Abstract
Purpose
In many neurodegenerative diseases abnormal concentrations of cytokines and chemokines affect neuronal integrity leading to cognitive impairments; altered levels of these molecules could also play a role in cancer- and cancer-treatment related cognitive difficulties. Patients receiving doxorubicin-based (with cyclophosphamide, or cyclophosphamide plus fluorouracil; AC/CAF) chemotherapy or cyclophosphamide, methotrexate, and fluorouracil (CMF) chemotherapy report experiencing cognitive difficulties; because these regimens work by different modes of action, it is possible that they differentially affect cytokine levels.
Methods
This secondary study examined the relationships between cytokine levels (i.e., IL-6, IL-8, and MCP-1) and type of chemotherapy among 54 early-stage breast cancer patients receiving AC/CAF or CMF. Cytokine levels were assessed at two time-points: prior to on-study chemotherapy cycle 2 (Cycle 2) and after 2 consecutive chemotherapy cycles (prior to on-study cycle 4; Cycle 4).
Main Results
Analyses of variance using Cycle 2 levels as a covariate (ANCOVA) were used to determine differences between chemotherapy groups. Levels of IL-6, IL-8, and MCP-1 increased in the AC/CAF group and decreased in the CMF group; the only significant between-group change was in IL-6 (p<0.05).
Conclusions
These preliminary results suggest that AC/CAF chemotherapy is more cytokine inducing than CMF; future studies should explore the distinct inflammatory responses elicited by different chemotherapy regimens.
Keywords: chemotherapy, cytokines, cancer, cognitive impairment, immune response
Introduction
Up to 75% of breast cancer patients experience some form of cognitive impairment related to chemotherapy, including problems with verbal and/ or visuospatial memory, difficulty learning, and difficulty in concentration and/or attention; these difficulties are commonly referred to as “chemobrain (1-9).” For many patients (up to 75%), difficulties with cognitive function begin during treatment, and in up to 35%, this impairment persists for many months or years following completion of treatment (5, 6, 8). These cognitive problems negatively affect activities of daily living such as performance at work (6), caring for and interacting with family members, accessing necessary medical care and conveying appropriate treatment decisions. Until the past decade this condition was largely under-studied. In fact, while patients have been experiencing these symptoms for years, only recently have investigators appreciated that cognitive difficulties can occur independently of other symptoms such as anxiety and sleep disturbance (10).
Elevated levels of peripheral blood and intrathecal cytokines and chemokines have been implicated in the pathogenesis of numerous diseases and disorders associated with cognitive difficulties including mild cognitive impairment, Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), and human immunodeficiency type 1 dementia. Increased levels of MCP-1, IL-6, and IL-8 have been demonstrated in patients with mild cognitive impairment (11-14). Additionally, traumatic brain injury (TBI) early in life and the later development of AD are positively correlated in some studies (15), suggesting that a heightened inflammatory status initiated by brain injury may contribute to AD-related cognitive impairments.
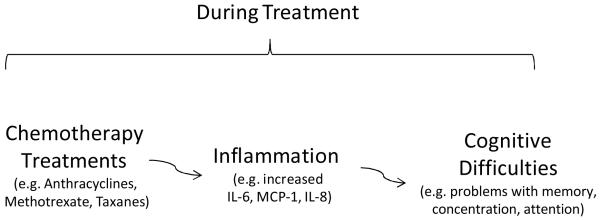
Recent literature suggests that elevated levels of peripheral pro-inflammatory cytokines may be related to cognitive problems in cancer patients (16, 17). Chemotherapy has been associated with increased levels of pro-inflammatory cytokines (e.g. IL-1 β) in patients treated for Hodgkin’s disease (18). Another study, in breast cancer patients receiving paclitaxel chemotherapy, revealed that levels of IL-6 increased 3 days after treatment compared to pretreatment levels, but no increase was seen in those who received CMF (19). Significant changes in markers of endothelial and platelet activation and inflammation were found in breast cancer patients receiving anthracycline-based treatment (20). Taken together, the existing literature on the association between inflammatory cytokines, cognitive difficulties, and chemotherapy lends support to the hypothesis that elevated levels of cytokines play an etiologic role in the development of cognitive difficulties in cancer patients (Figure 1). Furthermore, if this hypothesis is true, it would be important to know how changes in cytokine and chemokine levels vary by chemotherapy regimen.
