Abstract
Purpose
Melanoma is a heterogeneous disease where monotherapies are likely to fail due to variations in genomic signatures. B-RAF inhibitors have been clinically inadequate but response might be augmented with combination therapies targeting multiple signaling pathways. We investigate the pre-clinical efficacy of combining the multi-kinase inhibitor Sorafenib or mutated B-RAF inhibitor PLX4720 with Riluzole, an inhibitor of glutamate release that antagonizes GRM1 (metabotropic glutamate receptor1) signaling in melanoma cells.
Experimental Design
Melanoma cell lines that express GRM1 and either wild type B-RAF or mutated B-RAF were treated with Riluzole, Sorafenib, PLX4720 or the combination of Riluzole with Sorafenib or with PLX4720. Extra-cellular glutamate levels were determined by glutamate release assays. MTT assays and cell cycle analysis demonstrate effects of the compounds on proliferation, viability and cell cycle profiles. Western immunoblots and immunohistochemical staining showed apoptotic markers. Consequences on MAPK pathway were assessed by western immunoblots. Xenograft tumor models were used to determine the efficacy of the compounds in vivo.
Results
The combination of Riluzole with Sorafenib exhibited enhanced anti-tumor activities in GRM1 expressing melanoma cells harboring either wild type or mutated B-RAF. The combination of Riluzole with PLX4720 showed lessened efficacy compared with the Riluzole and Sorafenib combination in suppressing the growth of GRM1 expressing cells harboring the B-RAFV600E mutation.
Conclusions
The combination of Riluzole with Sorafenib appears potent in suppressing tumor proliferation in vitro and in vivo in GRM1 expressing melanoma cells regardless of B-RAF genotype and may be a viable therapeutic clinical combination.
Keywords: Melanoma, GRM1, Glutamate, Riluzole, Sorafenib, PLX4720
INTRODUCTION
The incidence of melanoma has risen rapidly in the past three decades and has become a significant health risk in the United States (1). The treatment of early stage melanoma is surgical resection, with over 85% of patients in the early stages of disease experiencing long-term survival. However, when melanoma metastasizes the prognosis is poor, with few patients diagnosed with stage IV disease surviving past five years (2). Standard cytotoxic chemotherapeutic regimens have failed to alter the outcome in patients with advanced disease (3) and only the use of biological therapies based on interleukin-2 (IL-2) demonstrate any effect in extending long-term survival (4). Over the past decade, our understanding of the genetic alterations that lead to melanomagenesis and melanoma progression has advanced rapidly. Key signaling pathways involved in the pathogenesis and progression of melanoma, including the MAPK, PI3K/AKT, Wnt, JNK, TGF-β, NFκB, and others (5–7) suggest a molecularly complex and heterogeneous disease.
Our group has added to the understanding of aberrant signaling in melanoma by discovering that the ectopic expression of a G-protein-couple-receptor, metabotropic glutamate receptor 1 (GRM1) in melanocytes was sufficient to induce spontaneous melanoma development in vivo with 100% penetrance (8). We also confirmed ectopic expression of GRM1 in a subset of human melanoma cell lines and biopsies (8). To date, we have examined over 175 human melanoma biopsies as well as 25 human melanoma cell lines and found that 80% of the cell lines and over 60% of the human biopsies test positive for expression of the receptor at the level of both RNA and protein (9), suggesting that GRM1 may be involved in the pathogenesis of a significant subset of human melanomas. Our work has recently been confirmed by a report demonstrating that transgenic mice with conditional expression of GRM1 in melanocytes developed pigmented lesions at 29 weeks after activation of the transgene with the incidence of subsequent melanoma being 100% at 52 weeks (10).
We have worked to unravel the causes and consequences of GRM1 signaling in this disease (9) as well as design therapeutic interventions that target GRM1-signaling. Earlier, we reported in vitro and in vivo pre-clinical findings using human melanoma cell lines that are wild type in B-RAF and N-RAS (C8161) or contain an N-RASQ61R mutation (WM239A). We demonstrated that MAPK signaling is critical in GRM1-mediated oncogenesis (9) and have also shown that activation of the receptor using known GRM1 agonists results in an up-regulation of the activated (phosphorylated) form of ERK (9). In addition, the majority of GRM1-expressing human melanoma cell lines tested exhibited elevated levels of extra-cellular glutamate which promotes growth by activation of a glutamate autocrine loop. Suppression of GRM1 signaling by either GRM1-antagonists or a reduction in the levels of GRM1 ligand, glutamate, with a glutamate release inhibitor Riluzole, resulted in decreased cell proliferation in vitro and tumorigenesis in vivo (9).
