Abstract
Purpose
The aim was to compare late-time extrapolation of plasma clearance (CL) from Tikhonov adaptively regularized gamma variate fitting (Tk-GV) and from mono-exponential (E1) fitting.
Methods
Ten 51Cr-ethylenediaminetetraacetic acid bolus IV studies in adults—8 with ascites—assessed for liver transplantation, with 12–16 plasma samples drawn from 5-min to 24-h, were fit with Tk-GV and E1 models and CL results were compared using Passing-Bablok fitting.
Results
The 24-h CL(Tk-GV) values ranged from 11.4 to 79.7 ml/min. Linear regression of 4- versus 24-h CL(Tk-GV) yielded no significant departure from a slope of 1, whereas the 4- versus 24-h CL(E1) slope, 1.56, was significantly increased. For CL(Tk-GV-24-h) versus CL(E1-24-h), there was a biased slope and intercept (0.85, 5.97 ml/min). Moreover, the quality of fitting of 24-h data was significantly better for Tk-GV than for E1, as follows. For 10 logarithm of concentration curves, higher r values were obtained for each Tk-GV fit (median 0.998) than for its corresponding E1 fit (median 0.965), with p < 0.0001 (paired t-test of z-statistics from Fisher r-z transformations). The E1 fit quality degraded with increasing V/W [volume of distribution (l) per kg body weight, p = 0.003]. However, Tk-GV fit quality versus V/W was uncorrelated (p = 0.8).
Conclusion
CL(E1) values were dependent on sample time and the quality of fit was poor and degraded with increasing ascites, consistent with current opinion that CL(E1) is contraindicated in ascitic patients. CL(Tk-GV) was relatively more accurate and the good quality of fit was unaffected by ascites. CL(Tk-GV) was the preferred method for the accurate calculation of CL and was useful despite liver failure and ascites.
Keywords: Renal function, Tikhonov regularization, Minimization of errors, Gamma variate, Ascites
Introduction
This paper presents a validation of a curve fit method for measuring plasma clearance (CL, ml/min). The CL method validated here uses Tikhonov adaptively regularized gamma variate (Tk-GV) fitting. The word “adaptive” is used to imply minimization of relative error of CL as opposed to an ordinary curve fit minimization (Appendix and [13]). In general, radiometric CL is indicated:
To detect nephrotoxicity of chemotherapy drugs, especially in children
For many chemotherapeutic agents’ dose calculations
For detection of renal failure in patients in whom (a) serum creatinine results might be misleading, (b) missing a decline in renal function might be disastrous e.g. single kidney, renovascular disease or renal transplant and (c) a 24-h clearance measurement is difficult, e.g., in the elderly or those with learning difficulties
For assessment of potential live donors for kidney transplantation
For the evaluation and follow-up of chronic renal disease
For the evaluation of single kidney function in conjunction with relative renal function measurements from static or dynamic radionuclide imaging [2]
However, utilization of radiometric CL methods is spotty with only occasional centres performing them, with needed but inconsistently applied CL-value correction factors, without quality control of the individual CL-values, and in certain cases, current methods are relatively contraindicated. For example, liver failure, ascites, oedema and low CL are identified in the 2004 British Nuclear Medicine Society Guidelines as having problematically inaccurate CL-values [2]. On the other hand, Xirouchakis et al. [14] outline the clinical importance of CL measurements in liver failure: “Renal dysfunction is a well-established predictor of mortality in both acute liver failure and cirrhosis, particularly following the development of complications, such as sepsis. Inulin clearance and other direct methods using injected exogenous radiolabelled substances [51Cr-EDTA (ethylenediamine tetra-acetic acid), 125I-Iothalamate, 99mTc-DTPA (diethylenetriamine penta-acetic acid)] are the most accurate to assess renal function.” Thus, the need for CL measurements in liver failure is clear, but current radiometric methods are inadequate for this purpose [2, 4, 6, 8, 9].
In this study, Tk-GV CL values will be obtained for ten liver failure patients most of whom (8/10) have ascites. Investigated here is whether the Tk-GV models overcome the need for CL-value correction factors and maintain good quality even in the presence of ascites. In prior published work, the Tk-GV method has been applied to 4-h data using 99mTc-DTPA and 169Yb-DTPA [13]. Tk-GV results provide quality control for each individual CL-value. The current paper is a first application of Tk-GV to 51Cr-EDTA data, and to sampling data extended to 24-h. There is no a priori guarantee that Tk-GV will either usefully fit an untried pharmaceutical or 24-h data so testing is needed. The Tk-GV results are contrasted with those from mono-exponential (E1) fitting. E1 models fit to this new data are, from prior work, fairly certain to be problematic [13], especially in ascites [2, 4, 6, 8, 9]. Thus, how poorly E1 performs for our 24-h data is also of interest.
Intravenous (IV) bolus-injected inert markers clear into the interstitium as well as in the urine. Consequently, CL is slightly faster than glomerular filtration rate (GFR) [3, 5, 13], e.g. CL ≈ 1.076 GFR [5]. After IV bolus injection, CL estimates from multiple plasma samples are calculated from the area under the plasma marker disappearance curve (AUC) from time equals zero to infinity, i.e. CL = dose/AUC. AUC has units of concentration min. and marker (e.g. drug) systemic exposure time is plasma clearance (CL) related [1, 13]. Even the most minimalistic technique for estimating AUC, e.g. numerical integration [5], requires the use of a curve fit to extrapolate concentrations from the time of last sample to infinite time. In this paper, the E1 and Tk-GV curve fit methods for finding AUC are tested to determine the accuracy of CL from curve fitting of 4- versus 24-h data for ten 51Cr-EDTA concentration curves. A key issue explored is how well clearances determined only from 4-h data predict the results determined from 24-h data. When the 4-h CL curve does a good job of predicting the 24-h concentrations, the model extrapolates the late-time behaviour well, and the AUC values give accurate clearances. Conversely, if the 4-h CL curve fits do not predict the 24-h concentrations, then the ability of the fitting procedure to predict accurately the late-time behaviour is in question and the clearance values predicted from the method are suspect.
