Abstract
Morphine is frequently used as an analgesic and sedative in preterm infants. Adult rats exposed to morphine have altered hippocampal neurochemical profile and decreased neurogenesis in the dentate gyrus of the hippocampus. To evaluate whether neonatal rats are similarly affected, rat pups were injected twice daily with 2 mg/kg of morphine or normal saline from postnatal days 3 to 7. On postnatal day 8, the hippocampal neurochemical profile was determined using in vivo 1H NMR spectroscopy. The mRNA and protein concentrations of specific analytes were measured in hippocampus, and cell division in dentate gyrus was assessed using bromodeoxyuridine. The concentrations of γ-aminobutyric acid (GABA), taurine and myo-insotol were decreased, while glutathione, phosphoethanolamine and choline-containing compounds concentrations were increased in morphine-exposed rats relative to control rats. Morphine decreased glutamic acid decarboxylase enzyme levels and myelin basic protein mRNA expression in the hippocampus. Bromodeoxyuridine labeling in the dentate gyrus was decreased by 60-70% in morphine-exposed rats. These results suggest that recurrent morphine administration during brain development alters hippocampal structure.
Keywords: 1H NMR spectroscopy, bromodeoxyuridine, γ-aminobutyric acid (GABA), hippocampus, morphine, taurine
Introduction
Critically ill preterm infants are frequently treated with prolonged courses of opiates to decrease pain and stress (Anand et al. 2004). While the efficacy of such treatment is debated, the clinical practice persists (Carbajal et al. 2005; Cignacco et al. 2008; Franck et al. 2000; Simons et al. 2003) as the effects of untreated pain are well established (Anand 2000; Duric and McCarson 2006). There are increasing concerns that opiates may have detrimental effects on neurodevelopmental outcomes. Neonatal morphine treatment with and without stress, is associated with short term changes in gene expression and cellular composition in the hippocampus (Juul et al. 2011; Vien et al. 2009) and long term neurobehavioral deficits in rodents (Boasen et al. 2009; McPherson et al. 2007).
In adult rats, the neurochemical profile of the hippocampus is altered during morphine administration (Corrigall 1983; Gao et al. 2007; Simonato 1996) and hippocampal-mediated learning is impaired (Bhutta et al. 2001; Spain and Newsom 1991), possibly because of decreased neurogenesis in the dentate gyrus of the hippocampus (Eisch et al. 2000; Lledo et al. 2006).
In order to evaluate the safety of morphine for sedation in the absence of pain, we used a neonatal rat model of morphine administration (McPherson et al. 2007). We hypothesized that neonatal morphine administration would alter the neurochemical profile of the developing hippocampus and decrease neurogenesis in the dentate gyrus. The metabolites indexing neuronal and glial integrity, energy substrates and energy sufficiency, phospholipid biosynthesis, and amino acids and neurotransmitters in the developing hippocampus were assessed using high field in vivo 1H NMR spectroscopy, followed by evaluation of mRNA and protein expression of relevant analytes in the hippocampus. We assessed cell proliferation in the dentate gyrus using bromodeoxyuridine (BrdU) histochemistry. We found that rat pups exposed to recurrent morphine administration had an altered neurochemical profile, decreased glutamic acid decarboxylase (GAD) and myelin basic protein (MBP) expressions in the hippocampus, and decreased incorporation of BrdU in the dentate gyrus.
Materials and Methods
Animals and Drug Treatment
Experiments were performed using male and female Sprague-Dawley rat pups. All procedures conformed to guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee. Pregnant rats were purchased (Harlan laboratories; Madison, WI) and allowed to deliver spontaneously. The day of delivery was designated postnatal day (P) 0. Litter size was limited to 10 by culling soon after birth. Rats were kept in humidity and temperature-controlled rooms with a 12-hour light/dark schedule and were allowed food and water ad libitum. Pups were weighed daily.
Rat pups in each litter were randomly assigned to control (n = 20) or morphine (n = 25) groups. From P3 to P7, pups in the morphine group received twice daily injections of 2 mg/kg morphine sulfate i.p. (Hospira; Lake Forest, IL; concentration 0.5 mg/mL) for a total of 10 injections. Control pups received equivalent volume of normal saline i.p. The morphine dose was based on previous studies (Boasen et al. 2009; McPherson et al. 2007) and results in blood concentrations similar to those achieved in human neonates on bolus or standard infusions (Carbajal et al. 2005; Scott et al. 1999).
On P7, some pups (n= 6 control, n=8 morphine) were injected with 100 mg/kg BrdU i.p. (Sigma Aldrich; St. Louis, MO) to evaluate cell division within the dentate gyrus (Eisch et al. 2000).
Physiological Monitoring
Rat pups in both groups were separated from the dams for 2 hours after each injection, until the morphine-exposed pups had fully recovered from the sedative effects of morphine (determined by the normalization of the righting reflex). Nesting temperature (34°C) (Stern and Lonstein 1996) was maintained during the separation period. The heart rate, pulse distension, respiratory rate and subcutaneous tissue oxygen saturation (SpO2) were continuously monitored in representative pups (n= 6 control, n=8 morphine) using a rodent pulse oximeter system (MouseOx®; Starr Life Sciences Corporation; Oakmount, PA), beginning five min before the injection and continuing until return to dams. The pulse oximeter probe was secured over the dorsal cervical region and data were recorded to a spreadsheet at 1 Hz. The number and duration of desaturation events, defined as SpO2 < 90%, were recorded. Alterations by more than 10% in baseline heart rate, pulse distention and respiratory rate were considered significant.