The US Food and Drug Administration (FDA) approved Riluzole, is a member of the benzothiazole class of compounds and acts as an inhibitor of glutamate release for the treatment of amyotrophic lateral sclerosis (ALS). The ability of Riluzole to block the release of the ligand (glutamate) for GRM1 allows it to act functionally as a putative antagonist and interfere with intracellular events that follow stimulation of this receptor. With a low toxicity profile (11, 12), Riluzole was deemed an excellent compound to use in preliminary studies on the effects of glutamate signaling inhibition on melanoma cells (9). To date, the reported modes of actions of Riluzole in humans are inhibition of glutamate release, inactivation of voltage-dependent Na+ channels, and interference with G-protein dependent signaling (11). In melanoma cells expressing GRM1, Riluzole has been shown to inhibit cell proliferation in vitro and in vivo (9) as well as migration and invasion (13). Recently, a Phase 0 clinical trial of Riluzole in patients with advanced melanoma was conducted with 34% of patients given Riluzole showing measurable clinical responses. Some tumors decreased in size by over 90% and exhibited suppression of MAPK and PI3K/AKT signaling pathways in post-treatment tumor samples (14). A recently completed Phase II trial showed no RECIST criteria responses, however, 42% of the patients exhibited stable disease suggesting that Riluzole has overall modest anti-tumor activity whose potential could be realized by combination with other anti-cancer agents (15).
As we continue with studies that target GRM1 signaling in melanoma, it is important to perform pre-clinical studies using potential therapeutic agents that reflect the genetic diversity of this disease. Mutations in B-RAF have been identified in 8% of all cancers including over 50% of melanomas (16). Most of these mutations are due to the substitution of a single amino acid at residue 600 in the B-RAF kinase domain resulting in constitutive activation of the RAF-MEK-ERK signaling pathway (16).
The small-molecule, multi-kinase inhibitor Sorafenib (17) has proven to be ineffective against melanoma as a single agent but its use in combinatorial therapies may prove more effective in the clinic. A recently described specific small molecule inhibitor specific to B-RAF kinase, PLX4720/PLX4032, was shown to have potent anti-melanoma activity in pre-clinical and clinical studies (18–20). However, its effectiveness has been hampered by the acquirement of drug resistance mechanisms including involvement of other RAF isoforms (21–24).
Given the high incidences of B-RAFV600E mutations and GRM1 expression in numerous melanomas, we investigate cellular responses for the combination of a RAF inhibitor with Riluzole, the putative antagonist of GRM1 signaling. Here, we provide data that demonstrates that combining inhibitors of RAF and GRM1 results in the suppression of human melanoma cell growth in vitro as well as tumorigenicity in vivo, suggesting that such a combinational therapy may be superior than either modality alone in melanoma patients. The following report describes in vitro and in vivo pre-clinical experiments using GRM1 expressing human melanoma cell lines that harbor the most common mutation B-RAFV600E, found in human melanomas. We demonstrate that the combination of Riluzole with Sorafenib appears potent in suppressing cell proliferation in vitro and in vivo in GRM1 expressing cells regardless of B-RAF status and may be a viable therapeutic clinical combination.
MATERIALS AND METHODS
Antibodies and Reagents
Antibodies against activated Caspase 3, Ki67, PARP, phospho- and total ERK, cleaved PARP, and Mcl-1 were obtained from Cell Signaling (Danvers, MA); antibody for α-tubulin, MTT cell viability assay solution 1 (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), iodonitrotetrazolium chloride, Riluzole was obtained from Sigma (St. Louis, MO); Sorafenib (LC labs, Woburn, MA) and PLX4720 was a gift from Plexxikon Inc., Berkeley, CA.
Cell Lines
UACC903 and UACC930 cells were provided by Dr. Jeffery Trent (The Translational Genomics Research Center, Phoenix, AZ) and 1205Lu cells were provided by Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA). C8161 cells were provided by Dr. Mary Hendrix (Children’sMemorial Research Center, Chicago, IL). SKMEL2, SKMEL 187 and A2058 cells were purchased from ATCC. The cells were maintained in RPMI plus 10% FBS. Human epidermal melanocytes (HEM) were maintained in medium 254 (Invitrogen) supplemented with human melanocyte growth supplement (Invitrogen). Human epithelial kidney cells (HEK) were maintained in DMEM plus 10% FBS.
MTT Assays, Cell Cycle Analysis and Glutamate release
MTT cell viability assays were performed as previously described (9). C8161, UACC903, 1205Lu, SKMEL 187 and A2058 melanoma cell lines were used in the MTT assays. Each cell line was cultured in 96 well plates (103 cells/well) with the following conditions: no treatment, vehicle alone (DMSO), Riluzole (1μM, 5μM, 10μM, 25μM, or 50μM), Sorafenib (1μM, 3μM, 5μM, or 7μM), or the combination of Riluzole (10μM) and Sorafenib (5μM), PLX4720 (0.01μM, 0.05μM, 0.1μM, or 0.3μM) or the combination of Riluzole (10μM) plus PLX4720 (0.05μM). Viable cells were measured every day for 4 or 7 days. For cell cycle analysis, UACC903, 1205Lu, and A2058 melanoma cell lines were used. Cell cycle analysis was performed at 24 and 48 hours of incubation of the cell lines in monolayer culture with no treatment, vehicle alone (DMSO), or 10μM Riluzole. Cells were harvested at each time point and examined using propidium iodide followed by flow cytometry performed by the Flow Cytometry Facility Core at Rutgers University as previously described (9). Amplex Red Glutamic Acid/Glutamate Oxidase assay kit (Invitrogen) was used to measure levels of glutamate.