On a lnE1 versus t plot, lnE1 models are, of course, straight lines. However, let C obs(t) be the observed concentrations. Then, on a lnC obs(t) versus t plot, the data are curved; thus E1 models do not fit data well. This same trend, i.e. curvature, is also seen in a plot of lnC obs(t) versus lnE1, and the Pearson r-value is less than 1. Indeed, from lnC obs(t) versus lnE1, the best R2-value that can be achieved by a linear fit (i.e. an E1 model of any kind, fit to any subset of the data) is the Pearson r-value squared, i.e. r 2, as this is the limitation of the data’s linearity. To achieve a better fit and to gain better predictability, one needs to use curves that follow the curvature of the lnC obs(t) versus t plot. As we shall see, such a curve is the Tk-GV model. If such a model successfully follows the data’s curvature, the observed log concentrations, lnC obs(t), will be much better correlated with the model predictions. In other words, in a plot of lnC obs(t) versus lnC Tk-GV(t), the relationship should be almost perfectly linear, unlike the lnC obs(t) versus lnC E1(t) plots that show curvature and thus imperfect correlation.
Materials and methods
The patients are part of a research project comparing methods of assessing renal function in cirrhosis approved by the Royal Free Hospital Research Ethics Committee. Thirteen patients with cirrhosis under assessment for liver transplantation had 51Cr-EDTA bolus IV studies. Of these, three patients did not have plasma samples drawn at various times, e.g. at 12- or 24-h, and were discarded. Of the remaining ten patients, four had hepatitis C-related cirrhosis (one also with hepatocellular carcinoma), three had alcoholic cirrhosis, two had primary biliary cirrhosis, and one had non-alcoholic, fatty liver disease-associated cirrhosis. Eight were men and two women: eight were white, one black and one South Asian Indian. The median weight was 79.5 kg (range 51.3–105.8), median serum bilirubin was 66 mmol/l (range 13–390), median serum albumin was 35 g/l (range 26–40) and median INR was 1.7 (range 1.5–2.4). Hepatic encephalopathy was present in eight and ascites in eight. The patients had 12–16 plasma samples drawn from 5-min to 24-h. Their concentration curves were fit with Tk-GV and E1 models and CL results were compared using Passing-Bablok fitting and paired (correlated) samples t-tests. A minimum of two samples determines an E1 solution, and fits to data from 5-min to 4-, 12- and 24-h are examined here. Also examined is a recommended clinical method of obtaining E1 functions from plasma samples at 2- and at 4-h [2]. Exponential models have uniform concentrations (i.e. are well mixed, aka, instant mixing) in one or more partial volumes. For Tk-GV fitting, a minimum of four samples are needed. The GV concentration, C(t), model used for Tk-GV fitting is  , where C(t) is the marker concentration, α is the dimensionless volume of distribution scaling factor with V = αCL/β, β is the renal elimination rate constant, K is a constant, and the mean residence time MRT = α/β. The Tk-GV method uses least relative error of CL as a minimization target (see the Appendix) and is more robust than GV fitting with least-squares regression. The fit results for well-posed GV CL-values (0 < α ≤ 1, β > 0, and fits from 5 or 10 min to at least 3-h of data) are extensively described elsewhere [13]. In specific, the 0 < α ≤ 1 constraint is only observed with Tk-GV fitting and does not occur with least-squares GV fitting. A Tk-GV model of an inert marker is a poorly mixed system with a concentration gradient such that the physical volume of the system is factor of α times smaller than the virtual volume (where the latter is CL/β). Moreover, the dynamic equilibrium concentration gradient is established by renal function and disappears, i.e. α → 1, only when renal function approaches zero [13].
, where C(t) is the marker concentration, α is the dimensionless volume of distribution scaling factor with V = αCL/β, β is the renal elimination rate constant, K is a constant, and the mean residence time MRT = α/β. The Tk-GV method uses least relative error of CL as a minimization target (see the Appendix) and is more robust than GV fitting with least-squares regression. The fit results for well-posed GV CL-values (0 < α ≤ 1, β > 0, and fits from 5 or 10 min to at least 3-h of data) are extensively described elsewhere [13]. In specific, the 0 < α ≤ 1 constraint is only observed with Tk-GV fitting and does not occur with least-squares GV fitting. A Tk-GV model of an inert marker is a poorly mixed system with a concentration gradient such that the physical volume of the system is factor of α times smaller than the virtual volume (where the latter is CL/β). Moreover, the dynamic equilibrium concentration gradient is established by renal function and disappears, i.e. α → 1, only when renal function approaches zero [13].
Passing-Bablok fitting is used here for comparison of methods to provide unbiased linear regression slope and intercept [7]. The Passing-Bablok method includes the cusum test for linearity [7], which is useful for comparison of methods because it detects nonlinear relationships. Cusum p > 0.1 does not suggest nonlinearity, 0.05 < p < 0.1 suggests nonlinearity, and p < 0.05 is highly suggestive of nonlinearity. Spearman rank correlation is used once for extracting a correlation in a slightly nonlinear relationship. Paired (i.e. two, correlated) samples t-testing is used here for comparison of methods using all the data. Two-sample t-testing is also applied to (paired) z-statistics from Fisher r-z transformation for detecting improved correlation. This transformation changes the otherwise non-normally distributed correlation coefficients (r) to more normally distributed z-statistics, using the inverse hyperbolic tangent function, z = atanh (r). C obs(t) versus ln(t) plots are used to explain modelling effects. However, for calculating model fit quality correlations, we use the logarithm of both the observed concentrations and the predicted concentrations from the E1 and Tk-GV models. To test how linear the lnC obs(t) versus lnC Model(t) relationship is, one simply calculates its Pearson r value. (The correlation coefficient of determination, R2, is then the Pearson r-value squared, i.e. r 2.) The test for ascites looks at the trend of fit quality, r, versus V/W, where V is the volume of distribution (ml) of the Tk-GV model, and W is the patient mass in kilograms. A model r-value that degrades with increasing ascites (as indexed to V/W) suggests a poorly behaved model. If the model r-value is unchanged by increasing ascites, the model is relatively bulletproof.