In vivo 1H NMR Spectroscopy
NMR data were obtained from anesthetized, spontaneously breathing rats (n= 6 control, n=7 morphine) on P8. Anesthesia was achieved by inhalation of isoflurane, 3% for induction and 1-2% for maintenance in an equal mixture of O2 and N2O. Measurements were performed using a horizontal bore 9.4 T/31 cm magnet (Varian/Magnex Scientific; Oxford, UK) interfaced to a Varian INOVA console (Varian, Inc.; Palo Alto, CA). In vivo 1H NMR spectra were acquired using previously described protocol (Tkac et al. 2003). Briefly, field homogeneity was optimized using the FASTMAP shimming technique (Gruetter and Tkac 2000). Ultra-short echo-time single-voxel STEAM sequence (echo time TE = 2 ms, repetition time TR = 5 s) combined with outer volume suppression and VAPOR water suppression (Tkac et al. 1999) was used to acquire spectra from a 5 μL (2.3 × 1.1 × 2.0 mm3) volume of interest centered in the left hippocampus. Positioning of the volume of interest was based on multislice fast spin-echo imaging (echo train length = 8, echo spacing = 15 ms, echo time = 48 ms, field of view = 2 cm × 2 cm, matrix = 256 × 256, slice thickness = 1 mm). The study of a single rat did not exceed 60 min.
Quantification of Neurochemicals
In vivo 1H NMR spectra were analyzed using LCModel with the spectrum of fast relaxing macromolecules included in the basis set (Pfeuffer et al. 1999; Provencher 1993; Tkac et al. 2003). Unsuppressed water signal was used as an internal reference assuming 88% brain water content (Tkac et al. 2003). Only metabolites, systematically quantified with Cramer-Rao lower bounds < 30%, were included in the final data analysis: ascorbate, creatine, phosphocreatine, γ-aminobutyric acid (GABA), glucose, glutamate, glutamine, glutathione, lactate, myo-inositol, N-acetylaspartate, N-acetylaspartylglutamate, phosphoethanolamine, taurine and the sum of glycerophosphocholine and phosphocholine (GPC+PC).
Tissue collection
Rats were killed on P8 using an overdose of pentobarbital (100 mg/kg i.p.). In animals used for mRNA and protein expressions (n=6 control, n=10 morphine) the brain was collected and hippocampus was dissected out as previously reported (Rao et al. 2009). Tissues were immediately frozen in liquid nitrogen and stored at −80°C until processed. Animals used for immunohistochemistry (n=6 control, n=8 morphine) underwent intracardial perfusion with normal saline, followed by 4% formaldehyde and sucrose in PBS. The harvested brains were kept overnight in 4% formaldehyde and 5% sucrose in PBS and then dehydrated by overnight serial passages in 20% sucrose in PBS, and 30% sucrose in PBS at 4°C. They were then flash-frozen and stored at −80°C. Twenty micrometer coronal sections along the anterior-posterior axis of the entire hippocampus were collected using a cryostat (Model CM1900; Leica Instruments GmbH; Nussloch, Germany) and mounted on slides (Superfrost Plus; Fisher Scientific; Pittsburgh, PA). Slides were stored at −20°C until histochemistry.
Quantitative RT-PCR (qPCR)
To evaluate the effect of morphine on programmed cell death and myelination within the hippocampus, mRNA expression of apoptosis inducing factor (AIF) (Rn00442540_mL) and myelin basic protein (MBP) (Rn00566745_m1), respectively, was determined using previously described methods (Rao et al., 2009). Samples were assayed in duplicate and normalized against ribosomal protein S18 (Rn01428915_g1).
Western Analysis
Concentrations of the GABA-producing enzymes GAD 65, GAD 67, and apoptosis inducing factor (AIF) in the hippocampus were determined using published methods (Tran et al. 2009; Rao et al. 2009). Ten microgram of protein from the homogenized hippocampus was separated on 4-12% gradient SDS-PAGE gels (Invitrogen, Carlsbad, CA) and blotted onto nitrocellulose membranes. The membranes were incubated with rabbit anti-GAD 65 & 67 (1:7500; Abcam, Cambridge, MA) or rabbit anti-AIF (1:1000); Abcam, Cambridge, MA) along with mouse anti-ß-actin (1:5000; Sigma Chemical Co., St. Louis, MO) antibodies overnight at 4°C. After incubation with secondary antibodies (1:2500) for 30 min at room temperature, the membranes were imaged (Odyssey Infrared Imaging System; Li-cor Biosciences, Lincoln, NE).
Histochemical Analyses
BrdU Staining and Analysis
Six brain sections per rat pup, representing the anterior to posterior axis of the hippocampus were used for determining BrdU incorporation. Slide 1 from every animal represented the most anterior brain section containing the hippocampus. Subsequent slides were posterior to this slide and were 60-200 micrometer apart from each other. Slides were placed in 2N HCl for 20 min, rinsed in PBS and incubated overnight with mouse monoclonal antibody to BrdU (1:30, Abcam Inc.; Cambridge, MA) at 4°C. Following rinses in PBS, sections were incubated with anti-mouse biotinylated secondary antibody for 30 min at room temperature. Tissues were rinsed in PBS and incubated in avidin-biotinylated enzyme complex (Vector Elite ABC kit; Vector Labs; Burlingame, CA) at room temperature. The reaction was visualized using a chromagen kit (Vector Labs; Burlingame, CA) and slides were coverslipped and mounted using Permount (Fisher Scientific; Pittsburgh, PA). Photomicrographs of dentate gyrus were obtained as previously described (Ennis et al. 2008). All BrdU-positive cells in granule cell layer and the hilus of the dentate gyrus were counted using the ImageJ program (ImageJ version 1.4g; Research Services Branch, National Institutes of Health, Bethesda, Maryland, USA).