Three Dimensional Anchorage Independent Assays
We performed three-dimensional colony assays using C8161, UACC903, SKMEL2 and 1205Lu human melanoma cell lines in the presence of vehicle (DMSO), Riluzole (10μM), Sorafenib (5μM), or the combination of Riluzole (10μM) and Sorafenib (5μM). The cells were suspended in 0.35% agar in RPMI supplemented with 10% FBS and plated on a layer of 0.75% agar in the same medium in 12-well culture plates (1 – 1.5 × 104 cells/well). Vehicle, Riluzole alone, Sorafenib alone, or Riluzole and Sorafenib, were added in the agar underlay, as well as to the cells suspended in agar on day 1. Every other day, the vehicle, or drug(s) was again added with 250μl of complete medium. After 14 days, the colonies were stained with iodonitrotetrazolium chloride (Sigma) and photographed. The numbers of colonies were counted using Image J software. Quantitation was performed by comparing the total number of colonies from three representative photomicrographs from each experiment. The histograms represent the average of three independent experiments.
Western Immunoblots
Protein lysates were prepared as described previously (9). Briefly, media was removed and cells were washed once with ice-cold phosphate buffered saline (PBS). After removal of PBS, the extraction buffer was added directly to the plates and cells were collected with a cell scraper. Cells were incubated on ice for 20 minutes. Cell debris was removed by centrifugation at 25,000 × g at 4°C for 20 minutes and supernatant taken for Western immunoblot analysis. Western Blotting was carried out with anti-PARP, anti-cleaved PARP, anti-phospho ERK, anti-total ERK and anti-α-tubulin antibodies.
Xenografts in Immunodeficient Nude Mice
The Institutional Review Board approved all animal studies for the Animal Care and Facilities Committee of Rutgers University. Nude mice were purchased from Taconic (Hudson, NY). Cells were injected into 2 dorsal sites of each mouse at 106 cells per site. Tumor size was measured twice a week with a Vernier caliper and calculated as described (9). Once tumor volumes reached 6–10mm3, mice were divided into no treatment and treatment groups. The treatment groups received either vehicle (DMSO), Riluzole (10mg/kg), Sorafenib (24mg/kg), PLX4720 (20mg/kg), or the combination of Riluzole (5mg/kg) and Sorafenib (12mg/kg) or Riluzole (5mg/kg) and PLX4720 (10mg/kg) by oral gavage daily. The doses of oral Riluzole, Sorafenib, and PLX4720 were based on published reports (11, 12, 20). The experiments were terminated when the xenografts on the no treatment group reached the maximum permitted size.
Immunohistochemistry
Tissue Analytical Services at the Cancer Institute of New Jersey performed immunohistochemical staining on excised tumor xenografts to detect changes in the number of apoptotic and proliferating cells (activated Caspase 3 and Ki-67, respectively).
RESULTS
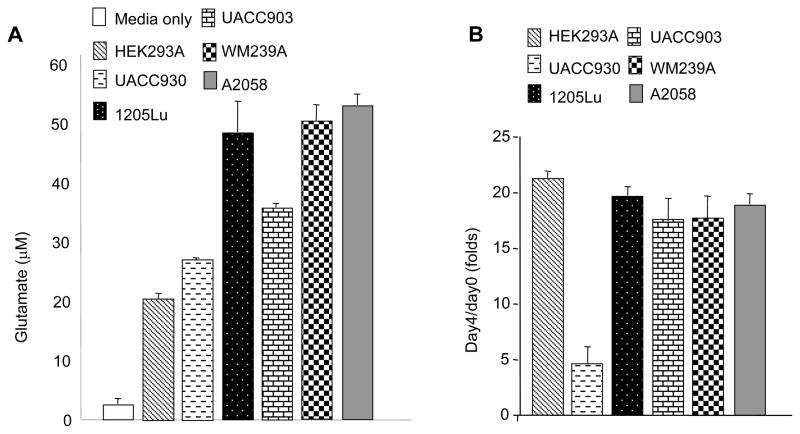
The oncogenic transformation of various cell types by ectopic expression of GPCRs is characterized by the development of autocrine and paracrine loops that enhance cellular proliferation (25). Three melanoma cell lines (UACC903, 1205Lu and A2058) containing the activating B-RAFV600E mutation exhibited elevated levels of extra-cellular glutamate similar to that previously described for wild type B-RAF melanoma cells, C8161 and WM239A (9) compared to cells that do not express the receptor or cells that contain a truncated, non-functioning GRM1 receptor, UACC930 melanoma cells (Figure 1A). MTT cell viability assays were performed to rule out that the increase in glutamate observed was not attributable to the cell lysis, establishing that the cells themselves must be excreting glutamate into their surroundings in an attempt to establish autocrine activity (Figure 1B). We next assessed the effects of the glutamate-release inhibitor, Riluzole, on the growth of human melanoma cells in monolayer culture. Standard MTT assays were performed using four GRM1-expressing melanoma cell lines expressing wild type forms of B-RAF and N-RAS (C8161 and SKMEL 187) or B-RAFV600E mutation (UACC903, 1205Lu, and A2058). We found that Riluzole at concentration of 25μM or 50μM significantly decreased the number of viable cells as compared to no treatment or vehicle (DMSO) treated cells (Figure 2A). Melanoma cells harboring a wild type B-RAF (C8161 and SKMEL 187) were found to be much more sensitive to Riluzole than those that contained a mutant copy of B-RAF (1205Lu, UACC903). This is in support of earlier reports that indicated that since both GRM1 and B-RAFV600E stimulate MAPK signaling, one of the key signaling pathways in human melanoma leading to metastasis, abolishing GRM1 signaling alone in cells that bear B-RAFV600E would not abolish over-activated MAPK (9).