Results
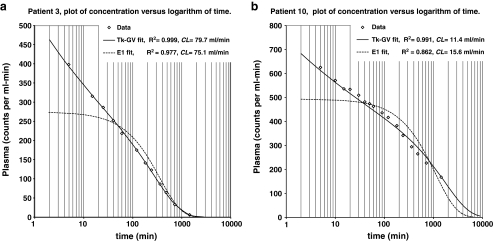
Figure 1 shows plots of 24-h concentrations of 51Cr-EDTA in counts per ml min, C(t), versus the logarithm of time, t, in min. Figure 1 plots show the highest and lowest CL(Tk-GV) cases (79.7 and 11.4 ml/min) in this series. These plots suggest an improvement in fit quality for 24-h data fits using Tk-GV functions compared to the E1 functions. C(t) versus ln(t) plots roughly linearize the early-time C(t). Figure 1 demonstrates that the exponential fit assumption of a constant concentration at time zero causes underestimation of the early concentrations of actual data. Let us define late-time C(t) as being after the concentration curve becomes nonlinear in ln(t), i.e. asymptotic to the C(t) = 0 axis. Figure 1a demonstrates that when an exponential fit includes the late-time concentrations, late-time concentrations are accurately fit. Figure 1b illustrates that when an exponential fit does not include late-time concentrations, the late-time concentrations are underestimated.
Fig. 1.
a Plot of 24-h concentrations of 51Cr-EDTA in counts per ml min, C(t), per logarithm of time, t, in min for patient 3’s data. For patient 3’s 24-h data, R2(Tk-GV) = 0.999 > R2 = 0.977 from a one exponential term model (E1). The CL(Tk-GV) for patient 3 is the highest CL in this series (79.7 ml/min). b Plot of 24-h concentrations, C(t), per logarithm of time, t, for patient 10’s data, having the lowest CL(Tk-GV) in this series (11.4 ml/min). Right-hand areas contribute more to AUC than those on the left do, for this plot type. For example in b, the AUC for the Tk-GV curve from 1,440 min to infinite time is 47.8% of the total area
For several reasons, the correlations (r) used for comparison of methods are calculated from the observed versus predicted logarithms of concentration values. These r-values (Table 1) are only indirectly related to r-values calculated without taking logarithms (e.g. the R2 in Fig. 1). Comparing methods, the improvement in correlation to logarithm concentration of lnC obs(t) and lnC Model(t) was highly significant (p < 0.0001) for Tk-GV functions compared to the E1 functions using paired samples t-testing of the z-values from z = atanh(r). Moreover, the r-values are better for Tk-GV than for E1 fits in each of the ten cases (Table 1). With a median 24-h Tk-GV fit of r = 0.998, there is little unexplained variance in the logarithm concentration data models (1−r 2 = 0.4%). This gives us confidence that extrapolation from 1,440 min to infinite time and from 5 min to zero time is accurate. Next, one uses Tk-GV fits to estimate the percentage of area under the curve (AUC) of concentration versus time not directly under the samples. Not surprisingly, there is a lesser percentage of extrapolated AUC for higher CL (e.g. Table 1, case 3, 5.4%), and a higher percentage for lower CL (case 10, 48.3%), and it takes longer than 24-h to clear 51Cr-EDTA in renal failure. However, the goodness of fit for Tk-GV allows us to have confidence that 24 h of AUC data is long enough to calculate accurate CL-values. An indication of CL(Tk-GV) precision is given as standard deviation (SD) of CL, which values range from 0.4 to 3.5 ml/min in Table 1. The Tk-GV method produces these estimates during processing. These estimates correspond to 95% confidence intervals for errors of 24-h CL-values that range from ±0.8 to ±6.9 ml/min.
Table 1.
From 24-h fitting to logarithms of concentrations for Tk-GV, CL and V (l) ± 1 standard deviation (SD) error, V as percent of W (weight-kg), and percentage of AUC from extrapolation from left tails (0 to 5 min) and right tails (1,440 min to infinite time). Also shown are correlation coefficients, r, for Tk-GV and E1 fitting
| Case | CL ±1 SD (ml/min) | V ±1 SD (l) | V/W (%) | AUC of tails (%) | Tk-GV r | E1 r all fits |
|---|---|---|---|---|---|---|
| 3 | 79.7 ± 0.4 | 29.7 ± 0.2 | 34.9 | 5.4 | 1.000 | 0.988 |
| 5 | 76.5 ± 1.6 | 38.9 ± 1.2 | 45.2 | 13.0 | 0.999 | 0.933 |
| 6 | 56.5 ± 0.9 | 21.3 ± 0.6 | 29.6 | 6.8 | 0.998 | 0.975 |
| 9 | 46.5 ± 0.8 | 18.6 ± 0.3 | 22.8 | 6.9 | 0.999 | 0.977 |
| 4 | 45.8 ± 0.8 | 21.9 ± 0.4 | 35.6 | 9.0 | 0.999 | 0.971 |
| 12 | 42.5 ± 2.1 | 26.9 ± 0.9 | 39.7 | 13.7 | 0.995 | 0.960 |
| 13 | 36.4 ± 3.1 | 41.9 ± 1.8 | 39.6 | 29.3 | 0.988 | 0.937 |
| 8 | 29.9 ± 3.5 | 60.8 ± 4.7 | 59.9 | 45.5 | 0.998 | 0.848 |
| 11 | 21.2 ± 0.8 | 17.7 ± 0.4 | 34.7 | 19.5 | 0.998 | 0.970 |
| 10 | 11.4 ± 0.9 | 23.4 ± 0.6 | 30.4 | 48.3 | 0.994 | 0.929 |
As per the “Materials and methods” section, lnC from E1 fit equations from any sample subsets have at best a quality of fit to lnC
obs(t) of the correlation coefficient evaluated at all the sample times (median as r = 0.965, see Table 1). The correlation of r-values from E1 24-h data fits with V/W (Table 1) is significantly negative,  , p = 0.0034. However, the correlation of r-values from 24-h Tk-GV fits to the data with the V/W from the same Tk-GV model is uncorrelated,
, p = 0.0034. However, the correlation of r-values from 24-h Tk-GV fits to the data with the V/W from the same Tk-GV model is uncorrelated,  , p = 0.8165.