Cell Death Analysis
Degenerating cells in the GCL and hilus of the dentate gyrus were stained in 2 slides per animal at least 300 micrometers apart using Fluoro-Jade B (FJB) histochemistry as in our previous study (Ennis et al. 2008). Sections were visualized under a fluorescence microscope and all FJB positive cells in the granule cell layer and hilus of the dentate gyrus were manually counted (n=6 control, n=8 morphine).
Immunofluorescence staining
GAD enzyme expression in the subregions of the hippocampus was evaluated using immunofluorescence. Brain sections (n=6 per group; 2 sections per rat pup at least 300 micrometers apart) were subjected to antigen retrieval using 10mM citric acid and 0.05% Tween 20 at pH 6.0 at 85°C for 10 min. To block endogenous peroxidases, sections were placed in 3% H2O2 in PBS with 1% Triton for 30 min. Nonspecific binding was blocked using 1% horse serum in PBS and 1% Triton for 30 min. Following overnight incubation with rabbit anti-GAD 65 & 67 (1:1500, Abcam Inc.; Cambridge, MA) at 4°C, tissues were rinsed in PBS and 1% Triton and incubated with secondary goat anti-rabbit IgG (Alexa Fluor 488; Invitrogen, Carlsbad, CA) for 30 min. Slides were coverslipped with fluorescence mounting media containing DAPI (Vector Laboratories; Burlingame, CA) (Ennis et al. 2008; Rao et al. 2009). Staining intensity in the hilus and granule cell layer of the dentate gyrus was determined using the ImageJ program as previously described (Rao et al. 1999).
To characterize the nature of the BrdU positive cells, brain sections (n=4 morphine group, 1 slide per pup) were co-labeled with markers of immature neuron (doublecortin) and astrocytes (glial fibrillary acidic protein [GFAP]). Sections were incubated overnight at 4°C with mouse anti-BrdU (1:400; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) and anti-rabbit doublecortin (1:1000, Abcam Inc.; Cambridge, MA) or GFAP (1:500, Novus Biologicals, LLC, Littleton, CO). Primary antibodies were tagged using a secondary antibody conjugated with a fluorophore (1:400, Alexa Fluor 488 (BrdU) or 1:250, Alexa Fluor 555 (doublecortin or GFAP). Sections were coverslipped with an aqueous mounting media and visualized using a fluorescence microscope.
Statistical analysis
Data were analyzed using a computer program (SPSS version 18; Chicago, IL). Kolmogorov-Smirnof test was used to test for normalcy of the data. General linear repeated measures analysis and Student’s t-test were used to compare control and morphine groups. Data are presented as mean ± SEM. An alpha of 0.05 was used to determine significance.
Results
Effect of Morphine on Body and Brain Weights
Morphine administration did not affect weight gain (control, 16.70 ± 0.50 g; morphine, 15.82 ± 0.34 g) or brain weight (control, 0.61 ± 0.03 g; morphine, 0.58 ± 0.02 g). There were no gender-specific effects.
Effect of Morphine on Cardiopulmonary Physiology
Oxygen desaturation (SpO2 <90%) without apnea was observed in 75% of the morphine-exposed rats. The longest duration of desaturation was one min. Over 90% of the desaturation episodes were < 20 sec in duration and most episodes (74%) occurred during the first two days of morphine exposure. Desaturations were not observed in the control group at any time and during the 20 min before the pups were returned to the dams in the morphine group. Heart rate, pulse distention and respiratory rates were unaffected by morphine administration. No evidence of opiate withdrawal (increased stretching, rolling, and increased head movements) (Jones and Barr 1995) was observed.
Effect of Morphine on Neurochemical Profile of the Hippocampus
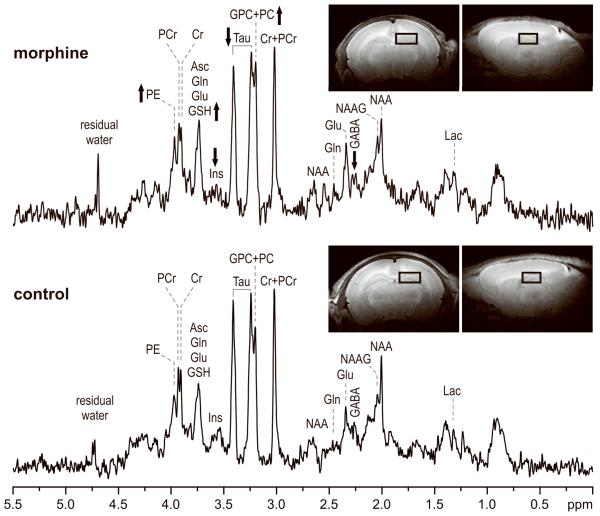
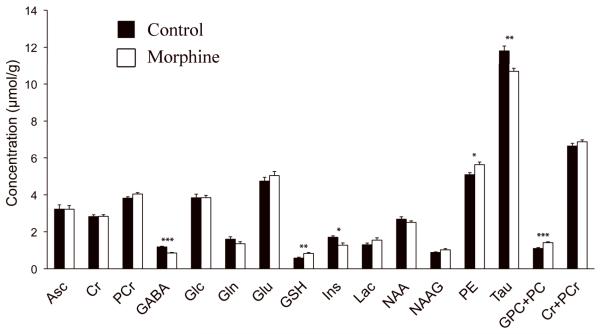
Representative in vivo 1H NMR spectra from the control and morphine-exposed rats are shown in Figure 1. Achieved spectral quality enabled quantification of 15 brain metabolites from each spectrum (Figure 2). The neurochemical profile of the hippocampus was altered in the morphine group relative to the control group. Decreased concentrations of GABA (−28%, p < 0.0005), myo-inositol (−25%, p < 0.002) and taurine (−10%, p < 0.005) and increased concentrations of glutathione (+43%, p < 0.02), phosphoethanolamine (+11%, p < 0.02) and GPC+PC (+29%, p < 0.001) were observed in the morphine group relative to the control group. These concentration differences were always larger than the estimated errors of metabolite quantification (Cramer-Rao lower bounds in concentration units).