Figure 1. Figure 1A: Elevated levels of glutamate in melanoma cells.
Levels of glutamate (μM) released in three B-RAFV600E human melanoma cell lines (UACC903, 1205Lu, and A2058) confirmed earlier results with WM239A with B-RAFV600D (9). GRM1 expression is detected in all lines except for HEK and UACC930 (9). B: MTT cell viability/proliferation assays showing viability of cells assayed for glutamate release. Y-axis is the absorbance at day4/day0, with day0 of each line set as 1.
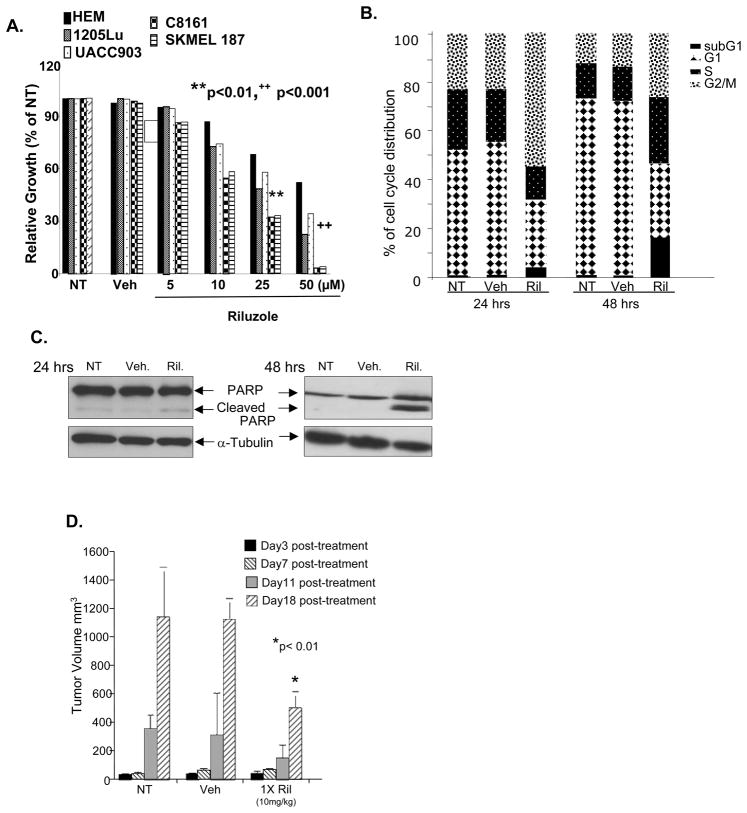
Figure 2. Figure 2A: Melanoma cells with mutated B-RAF are sensitive to Riluzole.
MTT assays of four melanoma cell lines. C8161 and SKMEL 187 cells have wild type B-RAF. UACC903 and 1205Lu have mutated B-RAFV600E. Treatment of all four lines with Riluzole (25 and 50μM) resulted in a decrease of viable cells in comparison to no treatment (NT) or vehicle (Veh, DMSO) controls. B: Cell cycle profiles of Riluzole (10μM) treated UACC903 representative of similar results obtained with two other mutated B-RAFV600E cell lines, 1205Lu and A2058. C: Western immunoblots of cell lysates made from parallel plates as described in B indicating elevated levels of PARP cleavage in UACC903 cells treated with Riluzole. Equal loading was demonstrated with α-tubulin. D: Xenografts of UACC903 cells treated with Riluzole (10 mg/kg) daily by oral gavage. Tumor volume (mm3) was an average of 12 mice per group ± S.D. * p<0.01 comparison between Riluzole treated with no treatment (NT) or vehicle (Veh, DMSO) controls.
We next obtained the cell cycle profiles of Riluzole (10μM) treated UACC903, 1205Lu, and A2058 melanoma cells to assess the effects that it had on cell cycle progression over time. All three cell lines yielded very similar results with an example of UACC903 shown. At 24 hours post-treatment about half of the cells were found to accumulate in the G2/M phase. By 48 hours there was a 10–20-fold shift of the cell population to the subG1 phase of the cycle, indicative of apoptotic cell response (Figure 2B). This apoptotic response was confirmed by an increase in the cleaved form of PARP by Western analysis. Control samples showed negligible amounts of cleaved PARP at 24 and 48 hours (Figure 2C). These results were very similar to our previous report demonstrating a similar G2/M cell cycle arrest followed by apoptotic shift in GRM1-expressing human melanoma cell lines harboring wild type B-RAF and N-RAS (C8161) or mutated N-RAS (WM239A) in the presence of Riluzole (9), suggesting that depletion of the ligand (glutamate) to the receptor, GRM1, by Riluzole induces cell cycle arrest and promotes apoptosis in GRM1 positive melanoma cells regardless of B-RAF genotype. To confirm this observation in vivo, we performed xenograft experiments using single agent Riluzole as described (9) (Figure 2D). Briefly, UACC903 cells were injected into the dorsal flanks of nude mice. Tumors were allowed to grow to approximately 6–10mm3 and mice were divided into groups to obtain relatively constant tumor volumes between each group (12 mice per group). Animals were treated daily with Riluzole or vehicle (DMSO) by oral gavage. At day 18, there was a substantial difference between the tumor sizes of Riluzole-treated animals compared to controls (Figure 2D). Though Riluzole on its own appears effective in inhibiting proliferation and inducing apoptosis in melanoma cells harboring activating B-RAF mutations in vivo, it is less effective at doing so than in melanoma xenografts harboring wild type B-RAF (9). Clinically, these observations suggest it is likely that administration of a single agent Riluzole will not be as effective in patients whose melanomas contain a mutated form of B-RAF.