Figure 1. Inflammation induced by chemotherapy as a possible mechanism of cognitive difficulties in breast cancer patients.
Multiple factors can contribute to cognitive difficulties in cancer patients. During treatment, inflammation caused by tissue damage from chemotherapy may contribute to cognitive difficulties in cancer patients.
Ultimately, an understanding of the factors contributing to chemotherapy-related cognitive impairment could aid in identifying patients at risk and might indicate potential therapeutic options for treating cognitive difficulties. Not all of the studies conducted to date have detected differences in all of the parameters thought to be altered in cancer-related cognitive difficulties, and the severity of these impairments also varies among the studies. While some of the discrepancy in study results may be methodological (e.g. variations in time-points, cognitive measures, control group selection and statistical analyses) it is possible that different types of treatment (e.g. various chemotherapy regimens) may impair cognitive functioning through diverse effects on the inflammatory pathways.
In the current study, we compared levels of IL-6, IL-8 and MCP-1 (Table 2) in patients receiving doxorubicin-based (AC/CAF) chemotherapy with levels in those receiving a combination of cyclophosphamide, methotrexate, and fluorouracil (CMF) chemotherapy. We chose to assess these cytokines because they have been implicated in other conditions associated with cognitive difficulties; IL-6 and IL-8 have been reported to increase in response to chemotherapy (19). We hypothesized that chemotherapy regimens would differentially alter expression of these three inflammatory molecules over two cycles of treatment.
Table 2.
Description of cytokines and chemokines with functions related to established immune actions and cognitive function
| Cytokine/Chemokine | Established Functions |
|---|---|
| MCP-1 |
|
| IL-6 |
|
| IL-8 |
|
METHODS
Participants
The current study is a secondary analysis of a randomized trial undertaken to investigate the effects of Paxil® (paroxetine) on fatigue in female breast cancer patients undergoing chemotherapy, as reported previously (21). The current study includes 54 female breast cancer patients from that trial who received either doxorubicin-based (AC/CAF) chemotherapy or a combination of cyclophosphamide, methotrexate, and fluorouracil (CMF) chemotherapy. Approval from the Institutional Review Board (IRB) was obtained prior to acquiring written informed consent and enrolling participants. To be eligible, patients in that trial were scheduled to receive 4 cycles of chemotherapy (2 weeks or more apart) and were not receiving radiation or interferon treatments. Patients may have had up to two chemotherapy treatments prior to consent; therefore, their first on-study treatment was not necessarily their first chemotherapy. Patients were excluded if they had a history of seizure, mania, or any condition requiring psychotropic medications. In this double-blinded study, patients were stratified by chemotherapy type and randomized to receive either 20 mg paroxetine or an identical appearing placebo starting on Day 7 of their first on-study treatment and concluding 7 days following their fourth on-study treatment. Patients were recruited from the University of Rochester Medical Center and two affiliates: Rochester General Hospital and Highland Hospital. Fifty-four patients who received either AC/CAF or CMF provided evaluable blood samples at both time-points and are included in this analysis.
Cognitive Questions/Items
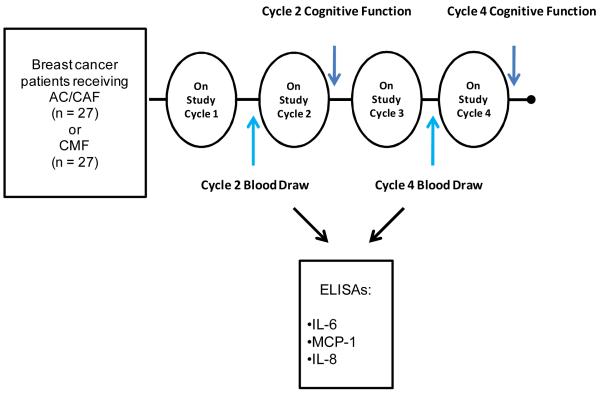
Five questions from the Fatigue Symptom Checklist (FSCL), a validated measure of fatigue (22), were chosen to indicate aspects of self-reported cognitive function at cycle 2 and cycle 4 of on-study chemotherapy. The FSCL was completed within the first week following each chemotherapy treatment (Figure 1). The cognitive items chosen were: “At the moment I feel:” (#1) heavy headed, (# 5) my thoughts are muddled, (# 11) difficulty thinking, (# 14) unable to concentrate, and (# 16) forgetful. The FSCL is based on a five-point scale with 1 being “absence of” and 5 being “a great deal”.