Figure 1.
In vivo 1H NMR spectra measured from the hippocampus of control and morphine groups on postnatal day 8. STEAM, TE = 2 ms, TR = 5 s, NT = 240. Processing: Gaussian multiplication σ = 0.10, FT, zero-order phase correction, water signal removal was not applied. Arrows represent the direction of the metabolite changes of morphine-exposed pups compared to control pups. Insets: Fast spin-echo images with the selected volume of interest centered on the left hippocampus. Abbreviations: Asc, ascorbate; Cr, creatine; GABA, γ-aminobutyric acid; Glc, glucose; Gln, glutamine; Glu, glutamate; GPC, glycerophosphocholine; GSH, glutathione; Ins, myo-inositol; Lac, lactate; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PC, phosphocholine; PCr, phosphocreatine; PE, phosphoethanolamine; Tau, taurine.
Figure 2.
Comparison of neurochemical profiles of rat pups exposed to morphine relative to littermate controls. Metabolite concentrations were measured from left hippocampus on postnatal day 8. Values are mean ± SEM, n = 6 control (black), 7 morphine (white). * p < 0.05, ** p < 0.01, *** p < 0.001. Abbreviations: Asc, ascorbate; Cr, creatine; PCr, phosphocreatine; GABA, γ-aminobutyric acid; Glc, glucose; Gln, glutamine; Glu, glutamate; GSH, glutathione; Ins, myo-inositol; Lac, lactate; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PE, phosphoethanolamine; Tau, taurine; GPC+PC, sum of glycerophosphocholine and phosphocholine.
Effect of Morphine on GAD Protein Expression
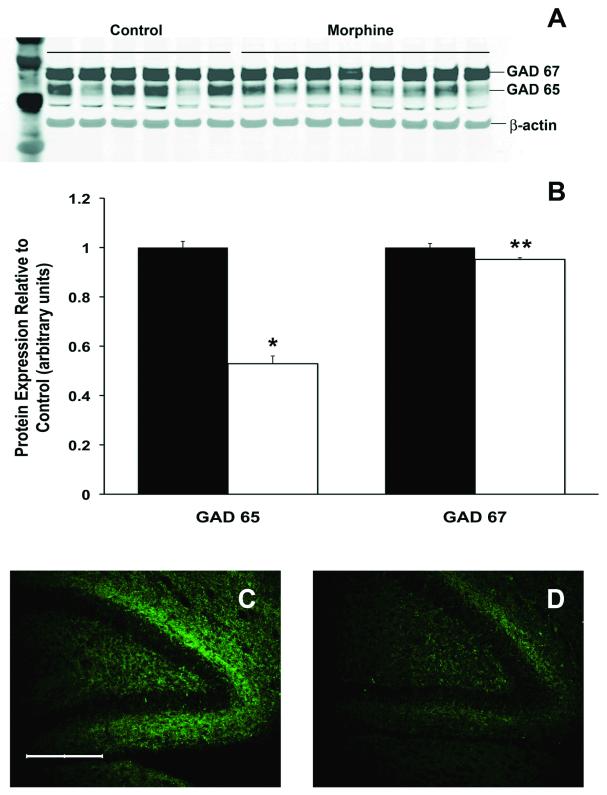
To investigate the potential reasons for decreased GABA concentration in the hippocampus, we compared the protein concentration of GABA-producing enzymes GAD 65 and 67 in morphine and control hippocampi. GAD 65 expression was 47% lower (p<0.001), and GAD 67 expression was 5% lower (p<0.05) in the morphine group compared with the control group (Figure 3A and 3B). In the dentate gyrus, morphine administration decreased combined GAD 65 & 67 immunoreactivity by 27% (p < 0.05); while a 35% decrease was noted in the granule cell layer (p < 0.01). A trend towards decreased GAD reactivity (26%) was seen in the hilus (p = 0.06) (Figure 3C and 3D).
Figure 3.
Glutamic acid decarboxylase (GAD) enzyme levels of rat pups exposed to morphine relative to littermate controls. The expression GAD 65 and GAD 67 protein levels using western blot (A) demonstrates that mophine-exposed rats have decreased levels of both isoenzymes relative to littermate controls (B). Values are mean ± SEM, n = 6 control (black), 6 morphine (white).* p < 0.001 and ** p < 0.05. Photomigrographs of GAD 65 & 67 protein immunostaining in the dentate gyrus of control (C) and morphine-exposed (D) pups demonstrates decreased intensity of GAD staining in the morphine group. Scale bar = 200 μm.