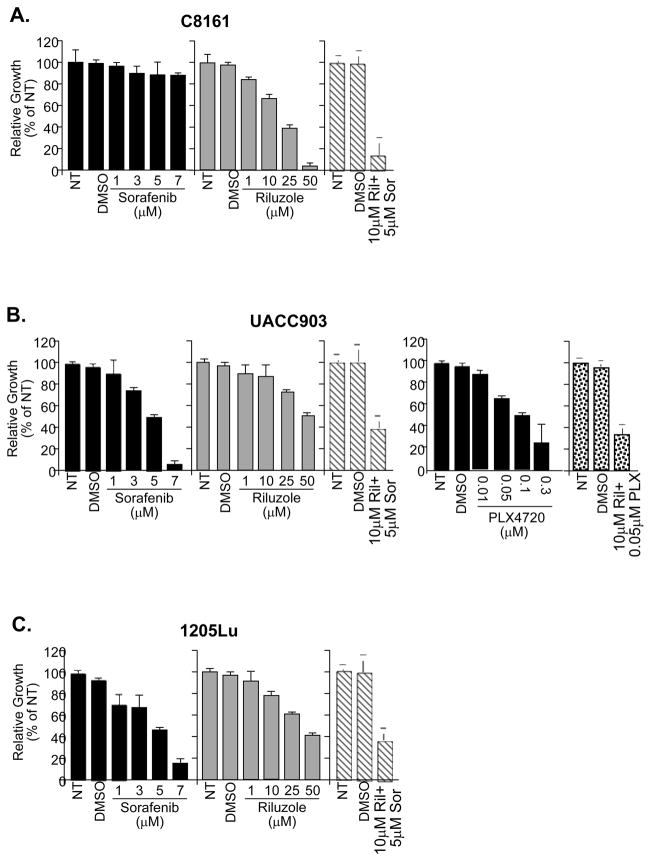
Tumors are composed of heterogeneous cell populations. For this reason, we started to explore potential combinatorial therapies that would include Riluzole as one of the components to treat heterogeneous tumor populations in an attempt to slow the progression of this disease. We choose Sorafenib a multi-kinase inhibitor which has been shown to inhibit RAF signaling, and whose toxicity profile is known in vivo (17, 26) and PLX4720, a recently described specific small molecule inhibitor for B-RAFV600E (18–20). We treated three GRM1-expressing human melanoma cell lines (C8161, UACC903 and 1205Lu) with Riluzole, Sorafenib, or a combination of both Riluzole and Sorafenib for seven days and assessed cell proliferation and viability using MTT assays (Figure 3). In the presence of Riluzole alone, C8161 cell line has the highest reduction in the number of viable cells confirming our earlier report (9) (Figure 3A). UACC903 and 1205Lu are also positive for GRM1 expression and harbor a mutated B-RAF (V600E). These cell lines were not as sensitive to Riluzole (Figures 3B and 3C). In the presence of Sorafenib, the opposite responses were observed; UACC903 and 1205Lu displayed a substantial decrease in the number of viable cells in comparison to C8161 cells. A combination of 10μM Riluzole with 5μM Sorafenib led to synergistic, inhibitory effect on the proliferation C8161 cells (Figure 3A), and an additive, inhibitory effect on UACC903 and 1205Lu cells (Figures 3B and 3C) when analyzed as described (27). To assess if combining PLX4720 with Riluzole would also yield the additive effect observed with Sorafenib, we treated UACC903 and C8161 cells with Riluzole, PLX4720 or the combination of both. The IC50 for PLX4720 in UACC903 cells was determined to be 0.1μM (Figure 3B). UACC903 cells treated with a combination of 10uM Riluzole and half the IC50, 0.05uM PLX4720 exhibited additive inhibitory activity when compared to either single agent alone (Figure 3B). As expected wild type B-RAF, GRM1 positive C8161 cells show only slight inhibition in cell proliferation with higher concentrations of PLX4720 (10uM) and no increase in efficacy when combined with Riluzole (data not shown).
Figure 3. Treatment of melanoma cells with three different inhibitors.