Measurement of MCP-1, IL-6, and IL-8
We were interested in evaluating whether cytokines/chemokines (i.e. IL-6, IL-8 and MCP-1, Table 1A) that are associated with inflammation and are markers for cognitive difficulties in MCI, Alzheimer’s disease and other neurological diseases may be differentially expressed depending on chemotherapy regimen. Non-fasting blood was collected prior to on-study cycle 2 and on-study cycle 4 (Figure 1). Blood was drawn and spun for serum collection and was stored aseptically at −80°. Cytokine levels were measured in serum samples using commercial colorimetric ELISA kits (BD Biosciences, San Diego, California) according to the manufacturer’s recommended assay procedure. Absorbance values are directly proportional to the amount of cytokine produced; these levels were determined using a recombinant cytokine standard for each ELISA.
Table 1.
Baseline characteristics for AC/CAF and CMF groups
| Characteristic | AC/CAF (n = 27) | CMF (n=27) | P-value |
|---|---|---|---|
| Age (years) | 52.85 (8.64) | 50.18 (9.62) | 0.29 |
|
| |||
| Ethnicity | |||
| White | 85% | 85% | 0.57 |
| Black | 11% | 15% | |
| Hispanic | 4% | 0% | |
|
| |||
| Karnofsky Performance Status | 88.15 | 90.20 | 0.36 |
|
| |||
| Menopausal Status | |||
| Pre-menopausal | 12% | 35% | 0.10 |
| Post-menopausal | 88% | 65% | |
|
| |||
| Intervention | |||
| Paxil | 48% | 44% | 1.00 |
| Placebo | 52% | 56% | |
Age and KPS scores are represented as means (±SD). Ethnicity, menopausal status, and Intervention are represented as percents.
Statistical Analyses
Data analyses were conducted using SPSS version 16.0 software. Descriptive statistics for the participants’ baseline values were calculated; percentages were calculated for categorical variables, and means and standard deviations for continuous variables. Independent sample t tests were performed on age, and chi-square with Fisher’s exact test was performed on intervention arm and menopausal (pre- and post-menopausal) status to determine if significant baseline differences existed. Means and standard errors of the mean (SEM) were calculated for all cytokines/chemokines at on-study cycle 2 and on-study cycle 4, and changes from on-study cycle 2 to on-study cycle 4 were also calculated. Because the distributions of IL-6 and IL-8 were skewed, these values were log-transformed to provide increased normality. Log-transformed values for IL-6 and IL-8 are used in all statistical analyses; levels of MCP-1 were normally distributed so raw values were used in the statistical analyses. To assess within group changes in cytokines/chemokines, paired t tests were conducted. To assess differences in cytokine/chemokine levels between chemotherapy groups, analysis of covariance (ANCOVA) was used incorporating baseline cytokine/chemokine level as a covariate.
Values for cognitive scores were dichotomized into yes/no responses. Wilcoxon signed rank tests were conducted to determine whether significant within-group differences in cognitive complaints between cycle 4 and cycle 2 existed for each chemotherapy group. Spearman’s rho was used to correlate change scores (cycle 4-cycle2) for cytokine/chemokines with dichotomized cognitive function responses (yes for difficulty/no for no difficulty). In all analyses, two-sided p values of ≤ 0.05 were considered statistically significant.
RESULTS
Twenty-seven patients who received AC/CAF and 27 patients who received CMF provided evaluable blood samples at baseline (prior to on-study cycle 2) and after 2 additional cycles of chemotherapy (prior to on-study cycle 4). Levels of MCP-1 were normally distributed, and levels of IL-6 and IL-8 were skewed. Log-transforming values of IL-6 and IL-8 increased normality; one pair of values for log-transformed IL-8 was considered an outlier (> 3 S.D. from the mean) and was removed from the analyses. Levels of MCP-1 were normally distributed, and one outlier pair was removed for analyses. No significant differences were present between chemotherapy groups in age or in the proportion of patients receiving Paxil®/Placebo or the proportion of patients who were pre-menopausal/post-menopausal (Table 1, p > 0.05). There were no differences (p > 0.05) in cytokine and chemokine levels at baseline or after 2 additional cycles of chemotherapy between patients receiving Paxil® and those randomized to placebo.