Effect of morphine on MBP mRNA expression
Elevation of GPC+PC and phosphoethanolamine levels are associated with delayed myelination (Rao et al., 2003). Therefore, hippocampal MBP mRNA levels were determined. Whole hippocampal MBP mRNA levels were 28% lower in the morphine group, relative to the control group (p < 0.05) (Figure 4).
Figure 4.
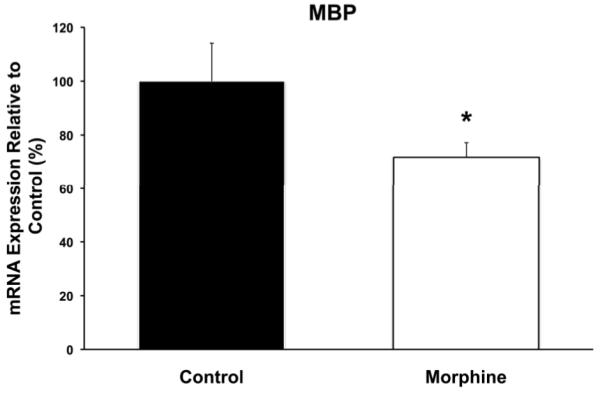
Hippocampal myelin basic protein (MBP) quantitative RT-PCR comparison of control and morphine animals on postnatal day 8. Hippocampal MBP mRNA level was decreased by 28% with morphine administration. Values are mean ± SEM relative to the expression in the control group, n = 6 control (black), n = 10 morphine (white). * p < 0.05.
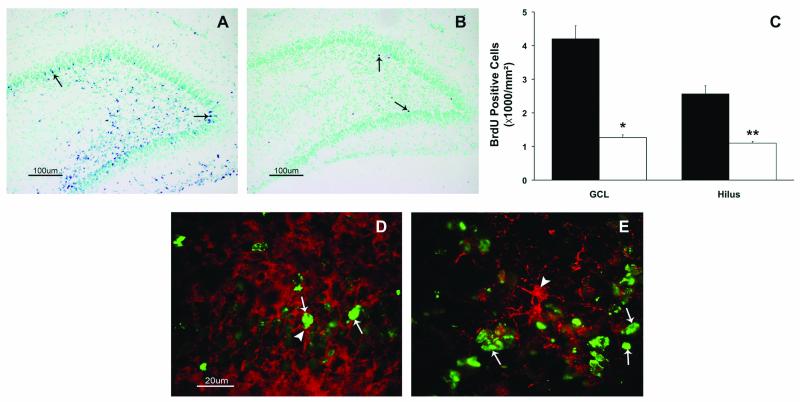
Effect of Morphine on Cell Division in the Dentate Gyrus
BrdU was incorporated into the cells in the granule cell layer and hilus of the dentate gyrus in both groups (Figure 5A and 5B). Pups exposed to recurrent morphine had 70% fewer BrdU positive cells in the granule cell layer and 60% fewer BrdU positive cells in the hilus (Figure 5C, p < 0.05). BrdU positive cells co-labeled with doublecortin but not with GFAP (Figure 5D and 5E) in the granule cell layer.
Figure 5.
Bromodeoxyuridine (BrdU) immunohistochemistry of the dentate gyrus. Brdu positive cells in the granular cell layer (GCL) and hilus of the dentate gyrus of postnatal day 8 pups in the control (A) and morphine (B) groups are shown. Arrows point to BrdU positive cells. Morphine administration decreased the number of BrdU positive cells (C) in the granule cell layer and the hilus of the dentate gyrus on postnatal day 8. Values are mean ± SEM, n = 6 control (black), 8 morphine (white). * p<0.01 and ** p<0.05. Co-labeling of BrdU positive cells (green, thin arrow) with doublecortin (D, red, thick arrow) and glial fibrillary acidic protein (E, red, thick arrow) show that most BrdU cells co-label with doublecortin. Scale bar = 100 μm (A and B), and = 20 μm (D and E).
Effect of Morphine on Cell Death
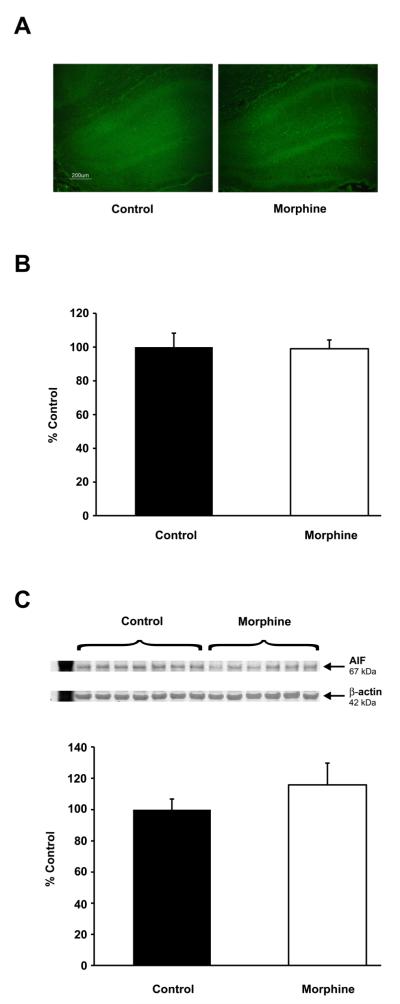
In the dentate gyurus, there was no difference in the number of FJB positive cells between the control and morphine groups (control, 1.65 ± 0.4, morphine, 2.6 ± 0.8) (Figure 6A). Whole hippocampal AIF mRNA and protein expressions were unchanged by morphine exposure (p > 0.05) (Figure 6B and 6C).