MTT assays of C8161 (3A, B-RAF and N-RAS wild type), UACC903 (3B, B-RAFV600E) and 1205Lu, (3C, B-RAFV600E) melanoma cells treated with different concentrations of Riluzole, Sorafenib, PLX4720 or the combination of the compounds at half of the concentration of each one alone. NT - No treatment; DMSO - Vehicle only treatment: Riluzole (Ril); Sorafenib (Sor); PLX4720 (PLX).
To further predict the results obtained in two-dimensional assays in a model more closely related to in vivo, we performed three-dimensional, anchorage-independence assays using four GRM1-positive melanoma cell lines: C8161 (wild type B-RAF and N-RAS), UACC903, 1205Lu (mutated B-RAFV600E), and SKMEL2 (mutated N-RASQ61R). In C8161 cells, we found that Riluzole at 10μM led to a 40% decrease in colony formation while Sorafenib alone had little effect (Figures 4A and 4B). However, the combination of Riluzole and Sorafenib had a substantial consequence resulting in a 70% decrease in colony formation (Figures 4A and 4B). In UACC903 cells, Riluzole alone had very little inhibitory activity while treatment with Sorafenib resulted in a 45% reduction in the number of colonies (Figures 4A and 4B). Furthermore, the combination of Riluzole and Sorafenib led to a drastic 90% decrease in the number of colonies in UACC903 (Figures 4A and 4B). In 1205Lu cells, Riluzole or Sorafenib alone yielded a 30% reduction in colony formation while the combination of both resulted in a 55% decrease in the number of colonies (Figures 4A and 4B). In SKMEL2, Riluzole alone had a modest effect, decreasing colony formation by 18% while Sorafenib was more efficacious at decreasing colony formation. The combination treatment yielded a 62% decrease (Figures 4A and 4B) compared to the control group. These observations further strengthen our hypothesis that a combination of Riluzole and Sorafenib would be able to inhibit tumor cell proliferation more effectively than either agent alone, regardless of the presence or absence of activating mutations in B-RAF or N-RAS in the cells. Given these findings, we performed combinatorial in vivo experiments using C8161, UACC903 and 1205Lu xenografts.
Figure 4. Inhibition of anchorage independent growth with Riluzole and Sorafenib alone and in combination.
A and B: Colony assays were performed with C8161 (B-RAF and N-RAS wild type), UACC903, 1205Lu (B-RAFV600E), and SKMEL2 (N-RASQ61R) GRM1 positive human melanoma cell lines. Representative photomicrographs were shown for each cell line. Colony number and size were determined using ImageJ software. Results are the mean of 3 independent experiments.
In the xenograft studies, all cell lines utilized express GRM1 but differ in B-RAF genotype with C8161 being wild type and UACC903 and 1205Lu containing the activating mutation. In C8161 xenografts, there was a significant decrease in the tumor volumes in animals treated with Riluzole alone confirming our previous report (9). Administration of Sorafenib on its own did not yield a significant decrease in tumor size and the combination of Riluzole with Sorafenib at half the dose used in either one alone yielded a considerable reduction in tumor volume (Figure 5A). In the human melanoma cell lines with mutated B-RAF; UACC903 and 1205Lu, differential responses were detected. UACC903 xenografts demonstrated very similar, statistically relevant responses with Riluzole or Sorafenib alone (Figure 5B). The combination of Riluzole and Sorafenib yielded a higher reduction in tumor volume than either compound alone (Figure 5B). 1205Lu xenografts were found to be more sensitive to Riluzole, Sorafenib or the combination of both reagents when compared to UACC903 xenografts (Figure 5C). It was noted that 1205Lu xenografts were more responsive to the combination therapy than UACC903 xenografts in spite of their common B-RAF V600E genotype indicating that other mutations persistent in these cells must influence their response. Additionally, immunohistochemical analyses were performed on excised xenografts using antibodies against the cleaved form of Caspase 3 to detect apoptotic cell death and Ki-67 to detect changes in cell proliferation. An example of excised UACC903 xenograft tumors is shown. Single agent Riluzole, Sorafenib or the combination of both compounds treated samples showed a substantial increase in the number of positive Caspase 3 cells in comparison to the controls (Figure 5E). Conversely, the number of Ki-67 positive cells was reduced in either single agent or combined treatments (Figure 5F). It is equally important to point out that Riluzole had a more potent effect on C8161 and 1205Lu cell lines despite the disparity in B-RAF status than UACC903. A combination of Riluzole and Sorafenib, though at half the concentration when used alone was effective against all three xenografts (Figures 5A, 5B and 5C). In vivo xenograft studies were also performed to evaluate the efficacy of Riluzole and PLX4720 combination in UACC903 cells. Surprisingly, PLX4720 alone was not as potent as Riluzole (Figure 5D), furthermore, when we combined half the doses of Riluzole and PLX4720 we did not detect further suppression of tumor progression as we observed with similar dosing with Riluzole and Sorafenib combination (compare Figure 5B with Figure 5D). Efficacy of combination Riluzole and PLX4720 against the wild type B-RAF melanoma cell line C8161 was not evaluated with PLX4720 in vivo as it has been shown by others to be ineffective in inducing apoptosis in vitro and in vivo (20) and has also been shown to promote cell growth through activation of the MAPK pathway in a C-RAF dependent manner (28).