, p = 0.8165.
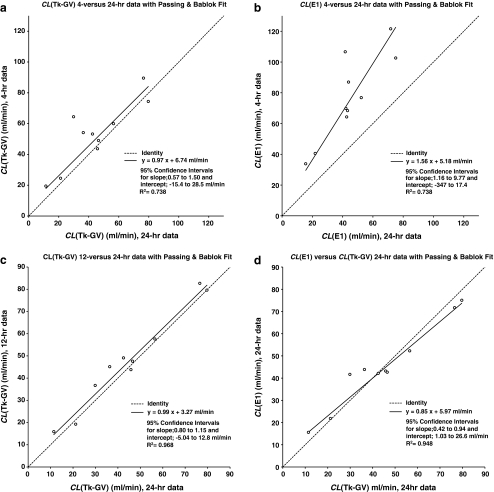
Changing sample subsets can produce different CL-values, especially for E1, as follows. With reference to Table 2 and Fig. 2a, the 4-h (vertical axis) versus 24-h (horizontal axis) CL(Tk-GV) plot yielded no significant Passing-Bablok departure from a line slope of 1 or an intercept of 0. This means that 4-h Tk-GV clearances unbiasedly predict 24-h Tk-GV clearances. However, the R2-value for this relationship is only 0.738, and the more powerful paired samples t-test shows a (borderline) significant bias (p = 0.04). This borderline significant mean difference is 8.6 ml/min greater CL measured for the 4-h data than for the 24-h data. Much of the variance for predicting 24-h CL from 4-h data is not related to the method of calculation but to the data themselves, i.e. from propagation of errors. For example, the furthest 4-h versus 24-h CL(Tk-GV) outlier of Fig. 2a, 64.4 versus 29.9 ml/min, is only a near outlier at 2.6 interquartile ranges (IQR) and would not usually require explanation. To decrease the 4- versus 24-h CL(Tk-GV) difference from 11.5 to 3.9 ml/min (1 SD), one carries the fits out to 12-h (instead of 4-h) of data when comparing to the 24-h CL. Note (Table 2) that for 12- versus 24-h, there is no significant departure from a line slope of 1 or an intercept of 0 and the R2 improves (0.968). However, the t-test, 3.1 ml/min mean bias, although small, is still (borderline) significant (p = 0.03).
Table 2.
Tk-GV and E1 line fit tests for slope, m =1, and intercept b = 0 and test for significance of different mean values of CL
| Passing-Bablok fit lines for | Parameter values | 95% CI | CI | Paired t test (Δ in ml/min) | R 2 | |||
|---|---|---|---|---|---|---|---|---|
| (h) = 4, 12, 24, 2&4 | (b in ml/min) | Low to high | Significance | Probability | Significance (Δ) | |||
| Tk-GV(4) = m•Tk-GV(24) + b | m 0.971 | 0.568 to 1.50 | NS | 0.0416 | B (8.6) | 0.738 | ||
| b | 6.7 | −15.4 to 28.5 | NS | |||||
| Tk-GV(12) = m•Tk-GV(24) + b | m | 0.986 | 0.795 to 1.15 | NS | 0.0337 | B (3.1) | 0.968 | |
| b | 3.3 | −5.0 to 12.8 | NS | |||||
| E1(4) = m•E1(24) + b | m | 1.56 | 1.16 to 9.77 | S | 0.0001 | S (32.1) | 0.738 | |
| b | 5.2 | −347 to 17.4 | NS | |||||
| E1(12) = m•E1(24) + b | m | 1.11 | 0.98 to 1.45 | NS | 0.0002 | S (9.1) | 0.951 | |
| b | 3.0 | −10.7 to 7.63 | NS | |||||
| E1(4) = m•Tk-GV(4) + b † | m | 1.31 | 1.04 to 1.62 | B | <0.0001 | S (23.9) | 0.928a | |
| b | 6.5 | −8.6 to 18.9 | NS | |||||
| E1(24) = m•Tk-GV(24) + b | m | 0.850 | 0.417 to 0.940 | S | 0.8386 | NS (0.4) | 0.948 | |
| b | 6.0 | 1.0 to 26.6 | B | |||||
| E1(2&4) = m•Tk-GV(24) + b | m | 1.01 | 0.626 to 1.59 | NS | 0.0008 | S (20.7) | 0.740 | |
| b | 16.4 | −7.0 to 36.0 | NS | |||||
CI confidence interval, R 2 is coefficient of determination, S significant (p < 0.01), NS not significant (p > 0.05), B borderline significant (0.01 < p < 0.05)
aNonlinear, 0.05 < p < 0.1 from cusum. However, R 2 (0.928) and the Spearman rank coefficient squared (0.929) are similar
Fig. 2.