Figure 6.
Morphine does not increase cell degeneration or death. Comparable number of degenerating/stressed (i.e. Fluoro-Jade B stained) cells were present in the dentate gyrus of control and morphine-exposed pups (A; scale bar = 200 μm). Similarly, apoptosis inducing factor (AIF) mRNA expression (B) and protein levels (C) were unaffected by morphine administration. Values are mean ± SEM relative to the expression in the control group, n = 6 control (black), n = 10 morphine (white), p > 0.05.
Discussion
We used a neonatal rat model of morphine exposure to model morphine-induced sedation in preterm infants in the absence pain or severe stress. We demonstrate that recurrent morphine administration under these circumstances alters the neurochemical profile, GAD enzyme concentrations, and MBP mRNA expression within the developing rat hippocampus, and decreased cell division in the dentate gyrus. These results suggest that recurrent morphine administration in the absence of pain or severe stress adversely affects hippocampus formation.
GABA neurotransmission is critical for hippocampal development (Scharfman et al. 2005; Tozuka et al. 2005) and is the major hippocampal excitatory neurotransmitter during the first postnatal week of rats (Cherubini et al. 1991). We found that hippocampal GABA concentration was decreased in pups exposed to morphine. The enzymes responsible for production of GABA, GAD 65 and 67, were also decreased. Maturation of the newly born neurons is delayed in mice lacking GAD 65 enzyme (Overstreet-Wadiche et al. 2006). Furthermore, GABAergic input is a regulator of neurogenesis (Tozuka et al. 2005) and blockade of GABA halts neurogenesis in the type 2 progenitor cell stage (Tozuka et al. 2005). Chronic morphine exposure was not associated with cell death in the present study nor in adult rodents (Eisch et al. 2000), although acute cell death was not investigated (Arguello et al. 2008). Thus, the lower GAD enzyme levels and GABA concentrations in the morphine group potentially indicate decreased GABAergic input to dentate gyrus progenitor cells and may be responsible for the decreased BrdU cell labeling in the dentate gyrus in the present study. A recent study in morphine-exposed adult mice supports this postulation (Arguello et al. 2008).
Neonatal exposure to morphine resulted in decreased hippocampal taurine and myo-inositol concentrations. This may suggest the presence of osmotic stress, since taurine and myo-inositol are major intracellular osmolytes in the brain (Hayes et al. 1980; Isaacks et al. 1994). In addition to being an osmolyte, taurine is essential for cell proliferation, migration and differentiation (Benitez-Diaz et al. 2003; Park et al. 2006; Takatani et al. 2003). Myo-inositol is also needed for cell division and production of giant depolarizing potentials (Kandler and Katz 1998; Tsukagoshi et al. 1966).
Collectively, decreased GABA, taurine and myo-inositol indicate potential for decreased giant depolarizing potentials, which is important for synapse formation in the developing hippocampus (Ben-Ari 2001; Ben-Ari et al. 1989; Ben-Ari et al. 2007; Overstreet-Wadiche et al. 2006). We speculate that these neurochemical changes portend altered synapse formation within the hippocampus that could explain the cognitive impairments seen at adulthood after neonatal morphine-exposure in rodents (Boasen et al. 2009; McPherson et al. 2007).
Increased hippocampal phosphoethanolamine and GPC + PC concentrations potentially reflect disrupted or delayed myelination due to morphine-exposure. The decreased MBP mRNA expression in this group is consistent with this interpretation. Phosphoethanolamine and GPC + PC are precursors of phospholipids (Pettegrew et al. 1990) and steadily decrease over the first month of life as myelination progresses in rats (Tkac et al. 2003). Similar neurochemical alterations were not seen in adult rats exposed to morphine (Gao et al. 2007), probably reflecting the lower myelination rate at adulthood.
Elevated glutathione concentration following repetitive morphine exposure is a novel finding. Glutathione is a major endogenous antioxidant in brain cells (Dringen 2000). We speculate that the elevated glutathione concentration seen 12-24 hours after the last dose of morphine is likely a compensatory response to previous oxidative stress. Morphine induces reactive oxygen species formation in various brain cells (Xu et al. 2006). In addition, opiates acutely decreases glutathione levels within the brain (Calderon-Guzman et al. 2009; Goudas et al. 1999; Guzman et al. 2006) and suppresses glutathione peroxidase activity (Xu et al. 2006). Repetitive hypoxic episodes may have also played a role; however, lower glutathione levels are more likely with chronic hypoxia (Raman et al. 2005).
Our study has certain limitations. The experimental protocol used in the present study is a model of sedation and not treatment of pain, which also adversely affects hippocampus development (Duric and McCarson 2006). Thus, our results are relevant to morphine exposure in the absence of pain or severe stress. However, minor stress due to handling, maternal separation and intraperitoneal injections were present. Except for brief desaturations soon after administration of morphine, exposure to morphine did not affect cardiopulmonary physiology and growth in the present study. While it is unlikely that the brief desaturations can fully explain the findings in our study, minor effects could not be excluded without additional experiments (for example, using lower doses of morphine).. However, a more severe hypoxia of longer duration is necessary to alter the neurochemical profile of the hippocampus during development (Douglas et al. 2007; Raman et al. 2005). While isoflurane’s effect on the brain is controversial (Johnson et al. 2008; Stratmann et al. 2010), the possibility of an interaction between morphine-exposed rats and isoflurane for the NMR results cannot be dismissed. However, the rest of the analyses, including GAD enzyme assays were performed in rats not exposed to isoflurane.