Figure 5. Suppression of human melanoma xenografts growth.
Xenografts of A: C8161 (B-RAF and N-RAS wild type), B: UACC903 (B-RAFV600E) and C: 1205Lu (B-RAFV600E). The groups were no treatment (NT), vehicle (Veh, DMSO), Riluzole (10mg/kg), Sorafenib (24mg/kg) or the combination of Riluzole (5mg/kg) and Sorafenib (12mg/kg). Tumor volume (mm3) was an average of 12 mice per group ± S.D. * p<0.01 when Riluzole or Sorafenib treated UACC903 samples were compared to NT or Veh controls at day 18. ** p<0.001 when Riluzole treated C8161 or the combination of Riluzole plus Sorafenib treated C8161, UACC903 or 1205Lu samples were compared to NT or Veh controls. D: Xenograft experiments of UACC903 treated with either Riluzole (10mg/kg), PLX4720 (20mg/kg) or a combination of Riluzole (5mg/kg) and PLX4720 (10mg/kg), * p<0.05 in comparison to NT or Veh. E and F: Excised UACC903 xenograft tumors from B and stained with activated cleaved Caspase-3 (E) and Ki67 (F). Arrows indicate clusters of Caspase-3 positive cells. Below each representative image is the average of positively stained cells from ten randomly selected fields. *p<0.01 comparison between Riluzole treated with no treatment (NT) or vehicle (Veh, DMSO) controls.
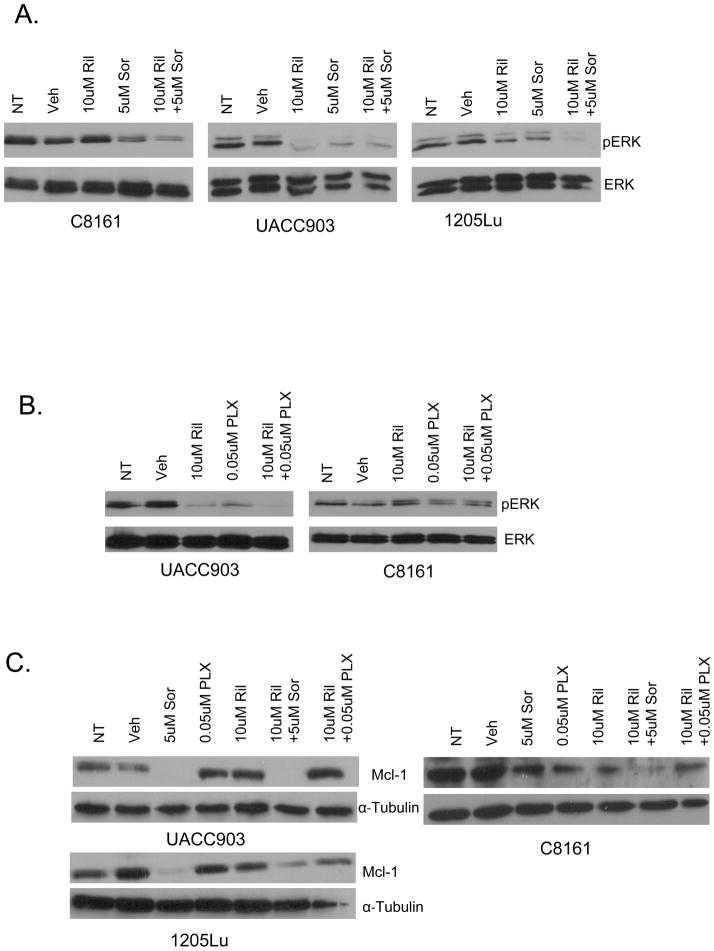
Pre-clinical and clinical trials performed with Sorafenib, PLX4720 and Riluzole demonstrated a reduction in levels of activated ERK supporting the notion that MAPK is a target for all three compounds (14, 17, 20). We performed Western immunoblots with protein lysates prepared from in vitro cultured cells or excised in vivo xenografts treated with Sorafenib, PLX4720 and Riluzole either alone or in combination as described above. Riluzole inhibits the MAPK pathway as measured by a decrease in levels of ERK phosphorylation in a cell line dependent manner (Figures 6A and 6B). Sorafenib was found to highly suppress ERK phosphorylation in UACC903 and 1205Lu cells than in C8161. The combination was however capable in suppressing ERK phosphorylation in all three cell lines. PLX4720 was only found to suppress ERK activity in the B-RAFV600E cell line UACC903 as a single agent or in combination but not in the C8161 cell line (Figure 6B). Protein lysates obtained with harvested xenografts showed similar results (data not shown). The effect of the combinational drugs on the pro-apoptotic protein Mcl-1, which has been shown to be down-regulated by Sorafenib (29, 30) was investigated as a possible target for additive and synergistic inhibition in tumor growth. A reduction in Mcl-1 levels was detected in Sorafenib treated UACC903 and 1205 LU cells while the combination of Riluzole and Sorafenib led to a reduction in Mcl-1 in all three cells lines (Figure 6C). PLX4720, however, does not down regulate the levels of Mcl-1 either by itself or in combination with Riluzole (Figure 6C).
Figure 6.