a The Tk-GV plasma clearance method, CL(Tk-GV), was applied to 4- and 24-h data and plotted. When this is fit with a Passing-Bablok regression line, there is no significant departure from unity slope and no significant y-intercept. b The 4-h data CL(E1), plotted against 24-h CL(E1), has a slope significantly >1, i.e. the 24-h CL(E1) values are overestimated by 4-h CL(E1) calculations. c
CL(Tk-GV) for 12- and 24-h data and no significant departure from a slope of 1 and an intercept of zero. d Even for 24-h data,  , with both the slope
, with both the slope  and the intercept
and the intercept  of the Passing-Bablok linear fit
of the Passing-Bablok linear fit
Significantly poorer results were obtained from the 4-h versus 24-h E1 fitting (see Table 2). Figure 2b shows these results plotted with a regression slope of 1.56, i.e. the 4-h CL-values are significantly larger than the 24-h CL-values with a very significant (p = 0.0001) mean difference of 32.1 ml/min. However, the R2 value for this plot is 0.738, whereas the R2 value (or Spearman rank sum squared, rs 2) for 4-h CL(Tk-GV) versus CL(E1) data would be significantly better, i.e. 0.928 (rs 2 = 0.929). That the R2 values of 4- versus 24-h CL(Tk-GV) and 4- versus 24-h CL(E1) are equal to 3 decimal places (i.e. 0.738), and 4-h CL(Tk-GV) and CL(E1) agree better with each other (0.928) than either 4-h CL agrees with its corresponding 24-h result (i.e. 0.738) appears to confirm error propagation as explaining much of the variance for predicting 24-h CL from 4-h data.
When the sample times included in the fit are increased to 12 h, then CL(E1-12-h) > CL(E1-24-h) in all cases. The paired t-test for this is highly significant (p = 0.0002, mean difference 9.1 ml/min), with an improved R2 (0.951), (see Table 2). From the 24-h data (Fig. 1d), the plot of CL(Tk-GV) versus CL(E1) has a significantly biased slope (0.85) and borderline significant intercept (5.97 ml/min, 0.01 < p < 0.05). Note that from Table 2, the t-test p = 0.8386 mean difference of 0.4 ml/min is not significant. In other words, on the average, CL(Tk-GV-24-h) values did not differ from CL(E1-24-h) values, but, for low CL-values, CL(E1) > CL(Tk-GV), and for high CL-values, CL(E1) < CL(Tk-GV).
The slope intercept (E1) 2- and 4-h plasma sample method results follow. The Passing-Bablok regression slope of CL(E1-2- & 4-h) versus CL(Tk-GV-24-h) has no bias detected for slope (1.01) and intercept (16.4 ml/min). However, the intercept is larger than for CL(Tk-GV-4-h) versus CL(Tk-GV-24-h) (6.7 ml/min). Indeed, paired t testing (Table 2) shows the mean bias (20.7 ml/min) to be highly significant (p = 0.0008) for CL(E1-2- & 4-h) versus CL(Tk-GV-24-h). This compares to the more moderate mean bias (8.6 ml/min) of borderline significance (p = 0.0337) for the CL(Tk-GV-4-h) versus CL(Tk-GV-24-h) plot. The Passing-Bablok regression of CL(E1-24-h) (vertical axis) versus CL(E1-2- & 4-h) (horizontal axis) has a significantly lesser slope (p < 0.01) than 1, i.e. 0.65. However, both CL(E1-24-h) and CL(E1-2- & 4-h) have r-values for fitting the logarithms of concentrations that are very significantly poorer (z-statistic t-test p < 0.0001) than CL(Tk-GV-24-h).
Discussion
The Tk-GV models have a less obvious dependence on sampling-time effects than E1 fits. They also have less bias for extrapolation and fit the data very significantly better (p < 0.0001). Of some concern is that the predictions of 24-h CL from 4-h CL data were imprecise (R2 = 0.738). This lower than optimal coefficient of determination is partly due to a restricted range of CL-values from examining low renal function patients only, may partly be due to biological fluctuation of CL in time and is due to the propagation of errors of measured concentrations, which in turn is due to errors in sample measurements, e.g. see [2]. In early prospective clinical experience, CL(Tk-GV) has been implemented for four-sample 4-h 99mTc-DTPA, with some notable features being that the ease of use, stability of the solutions and that the error calculations help to improve sampling technique and inspire confidence in the results. Some of the sources for error are easily addressed. For example, extravasation in the injection site is reduced by applying the flush-bolus technique and checked for by imaging of the injection site [12]. Whatever the causes for error, use of Tk-GV has the advantage of increased accuracy for extrapolated CL (defined as lack of bias) while having precision (defined as R2 for CL extrapolation) as good as or better than that of E1 fitting.
Fractal structures have been postulated as causing a gamma variate (GV) shape for inert substance CL-curves [13]. However, it is only when a GV is found using a CL relative-error minimizing technique that GV (1) accurately predicts the late-time concentration and (2) consistently forms a model with a dynamic concentration gradient established by renal function. The results here suggest that the 24-h CL(Tk-GV) values are a standard against which to judge clearance, because of the very good quality of all of the curve fits (median r = 0.998) (see Fig. 1 and Table 1). Moreover, prediction of 24-h CL(Tk-GV) from 4- and 12-h CL(Tk-GV) was good, suggesting that 24-h Tk-GV fits should extrapolate concentration correctly at even later times. In particular for six statistical tests, CL(Tk-GV) extrapolation was unbiased for four tests and borderline biased (i.e. 0.01 < p < 0.05) for two, whereas CL(E1) was significantly biased in three of six tests. The 4-h CL(Tk-GV) versus CL(E1) (Table 2) is somewhat nonlinear (cusum probability, 0.05 < p < 0.1 [7]) and the clinically used slope intercept method with 2- and 4-h plasma samples differs from 24-h CL(Tk-GV) by a constant (mean difference 20.7 ml/min, p = 0.0008), so there is no “quick-fix” linear transformation of CL(E1) values to estimate CL(Tk-GV). This is discordant with the literature suggesting that CL(E1-2- & 4-h) may be corrected by multiplying by 0.87, i.e. the Chantler correction factor, and does not suggest subtracting a constant [2]. However, it is easy enough to see how such an impression could arise as the Passing-Bablok regression of CL(E1-24-h) (vertical axis) versus CL(E1-2- & 4-h) (horizontal axis) only has a significant slope (0.65, p < 0.01 of slope =1) and an insignificant intercept. However, both CL(E1-24-h) and CL(E1-2- & 4-h) have r-values for fitting the logarithms of concentrations that are very significantly poorer (z-statistic t-test p < 0.0001) than CL(Tk-GV-24-h). Thus, subtraction of a 16.4 (or 20.7) ml/min constant from the CL(E1-2- & 4-h) values may be the more accurate correction, at least for our patients with ascites [and if one accepts that CL(Tk-GV-24-h) is a gold standard].