In summary, recurrent morphine administration altered the neurochemical profile, GABA synthesis and cell division of the developing rat hippocampus. We speculate that morphine delays maturation of the hippocampus. These changes may explain the enduring neurobehavioral deficits seen in adulthood following neonatal morphine administration.
Acknowledgments
The assistance of Jeff Long, Ph.D. with statistical analysis, Kari Roberts, M.D. and Sandra Juul, M.D., Ph.D. for their critical review of manuscript are gratefully acknowledged. This work was supported in part by grants from The Center for Neurobehavioral Development, Viking Children’s Fund Fellow Grants-in-Aid, The Academic Health Center University of Minnesota, NIH P41 RR08079, P30 NS057091 and WM Keck Foundation.
Grant Information: Grant Sponsor: The Center for Neurobehavioral Development; University of Minnesota, Grant Number: NA Grant Sponsor: Viking Children’s Fund Fellow Grants-in-Aid; Grant Number: NA Grant Sponsor: The Academic Health Center, University of Minnesota; Grant Number: NA; Grant sponsor: NIH P41 RR08079; Grant sponsor: P30 NS057091; Grant sponsor: WM Keck Foundation
References
- Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med. 2000;6(9):971–973. doi: 10.1038/79658. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363(9422):1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- Arguello AA, Harburg GC, Schonborn JR, Mandyam CD, Yamaguchi M, Eisch AJ. Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neu roscience. 2008;157(1):70–79. doi: 10.1016/j.neuroscience.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24(6):353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87(4):1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Benitez-Diaz P, Miranda-Contreras L, Mendoza-Briceno RV, Pena-Contreras Z, Palacios-Pru E. Prenatal and postnatal contents of amino acid neurotransmitters in mouse parietal cortex. Dev Neurosci. 2003;25(5):366–374. doi: 10.1159/000073514. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Rovnaghi C, Simpson PM, Gossett JM, Scalzo FM, Anand KJ. Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiol Behav. 2001;73(1-2):51–58. doi: 10.1016/s0031-9384(01)00432-2. [DOI] [PubMed] [Google Scholar]
- JF Boasen, McPherson RJ, Hays SL, Juul SE, Gleason CA. Neonatal stress or morphine treatment alters adult mouse conditioned place preference. Neonatology. 2009;95(3):230–239. doi: 10.1159/000165379. [DOI] [PubMed] [Google Scholar]
- Calderon-Guzman D, Osnaya-Brizuela N, Garcia-Alvarez R, Hernandez-Garcia E, Juarez-Olguin H. Oxidative stress induced by morphine in brain of rats fed with a protein deficient diet. Hum Exp Toxicol. 2009;28(9):577–582. doi: 10.1177/0960327109102798. [DOI] [PubMed] [Google Scholar]
- Carbajal R, Lenclen R, Jugie M, Paupe A, Barton BA, Anand KJ. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics. 2005;115(6):1494–1500. doi: 10.1542/peds.2004-1425. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cignacco E, Hamers JP, van Lingen RA, Zimmermann LJ, Muller R, Gessler P, Nelle M. Pain relief in ventilated preterms during endotracheal suctioning: a randomized controlled trial. Swiss Med Wkly. 2008;138(43-44):635–645. doi: 10.4414/smw.2008.12288. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Opiates and the hippocampus: a review of the functional and morphological evidence. Pharmacol Biochem Behav. 1983;18(2):255–262. doi: 10.1016/0091-3057(83)90371-4. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP. Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1254–1259. doi: 10.1152/ajpregu.00404.2006. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62(6):649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7(8):544–555. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97(13):7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis K, Tran PV, Seaquist ER, Rao R. Postnatal age influences hypoglycemia-induced neuronal injury in the rat brain. Brain Res. 2008;1224:119–126. doi: 10.1016/j.brainres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck LS, Boyce WT, Gregory GA, Jemerin J, Levine J, Miaskowski C. Plasma norepinephrine levels, vagal tone index, and flexor reflex threshold in premature neonates receiving intravenous morphine during the postoperative period: a pilot study. Clin J Pain. 2000;16(2):95–104. doi: 10.1097/00002508-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Gao H, Xiang Y, Sun N, Zhu H, Wang Y, Liu M, Ma Y, Lei H. Metabolic changes in rat prefrontal cortex and hippocampus induced by chronic morphine treatment studied ex vivo by high resolution 1H NMR spectroscopy. Neurochem Int. 2007;50(2):386–394. doi: 10.1016/j.neuint.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Goudas LC, Langlade A, Serrie A, Matson W, Milbury P, Thurel C, Sandouk P, Carr DB. Acute decreases in cerebrospinal fluid glutathione levels after intracerebroventricular morphine for cancer pain. Anesth Analg. 1999;89(5):1209–1215. [PubMed] [Google Scholar]
- Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Guzman DC, Vazquez IE, Brizuela NO, Alvarez RG, Mejia GB, Garcia EH, Santamaria D, de Apreza MR, Olguin HJ. Assessment of oxidative damage induced by acute doses of morphine sulfate in postnatal and adult rat brain. Neurochem Res. 2006;31(4):549–554. doi: 10.1007/s11064-006-9053-7. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Stephan ZF, Sturman JA. Growth depression in taurine-depleted infant monkeys. J Nutr. 1980;110(10):2058–2064. doi: 10.1093/jn/110.10.2058. [DOI] [PubMed] [Google Scholar]