Changes in Cytokines/Chemokines by Chemotherapy Regimen
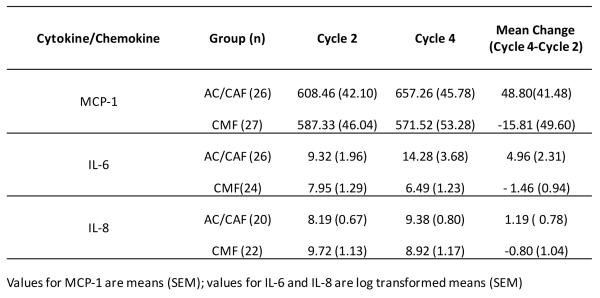
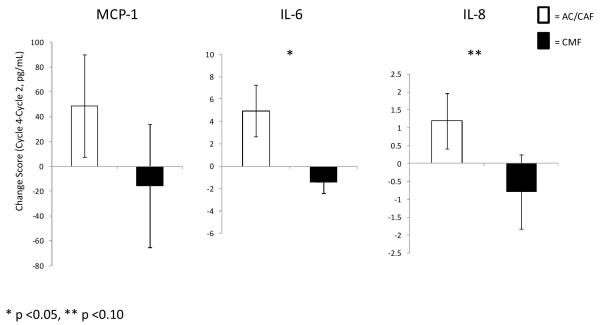
Levels of IL-6, IL-8, and MCP-1 all increased in the AC/CAF group from on-study cycle 2 (baseline) to on-study cycle 4 (mean change for IL-6 = 4.96, IL-8 = 1.19, and MCP-1 = 48.80 pg/mL); levels of these same molecules decreased in the CMF group (mean change for IL-6 = −1.46, IL-8 =−0.80, and MCP-1 =−15.81; Figure 3A). The only significant within-group change was an increase in IL-6 in the AC/CAF group (p=0.042). IL-8 was nearly significantly increased in this group (p=0.060), and IL-6 was nearly significantly decreased in the CMF group (p=0.054). Figure 3B represents changes (Cycle 4 minus Cycle2) in cytokine/chemokine levels by chemotherapy regimen.
Figure 3.
(A) Comparison of cytokine and chemokine levels among chemotherapy groups
(B) Mean changes in MCP-1, IL-6 and IL-8 according to chemotherapy regimen
ANCOVA analyses, incorporating baseline IL-6, revealed a significant treatment regimen difference for IL-6 at cycle 4 (F=9.04, p=0.004). ANCOVA analyses for IL-8 revealed a borderline trend for significance (F=3.94, p=0.054). No significant treatment regimen effects were observed for MCP-1 (F=1.46, p=0.232).
Self-Reported Cognitive Items and Relationship to Cytokines/Chemokines
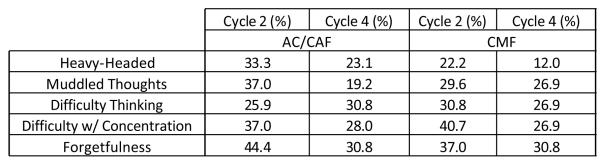
The percentages of those with self-reported cognitive complaints of heavy-headed feelings, muddled thoughts, difficulty thinking, difficulty in concentrating, and forgetfulness are reported in Figure 4A. The proportion of patients with complaints of heavy-headed feeling, muddled thoughts and forgetfulness was higher in the AC/CAF group at cycle 2; difficulty thinking and difficulty with concentration were higher in subjects receiving CMF. At cycle 4, heavy-headedness, difficulty thinking, and difficulty with concentration were all higher in the AC/CAF group; muddled thoughts were higher in the CMF group, and forgetfulness was the same in both groups. Overall, both groups reported less cognitive complaints at cycle 4 than at cycle 2, with the exception of an increase in difficulty thinking in the AC/CAF group. None of the within-group differences were significant except for the improvement in muddled thoughts in the AC/CAF group (F=−2.2, p=0.025).
Figure 4.