A final caveat is that eight of ten patients had ascites. Ascites has been associated with increased CL for compartmental models by 16–20 ml/min [4, 6, 9] and is considered by some to relatively contraindicate CL testing due to gross inaccuracy [2]. However, ascites seems to have had little effect on the CL(Tk-GV)-values here. This is significant and it needs to be emphasized that the Tk-GV method is valid in patients with low GFR and with ascites. This comes about as the Tk-GV volumes of distribution (V) (and V/W, Table 1) are larger than usual (e.g. see [2]). However, that is an expected result for ascites (also see [8]), as follows. For a bolus clearance model, MRT=V/CL, where MRT is the mean residence time. If in that equation CL is held constant, then for a longer residence time, V must be larger, and for a good model, that is an expected outcome of ascites. V/W is our measure of ascites from the 24-h Tk-GV model. The correlation of r-values from E1 24-h data fits with V/W (Table 1) is significantly negative,  , p=0.0034. However, the correlation of r-values from 24-h Tk-GV fits to the data with V/W from the same Tk-GV model is insignificant,
, p=0.0034. However, the correlation of r-values from 24-h Tk-GV fits to the data with V/W from the same Tk-GV model is insignificant,  , p = 0.8165. For
, p = 0.8165. For  , we have not caused a correlation by reusing parameters, because the only sharing between models is from the characteristics of the data themselves. For
, we have not caused a correlation by reusing parameters, because the only sharing between models is from the characteristics of the data themselves. For  , we have reused parameters, and despite this, there is no correlation. We conclude that using V/W (from 24-h Tk-GV) as an index of the severity of ascites, E1 fits degrade as ascites increases, and Tk-GV fit quality is not measurably affected. This finding confirms the observation that use of E1 (or E1 extrapolation) is problematic in ascites [4, 6, 8, 9] and presents a solution to this problem using Tk-GV. To inspect this in detail, let us examine the GV rate equation,
, we have reused parameters, and despite this, there is no correlation. We conclude that using V/W (from 24-h Tk-GV) as an index of the severity of ascites, E1 fits degrade as ascites increases, and Tk-GV fit quality is not measurably affected. This finding confirms the observation that use of E1 (or E1 extrapolation) is problematic in ascites [4, 6, 8, 9] and presents a solution to this problem using Tk-GV. To inspect this in detail, let us examine the GV rate equation,
 |
1 |
where (α−1)/t is definite negative, is associated with tissue clearance, and gets slower and slower in time (it is proportional to 1/t). CL>GFR implies α≤1, where α>1 is a common [8], but ill-posed solution for CL for GV fitting [13]. Here, using Tk-GV fitting, the entire range of α for our 24-h data is from 0.62 to 0.88, i.e. α is well behaved. So α does not change by much to accompany an increase of fluid volume. Here, a low α=0.67 coincides with the most inflated volumes of distribution as a percentage of patient mass 59.9% (case 8, Table 1).
Normally slow or pathologically slow clearance areas in the body include ascites or abdominal fluid, some adenomas and neoplasias, some chemotherapy, pleural or pericardial fluid, lymphatic circulation and lymphoedema, cerebrospinal fluid circulation, synovial fluid, circulation in aqueous humour, abscess, cellulitis, fibrous dysplasia, etc. The entire spectrum of slow tissue marker leakage rates is included in GV rate Eq. 1 because for all t>(1−α)/β, the rates of losses of marker into tissue are slower than renal loss. That is, tissue marker leakage rates converging to zero (i.e. to an infinite half-life) are an integral part of the GV model. By contrast, an infinite sum of exponential terms would be needed to approximate the effect of the temporal spectrum of the single, gradually decreasing rate term of the GV model.
Why only compare Tk-GV to E1 fitting? Why not then test E2, E3, E4 or En (i.e. sums of exponential terms) in general? The reason is that a robust model is strongly desirable, and E(n > 1) is not stable for regression analysis unless the number of samples is substantially greater than n [10, 13]. Although methods exist for stabilizing E2 models (e.g. see [11]), in general for regression, En is not robust, indeed ordinary least-squares GV function fitting is also not robust. However, GV fitting can be made robust by regularization (as in Tk-GV [13]). It is at best tedious and at worst impossible to obtain four (or more) stable parameter estimates with an unstable E2 (or higher) regression model. By contrast, a single attempt suffices to obtain the globally converged Tk-GV model’s three parameters, with superior quality curve fits, better extrapolation to late-time concentrations from fits to early concentration data and using fewer samples [13]. For practical measurements, one strongly desires either a simple robust model (e.g. E1 with correction factors) or another robust model (e.g. Tk-GV with no need of correction factors). However, as shown in the results above (Table 2) the magnitude and the type of the correction factors needed for E1 CL-values are a function of the times used to obtain the samples. Even if it were clear how to correct E1 CL-values (and it is not), any such timing correction formulae would have empirical coefficients. For example, these formulae would not apply to both small and large mammals without allometric rescaling. Moreover, E1 fit quality degrades in ascites, rendering the utility of the model questionable. In summary, making “corrections” to an inherently flawed exponential term model is not optimal. The better approach using Tk-GV better fits the observed real-life data, without corrections needed.