- Isaacks RE, Bender AS, Kim CY, Prieto NM, Norenberg MD. Osmotic regulation of myo-inositol uptake in primary astrocyte cultures. Neurochem Res. 1994;19(3):331–338. doi: 10.1007/BF00971582. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Young C, Olney JW. Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol. 2008;20(1):21–28. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- Jones KL, Barr GA. Ontogeny of morphine withdrawal in the rat. Behav Neurosci. 1995;109(6):1189–1198. doi: 10.1037//0735-7044.109.6.1189. [DOI] [PubMed] [Google Scholar]
- Juul SE, Beyer RP, Bammler TK, Farin FM, Gleason CA. Effects of neonatal stress and morphine on murine hippocampal gene expression. Pediatric research. 2011;69(4):285–292. doi: 10.1203/PDR.0b013e31820bd165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18(4):1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7(3):179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- McPherson RJ, Gleason C, Mascher-Denen M, Chan M, Kellert B, Juul SE. A new model of neonatal stress which produces lasting neurobehavioral effects in adult rats. Neonatology. 2007;92(1):33–41. doi: 10.1159/000100084. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26(8):2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Lee H, Park KK, Kim HW, Park T. Taurine-responsive genes related to signal transduction as identified by cDNA microarray analyses of HepG2 cells. J Med Food. 2006;9(1):33–41. doi: 10.1089/jmf.2006.9.33. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Panchalingam K, Withers G, McKeag D, Strychor S. Changes in brain energy and phospholipid metabolism during development and aging in the Fischer 344 rat. J Neuropathol Exp Neurol. 1990;49(3):237–249. doi: 10.1097/00005072-199005000-00005. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Tkac I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time (1)H NMR spectra of the rat brain. J Magn Reson. 1999;141(1):104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Brain Res Dev Brain Res. 2005;156(2):202–209. doi: 10.1016/j.devbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Rao R, de Ungria M, Sullivan D, Wu P, Wobken JD, Nelson CA, Georgieff MK. Perinatal brain iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. J Nutr. 1999;129(1):199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- Rao R, Sperr D, Ennis K, Tran P. Postnatal age influences hypoglycemia-induced poly(ADP-ribose) polymerase-1 activation in the brain regions of rats. Pediatr Res. 2009;66(6):642–647. doi: 10.1203/PDR.0b013e3181bbce69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Scott CS, Riggs KW, Ling EW, Fitzgerald CE, Hill ML, Grunau RV, Solimano A, Craig KD. Morphine pharmacokinetics and pain assessment in premature newborns. J Pediatr. 1999;135(4):423–429. doi: 10.1016/s0022-3476(99)70163-0. [DOI] [PubMed] [Google Scholar]
- Simonato M. The neurochemistry of morphine addiction in the neocortex. Trends Pharmacol Sci. 1996;17(11):410–415. doi: 10.1016/s0165-6147(96)10047-x. [DOI] [PubMed] [Google Scholar]
- Simons SH, van Dijk M, van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, Bunkers C, Smink E, Anand KJ, van den Anker JN, Tibboel D. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290(18):2419–2427. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- Spain JW, Newsom GC. Chronic opioids impair acquisition of both radial maze and Y-maze choice escape. Psychopharmacology (Berl) 1991;105(1):101–106. doi: 10.1007/BF02316870. [DOI] [PubMed] [Google Scholar]
- Stern JM, Lonstein JS. Nursing behavior in rats is impaired in a small nestbox and with hyperthermic pups. Dev Psychobiol. 1996;29(2):101–122. doi: 10.1002/(SICI)1098-2302(199603)29:2<101::AID-DEV2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Stratmann G, Sall JW, Bell JS, Alvi RS, May LV, Ku B, Dowlatshahi M, Dai R, Bickler PE, Russell I, Lee MT, Hrubos MW, Chiu C. Isoflurane does not affect brain cell death, hippocampal neurogenesis, or long-term neurocognitive outcome in aged rats. Anesthesiology. 2010;112(2):305–315. doi: 10.1097/ALN.0b013e3181ca33a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatani T, Takahashi K, Itoh T, Hirata M, Yamamoto Y, Ohmoto M, Schaffer SW, Azuma J. Cellular characterization of taurine transporter in cultured cardiac myocytes and nonmyocytes. Adv Exp Med Biol. 2003;526:25–31. doi: 10.1007/978-1-4615-0077-3_4. [DOI] [PubMed] [Google Scholar]
- Tkac I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med. 2003;50(1):24–32. doi: 10.1002/mrm.10497. [DOI] [PubMed] [Google Scholar]
- Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47(6):803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi S, Lembach K, Charalampous FC. Metabolic functions of myo-inositol. 3. Utilization of purine bases and nucleosides for nucleic acid biosynthesis in inositol-deficient KB cells. J Biol Chem. 1966;241(2):388–394. [PubMed] [Google Scholar]
- Vien TN, Gleason CA, Hays SL, McPherson RJ, Chavkin C, Juul SE. Effects of Neonatal Stress and Morphine on Kappa Opioid Receptor Signaling. Neonatology. 2009;96(4):235–243. doi: 10.1159/000220763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Wang Z, Li G, Li B, Lin H, Zheng R, Zheng Q. Heroin-administered mice involved in oxidative stress and exogenous antioxidant-alleviated withdrawal syndrome. Basic Clin Pharmacol Toxicol. 2006;99(2):153–161. doi: 10.1111/j.1742-7843.2006.pto_461.x. [DOI] [PubMed] [Google Scholar]