(A) Characterization of Cognitive Difficulties in Breast Cancer Patients Undergoing Chemotherapy
(B-C) Correlations between Changes in Cognitive Function and Cytokine/Chemokine Levels in AC/CAF and CMF Chemotherapy Groups
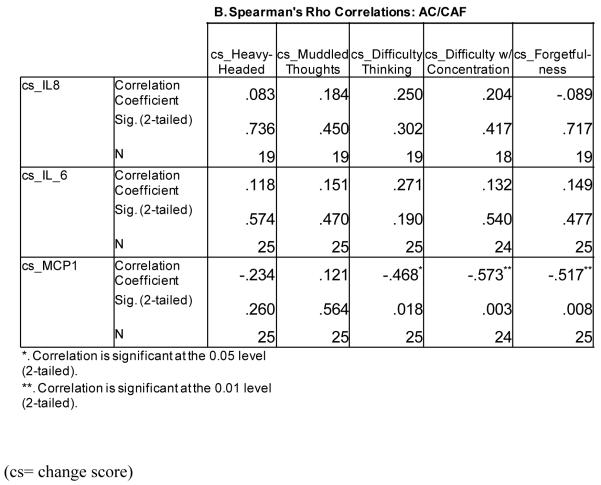
We performed preliminary correlation analyses of the relationships between these five cognitive areas and MCP-1, IL-6, and IL-8 for each chemotherapy group taking into consideration that the immune markers were measured prior to cycle 2 and cycle 4 and our cognitive assessment was done after chemotherapy treatment. In those who received AC/CAF, changes in IL-6 were positively correlated with changes in all five cognitive assessment items; however, none of these changes was significant (Figure 4B). For IL-8, positive correlations existed for all cognitive items except forgetfulness; none of these was significant. For MCP-1, we found negative correlations between heavy-headedness, difficulty thinking, difficulty with concentration, and forgetfulness; the later three were statistically significant. A positive correlation was found for MCP-1 and muddled thoughts; however, this was not significant.
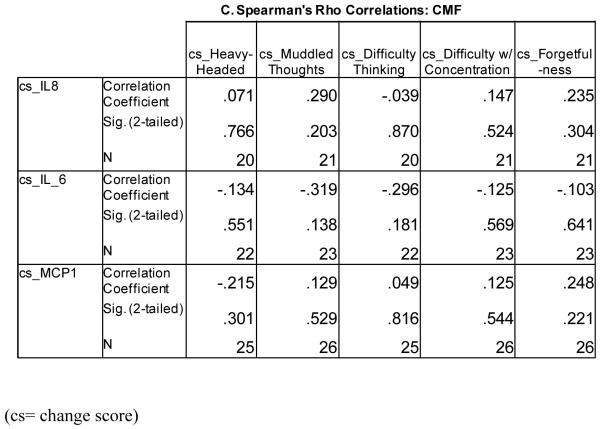
For those receiving CMF, none of the correlations were significant (Figure 4C). Negative correlations were observed for IL-6 with each of the five cognitive items. For changes in IL-8, positive correlations were observed for changes in all four questions except for difficulty thinking which was negatively correlated with changes in IL-8. For changes in MCP-1, positive correlations were observed with changes in all cognitive questions except a negative correlation with changes in heavy-headedness.
Discussion
We assessed the effects of two different chemotherapy regimens on cytokine IL-6 and chemokines MCP-1 and IL-8 to understand possible effects of chemotherapy regimens on inflammatory processes that may be implicated in chemotherapy-related cognitive difficulties. To our knowledge, this is the first study to compare the effects of two different chemotherapy regimens on these three cytokines in breast cancer patients undergoing chemotherapy. We observed that MCP-1, IL-6, and IL-8 all increased in the doxorubicin-based chemotherapy group but decreased in the CMF group over two cycles of treatment.
Both chemotherapy regimens included cyclophosphamide with or without fluorouracil. The major difference between our chemotherapy groups was adriamycin in the AC/CAF group and methotrexate in the CMF group. Therefore, differences in cytokine/chemokine responses are likely due to these specific agents.