Is 24-h a long enough data collection time to determine CL in renal failure (CL < 15 ml/min)? The results suggest that if we use E1 functions for fitting, the answer would be no. It is common to plot logarithm concentration versus time, which would linearize lnC(t)≈−at + b, i.e. an E1 function. In fact, note in Fig. 1 that C(t) at early times is approximately linear in lnt, i.e. C(t)≈−alnt + b. This latter plot type helps visually confirm the correlation results, in that E1 functions have the wrong shape and do not follow the concentration curves well. Adding more exponential terms does not solve the curve shape problem. Let us define late-time C(t) as being after the concentration curve becomes nonlinear in ln(t), i.e. asymptotic to the C(t)=0 axis. Figure 1b demonstrates that when an exponential fit does not include late-time concentrations, the late-time concentrations are underestimated. This explains the overestimation of CL (from underestimation of AUC) for (all choices of) E1 early-time extrapolations to later-time fits in Table 2. However, with the better-fitting Tk-GV functions, the extrapolation errors are not so severe, as suggested by extrapolation testing of early data. Using Tk-GV for the lowest CL value (11.4 ml/min, case 10, Fig. 1b), one predicts that it would take 6 days of data collection for the concentration to go below 1% of the concentration measured in the 5-min sample. Adding a “terminal” exponential to the 1-day data collection would underestimate the concentration at day 6, as exponentials go to zero too quickly to mimic late concentration. Adding another early exponential term to the Fig. 1 E1 fits would not remove the exponential fit assumption of a constant concentration at time zero causing underestimation of the early concentrations of actual data. On the other hand, finding the AUC is not especially challenging when the data collected include all the concentrations that contribute to them, and any arbitrary interpolative curve fitting, or numerical integration [5], will suffice for that purpose. Especially as relates to early-time only data collections, proper extrapolation is not as arbitrary as interpolation and must be validated by extrapolation testing, done here for Tk-GV. Thus, if a 4- or 12-h test is desired for finding CL, Tk-GV fitting appears to provide an accurate (i.e. relatively unbiased) method of predicting CL for 24-h, even in the presence of ascites.
Conclusion
CL(E1) values were dependent on sample time and the quality of fit was poor and degraded with increasing ascites, consistent with current opinion that CL(E1) is contraindicated in ascitic patients. CL(Tk-GV) was relatively more accurate and the good quality of fit was unaffected by ascites. CL(Tk-GV) was the preferred method for the accurate calculation of CL and was useful despite liver failure and ascites.
Acknowledgments
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- α
Shape parameter of GV, volume scale factor, and plasma leak term Eq. 1
- AUC
Area under the curve of concentration in time, i.e.

- β
Rate constant for GV (per min)
- CL
Plasma clearance, D/AUC
- Cobs(t)
Observed concentration, in Bq/ml, often scaled as dose percent per ml
- C(t)
Continuous function for estimation of C obs(t)
- CV
Coefficient of variation, SD/mean
- D
The dosage in Bq or per cent
- E1, E2, En
Sum of exponential terms model with 1, 2 or n terms, also, 1, 2, or n-compartment model
- GV
Gamma variate,

- Γ
Tikhonov matrix [do not confuse with Γ(x)]
- Γ(x)
The gamma function,
 , is a generalization of the factorial,
, is a generalization of the factorial, 
- K
Constant of proportionality for GV
- MRT
Mean residence time
- Ψ(α)
(Psi) digamma function of α is


SDs of
 for Tk-GV Eq. 4
for Tk-GV Eq. 4

SD of V from Tk-GV Eq. 5
- SD
Standard deviation
- Tk-GV
Tikhonov regularization of GV fit of CV of CL
- V
Volume of distribution of marker (ml or l)
Appendix
Tikhonov regularization
For an overdetermined system of linear equations, Ax = b, the Tikhonov regularization (Tk) of this problem introduces the penalty function Γx and seeks to find a solution that minimizes  . This latter is the square of a norm of the residuals,
. This latter is the square of a norm of the residuals,  , plus the square of a norm of the product of the Tikhonov matrix, Γ, with the x fit parameters (unknowns). The more general ΓTΓ regularizing term is often, as it is here, replaced by λ
I, where I is the identity matrix, and λ is a Lagrange (i.e. constraint) multiplier, also commonly called the shrinkage, Tikhonov or damping factor.
, plus the square of a norm of the product of the Tikhonov matrix, Γ, with the x fit parameters (unknowns). The more general ΓTΓ regularizing term is often, as it is here, replaced by λ
I, where I is the identity matrix, and λ is a Lagrange (i.e. constraint) multiplier, also commonly called the shrinkage, Tikhonov or damping factor.
A constraint on lnK
A most common constraint for regression is to require the fit function to pass through the data mean point [aka the centroid, ( )]. Because the logarithm of concentrations is the more homoscedastic quantity, it is common to fit the logarithms of marker concentrations rather than the concentrations themselves. Thus, for the Tk-GV method, the GV function is written
)]. Because the logarithm of concentrations is the more homoscedastic quantity, it is common to fit the logarithms of marker concentrations rather than the concentrations themselves. Thus, for the Tk-GV method, the GV function is written  , where the constant term
, where the constant term  need not be independent, but can be determined from the other fit parameters using a mean value constraint. Taking averages over the data
need not be independent, but can be determined from the other fit parameters using a mean value constraint. Taking averages over the data
 |
2 |
such that  ,
,  and
and  are data constants, where
are data constants, where  is the mean value of the logarithms of the concentrations,
is the mean value of the logarithms of the concentrations,  is the mean of the logarithms of the sample times and
is the mean of the logarithms of the sample times and  is the mean of the sample times. Then, Eq. 2 is used to remove K from the formula for CL, and an expression is derived for the errors in CL with only α and β as independent parameters, as follows
is the mean of the sample times. Then, Eq. 2 is used to remove K from the formula for CL, and an expression is derived for the errors in CL with only α and β as independent parameters, as follows
 |
3 |
where the gamma function,  .
