467-8624.1997.tb01967.x. [DOI] [PubMed] [Google Scholar]
- Nisbett RE. Intelligence and how to get it. New York, NY: Norton; 2009. [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proceedings of the National Academy of Sciences, USA. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The origins of intelligence in children. Oxford, England: International Universities Press; 1952. [Google Scholar]
- Ramey CT, Ramey SL. Early intervention and early experience. American Psychologist. 1998;53:109–120. doi: 10.1037//0003-066x.53.2.109. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacology and Therapeutics. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Gershoff ET, Aber JL. Testing equivalence of mediating models of income, parenting, and school readiness for White, Black, and Hispanic children in national sample. Child Development. 2007;78:96–115. doi: 10.1111/j.1467-8624.2007.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Jones SM, Li-Grining CP, Zhai F, Bub K, Pressler E. CSRP’s impact on low-income preschoolers’ pre-academic skills: Self-regulation as a mediating mechanism. Child Development. 2011 doi: 10.1111/j.1467-8624.2010.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Jones SM, Li-Grining C, Zhai F, Metzger MW, Solomon B. Targeting children’s behavior problems in preschool classrooms: A cluster-randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77:302–316. doi: 10.1037/a0015302. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Schweinhart LJ, Montie J, Xiang Z, Barnett WS, Belfield CR, Nores M. Lifetime effects: The HighScope Perry Preschool study through age 40. (Monographs of the HighScope Educational Research Foundation, 14) Ypsilanti, MI: HighScope Press; 2005. [Google Scholar]
- Sroufe LA. The organization of emotional life in the early years. NY NY: Cambridge: 1996. [Google Scholar]
- Stifter CA, Braungart J. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31:448–455. [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH, Baumwell L. Maternal responsiveness and children’s achievement of language milestones. Child Development. 2001;72:748 – 767. doi: 10.1111/1467-8624.00313. [DOI] [PubMed] [Google Scholar]
- Tang A, Akers K, Reeb B, Romeo R, McEwen BS. Programming, social, cognitive, and neuroendocrine development by early exposure to novelty. Proceedings of the National Academy of Sciences. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Willoughby M, Blair C, Wirth RJ, Greenberg M the FLP Investigators. The measurement of executive function at age 3: Psychometric properties and criterion validity of a new battery of tasks. Psychological Assessment. 2010;22:306–317. doi: 10.1037/a0018708. [DOI] [PubMed] [Google Scholar]
- Zelazo PD. The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nature Protocols. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Müller U. Executive function in typical and atypical development. In: Goswami U, editor. Handbook of childhood cognitive development. Oxford: 2002. pp. 445–469. [Google Scholar]
- Zelazo PD, Müller U, Frye D, Marcovitch S. The development of executive function in early childhood. Monographs of the Society for Research on Child Development. 2003;68(3):vii–137. doi: 10.1111/j.0037-976x.2003.00260.x. [DOI] [PubMed] [Google Scholar]