Adriamycin, an anthracycline that intercalates DNA preventing cell replication, is associated with an increase in cytokine IL-1β in Hodgkin’s patients receiving chemotherapy regimens containing this agent (18). More specific evidence that adriamycin directly contributes to an inflammatory response comes from an animal study, in which adriamycin was given to non-tumor-bearing mice. Peripheral and brain-inherent levels of cytokine TNF-α were increased (23) even though adriamycin was not found to cross the blood brain barrier. The peripheral increase in TNF-α was also associated with an increase in brain reactive oxygen species suggesting damage; when a TNF-α blocking antibody was given, adriamycin-induced brain oxidation levels were decreased. Overall, this study suggests that the inflammatory reponses following adriamycin directly led to an elevation in brain oxidation which could result in cognitive difficulties. In fact, other animal studies have shown that adriamycin leads to impairment in performing cognitive tasks (24).
Unlike adriamycin, methotrexate, an anti-metabolite that inhibits the synthesis of purine and pyrimidine precursors, appears to have anti-inflammatory properties (25). Specifically, methotrexate can inhibit growth of monocytes and macrophages (26)—cells which can produce the cytokines and chemokines assessed in this paper. For example, IL-6 expression by human monocytes is reduced following exposure to methotrexate (27).
It is difficult to interpret the inverse but significant relationships between changes in MCP-1 levels and changes in difficulty thinking, difficulty with concentration, and forgetfulness in those receiving AC/CAF. One explanation from our results is that changes leading to reduced MCP-1 levels are associated with cognitive decline and vice versa. However, since serum cytokine levels fluctuate over time, this observation could be incomplete. It is also possible that MCP-1, which is an early-stage mediator of inflammation, could have led to signaling via other inflammatory pathways that are more indicative of cognitive difficulties at the cycle 2 to cycle 4 time-points. For example, a recent study investigated serum MCP-1 levels in those who had a diagnosis of MCI and Alzheimer’s disease. Those with MCI had high levels of MCP-1 compared to healthy controls; however, Alzheimer’s cases had decreased levels of MCP-1 compared to both MCI and healthy controls (13). This result could explain our findings if more severe cognitive difficulties are associated with reductions in MCP-1 but perhaps increases in other inflammatory molecules.
The main strength of this study was our measurement of cytokines and chemokines in patients treated with two distinct chemotherapy regimens over two cycles of treatment; therefore, we could assess changes in levels of these molecules over the two cycles of chemotherapy and determine whether those changes differed by regimen. The study would have been strengthened with biomarker measurements prior to any treatment, and prior to and after each cycle of chemotherapy. The assessment of additional inflammatory mediators and their specific signaling pathways could have provided a greater understanding of the complexity of inflammatory responses following administration of various chemotherapy regimens.
We are limited in our interpretation of the cytokine/chemokine results as they relate to cognitive difficulties in these cancer patients. First, our cytokine/chemokine measurements were done prior to chemotherapy and our cognitive assessment was performed after chemotherapy. Secondly, our cognitive questions were taken from the FSCL, which is a measure designed to assess fatigue. Additionally, our study was not adequately powered to address the relationship between cytokine/chemokine levels and cognitive function. Therefore, it is difficult to explain the significant relationships between lower MCP-1 levels (prior to chemotherapy) and greater difficulty with thinking, concentration, and forgetfulness (memory) in those receiving AC/CAF. Lastly, we did not have information on stage of disease or other treatment information (e.g. corticosteroid, hormone treatments) that may have affected cytokine levels in these patients.
Nonetheless, this study revealed that specific chemotherapy regimens can lead to different responses in chemokine/cytokine levels. The results of this study are preliminary and hypothesis-generating and need to be confirmed in larger studies that specifically evaluate multiple inflammatory mediators at multiple time-points with concurrent neuropsychological assessments and/or validated cognitive questionnaires. These studies will clarify the influence of specific chemotherapy regimens on distinct inflammatory pathways and the effects of those pathways on the development and progression of cognitive impairment in cancer patients. In this study, patients had similar levels of self-reported cognitive complaints, however, cytokines increased over time in the adriamycin-based chemotherapy group but decreased over time in those receiving CMF. Only a greater-powered study can address whether there may be multiple inflammatory mechanisms involved, and/or other contributing pathways (e.g. endocrine), in the development of chemotherapy-related cognitive difficulties. Identifying all of these related pathways may allow for the development of tailored interventions in patients with cognitive impairments.
Figure 2.