.
Error propagation and the error adaptive equation
One applies error propagation (Taylor series expansion) to the right-hand CL expression of Eq. 3 with respect to α and β yielding
 |
4 |
where Ψ(α) is the digamma function of α and  , the subscripted s variables are the standard deviations of the subscripted quantities and (s
CL/CL)2 is the squared coefficient of variation CV2 of CL. Minimizing the right-hand side of Eq. 4 as a function of the shrinkage, λ, selects a λ value that produces the CL value with the smallest relative error achievable. Also, minimizing the relative error in CL is indispensable for making reliable measures of CL when CL is small.
, the subscripted s variables are the standard deviations of the subscripted quantities and (s
CL/CL)2 is the squared coefficient of variation CV2 of CL. Minimizing the right-hand side of Eq. 4 as a function of the shrinkage, λ, selects a λ value that produces the CL value with the smallest relative error achievable. Also, minimizing the relative error in CL is indispensable for making reliable measures of CL when CL is small.
The squared coefficient of variation, CV2, of the individual volumes of distribution, i.e. (s V/V)2, is calculated from the SDs produced from minimizing Eq. 4 substituted into
 |
5 |
Equation 5 is from application of the propagation of error formula to the substitution of Eq. 3 into V=(α/β)CL.
References
- 1.Calvert AH, Egorin MJ. Carboplatin dosing formulae: gender bias and the use of creatinine-based methodologies. Eur J Cancer. 2002;38:11–16. doi: 10.1016/S0959-8049(01)00340-9. [DOI] [PubMed] [Google Scholar]
- 2.Fleming JS, Zivanovic MA, Blake GM, Burniston M, Cosgriff PS, British Nuclear Medicine Society Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun. 2004;25:759–769. doi: 10.1097/01.mnm.0000136715.71820.4a. [DOI] [PubMed] [Google Scholar]
- 3.Florijn KW, Barendregt JNM, Lentjes EGW, van Dam W, Prodjosudjadi W, van Saase JL, et al. Glomerular filtration rate measurement by “single-shot” injection of inulin. Kidney Int. 1994;46:252–259. doi: 10.1038/ki.1994.267. [DOI] [PubMed] [Google Scholar]
- 4.Henriksen JH, Brøchner-Mortensen J, Malchow-Møller A, Schlichting P. Over-estimation of glomerular filtration rate by single injection [51Cr]EDTA plasma clearance determination in patients with ascites. Scand J Clin Lab Invest. 1980;40:279–284. doi: 10.3109/00365518009095579. [DOI] [PubMed] [Google Scholar]
- 5.Moore AE, Park-Holohan SJ, Blake GM, Fogelman I. Conventional measurements of GFR using 51Cr-EDTA overestimate true renal clearance by 10 percent. Eur J Nucl Med Mol Imaging. 2003;30:4–8. doi: 10.1007/s00259-002-1007-y. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen SS, Havsteen H, Petersen LK, Nielsen LE, Rehling M. Patients with ovarian cancer have elevated (51)Cr-EDTA plasma clearance early post-operatively. Nucl Med Commun. 2002;23:917–920. doi: 10.1097/00006231-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 8.Perkinson AS, Evans CJ, Burniston MT, Smye SW. The effect of improved modelling of plasma clearance in paediatric patients with expanded body spaces on estimation of the glomerular filtration rate. Physiol Meas. 2010;31:183–192. doi: 10.1088/0967-3334/31/2/005. [DOI] [PubMed] [Google Scholar]
- 9.Rehling M, Stadeager C, Henriksen JH, Siemsen O, Krintel JJ, Malchow-Møller A, et al. Measurement of glomerular filtration rate in patients with ascites. In: Thomsen HS, Nally JP Jr, Britton K, Frùkiñr J, et al., editors. Radionuclides in nephrourology. Copenhagen: FADL; 1998. pp. 114–118. [Google Scholar]
- 10.Russell CD. Optimum sample times for single-injection, multisample renal clearance methods. J Nucl Med. 1993;34:1761–1765. [PubMed] [Google Scholar]
- 11.Russell CD, Taylor AT, Dubovsky EV. A Bayesian regression model for plasma clearance. J Nucl Med. 2002;43:762–766. [PubMed] [Google Scholar]
- 12.Wesolowski CA, Hogendoorn P, Vandierendonck R, Driedger AA. Bolus injections of measured amounts of radioactivity. J Nucl Med Technol. 1988;16:1–4. [Google Scholar]
- 13.Wesolowski CA, Puetter RC, Ling L, Babyn PS. Tikhonov adaptively regularized gamma variate fitting to assess plasma clearance of inert renal markers. J Pharmacokinet Pharmacodyn. 2010;37:435–474. doi: 10.1007/s10928-010-9167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xirouchakis E, Marelli L, Cholongitas E, Manousou P, Calvaruso V, Pleguezuelo M, et al. Comparison of cystatin C and creatinine-based glomerular filtration rate formulas with 51Cr-EDTA clearance in patients with cirrhosis. Clin J Am Soc Nephrol. 2011;6:84–92. doi: 10.2215/CJN.03400410. [DOI] [PMC free article] [PubMed] [Google Scholar]