Study Schema
Acknowledgements
We would like to thank the following funding sources: NCI R25CA10618 (GRM; MCJ is a fellow) and DOD DAMD17-96-C-6106 (GRM). Additionally, we thank Mr. Eric Hernady for technical assistance on cytokine ELISAs.
Footnotes
Footnote: This paper was presented as an invited lecture at the MASCC/ISOO 2010 International Symposium in Vancouver, Canada.
REFERENCES
- 1.Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve. J Clin Oncol. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An Update on Cancer- and Chemotherapy-Related Cognitive Difficulties: State of the Field and Possible Mechanisms. Sem. Onc. 2010 doi: 10.1053/j.seminoncol.2011.03.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahles TA, Saykin AJ. Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer. 2002;3(Suppl 3):S84–90. doi: 10.3816/cbc.2002.s.018. [DOI] [PubMed] [Google Scholar]
- 4.Ahles TA, Saykin A. Cognitive effects of standard-dose chemotherapy in patients with cancer. Cancer Invest. 2001;19(8):812–20. doi: 10.1081/cnv-100107743. [DOI] [PubMed] [Google Scholar]
- 5.Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res Treat. 2009;116(1):113–23. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- 6.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–9. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 7.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 116(14):3348–56. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 8.Vardy J, Wong K, Yi QL, Park A, Maruff P, Wagner L, et al. Assessing cognitive function in cancer patients. Support Care Cancer. 2006;14(11):1111–8. doi: 10.1007/s00520-006-0037-6. [DOI] [PubMed] [Google Scholar]
- 9.Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26(7):955–69. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 10.Janelsins MC, Roscoe JA, Palesh OG, Mustian KM, Peppone LJ, Heckler CE, Morrow GR. Cognitive difficulties, fatigue, and sleep in cancer patients at pre-chemotherapy, post-chemotherapy, and at three months follow-up. Support Care Cancer. 2010 [Abstract] [Google Scholar]
- 11.Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42(3):233–40. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63(4):538–43. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 13.Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corra B, et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol Aging. 2006;27(12):1763–8. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68(22):1902–8. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 15.Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: a review. Prog Brain Res. 2007;161:303–16. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- 16.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–25. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 18.Villani F, Busia A, Villani M, Vismara C, Viviani S, Bonfante V. Serum cytokine in response to chemo-radiotherapy for Hodgkin’s disease. Tumori. 2008;94(6):803–8. doi: 10.1177/030089160809400605. [DOI] [PubMed] [Google Scholar]
- 19.Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25(3):94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Mills PJ, Ancoli-Israel S, Parker B, Natarajan L, Hong S, Jain S, et al. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain Behav Immun. 2008;22(1):98–104. doi: 10.1016/j.bbi.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roscoe JA, Morrow GR, Hickok JT, Mustian KM, Griggs JJ, Matteson SE, et al. Effect of paroxetine hydrochloride (Paxil) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Res Treat. 2005;89(3):243–9. doi: 10.1007/s10549-004-2175-1. [DOI] [PubMed] [Google Scholar]
- 22.Yoshitake H. Three caracteristic patterns of subjective fatigue symptoms. Ergonomics. 1978;21(3):231–3. doi: 10.1080/00140137808931718. [DOI] [PubMed] [Google Scholar]
- 23.Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, et al. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis. 2006;23(1):127–39. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Liedke PE, Reolon GK, Kilpp B, Brunetto AL, Roesler R, Schwartsmann G. Systemic administration of doxorubicin impairs aversively motivated memory in rats. Pharmacol Biochem Behav. 2009;94(2):239–43. doi: 10.1016/j.pbb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Cutolo M, Sulli A, Pizzorni C, Seriolo B, Straub RH. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001;60(8):729–35. doi: 10.1136/ard.60.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutolo M, Bisso A, Sulli A, Felli L, Briata M, Pizzorni C, et al. Antiproliferative and antiinflammatory effects of methotrexate on cultured differentiating myeloid monocytic cells (THP-1) but not on synovial macrophages from patients with rheumatoid arthritis. J Rheumatol. 2000;27(11):2551–7. [PubMed] [Google Scholar]
- 27.Bouma MG, Stad RK, van den Wildenberg FA, Buurman WA. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol. 1994;153(9):4159–68. [PubMed] [Google Scholar]