Effects of Riluzole, Sorafenib and PLX4720 on MAPK pathway and Mcl-1 levels. Western immunoblots examining the effect of A. Riluzole - Ril (10uM), Sorafenib - Sor (5uM) or combination of Riluzole (10uM) and Sorafenib (5uM) on MAPK pathway activation as indicated by levels of ERK phosphorylation in C8161 (B-RAF and N-RAS wild type), UACC903 and 1205Lu (B-RAFV600E) cell lines in vitro. B. Western immunoblots examining the effects of Riluzole (10uM), PLX4720 - PLX (0.05uM) or the combination 10uM and 0.05uM respectively on UACC903 and C8161 cell lines. Samples were normalized to total ERK. NT-No treatment, Veh - Vehicle. C. Western immunoblots examining the effects of Riluzole -Ril (10uM), Sorafenib – Sor (5uM) PLX4720- PLX (0.05uM) on UACC903 and 1205 Lu or 10uM on C8161 or the combination of 10um Riluzole and 5um Sorafenib or 10uM Riluzole and 0.05m PLX4720/10uM PLX4720 on levels of Mcl-1.
DISCUSSION
Several groups have proposed the concept that the glutamatergic system may play a role in tumor biology and intriguing links between neurodegenerative diseases and cancer have been put forth by several investigators (31, 32). For instance, the incidence of melanoma among patients with ALS or Parkinson’s disease is 2–3 times higher than that of the general population in multicenter studies in Australia and North America (33). These observations are in line with earlier reports that elevated levels of extracellular glutamate have been detected in several human disorders including gliomas, multiple sclerosis, Alzheimer’s disease, Parkinson and ALS (34, 35), suggesting that the common root of many of these diseases may be glutamate.
Metabotropic glutamate receptors (GRMs) are members of the seven-transmembrane domain G-protein-coupled receptor (GPCR) family (36). GRMs are divided into three groups based on sequence homology, agonist selectivity, and effecter coupling with all GRMs having glutamate as their natural ligand. GRM1 and GRM5 comprise Group I GRMs and are mainly involved in excitatory responses induced by strong presynaptic stimulation. Group I GRMs are coupled to a Gαq-like protein and stimulate phospholipase C beta (PLCβ) (37). It has been reported that in melanoma cells GRM1 stimulation results in the activation of PLCβ, which in turn converts phosphatidylinositol to two second messengers, inositol triphosphate (IP3) and diacylglycerol (DAG). DAG activates protein kinase C (PKC), which could stimulate both MAPK and PI3K/AKT pathways (38, 39). Activation of these two major signaling cascades is central for transformed cell survival, migration, invasion, epithelial-mesenchymal transition (EMT), and angiogenesis (40).
Our group described a heretofore-unknown component of melanoma pathogenesis. A transgenic murine model of melanoma was constructed by the ectopic expression of GRM1 in melanocytes (8). These mice spontaneously develop melanocytic lesions very similar to human melanoma. We have expanded these original studies and have now shown that over 60% of human melanomas express the human form of this receptor and that activation of this receptor results in activation of the MAPK and PI3K/AKT pathways in a B-RAF and N-RAS-independent fashion (9). The functionality of GRM1 in GRM1-expressing human melanoma cells was demonstrated by the responsiveness of these cells to stimuli and inhibitors of GRM1 (9). Studies by others showed that wild type GPCRs can become tumorigenic when exposed to an excess of locally produced or circulating ligands and agonists (41, 42) while other GPCRs harboring mutations in key conserved residues can have transforming activity even in the absence of their ligands (43). It has also been found that the level of expression of GPCRs is not as important to oncogenesis as the fact that the receptor is expressed (44). Based on these earlier results we assessed levels of GRM1-ligand, glutamate, and we detected elevated glutamate levels in all GRM1-expressing human melanoma cell lines (9). Depletion of glutamate in human melanoma cells was performed using an inhibitor of glutamate release, Riluzole, led to reduced extracellular glutamate level and inhibited the proliferation of GRM1 positive cells, presumably as a result of interfering with autocrine loops through which glutamate exerts its growth promoting abilities. Riluzole, being FDA approved for the treatment of ALS was deemed an excellent compound to use in preliminary studies that could be translated clinically on the effects of glutamate signaling inhibition on melanoma cells (9).
The Phase 0 and Phase II clinical trials with Riluzole, which functionally as a putative antagonist of GRM1 signaling has modest anti-tumor activity as a single agent (14, 15). It is possible that activating mutations in B-RAF, or other unidentified genetic factors, affect how GRM1 expressing tumor cells respond to Riluzole therapy since GRM1 signals through other pathways, such as Wnt-β-catenin (45), in addition to the MAPK and PI3K/AKT pathways (9, 39, 46, 47). We therefore extended our pre-clinical studies to include melanoma cells carrying the most commonly known mutations in B-RAF, (V600E). We found that melanoma cells, which harbor the B-RAFV600E mutation, were less sensitive to the single agent Riluzole in both in vitro MTT cell viability cell proliferation and anchorage independent colony assays. We began to examine different combinations of Riluzole and other inhibitors of downstream targets. We utilized Sorafenib, a small molecule inhibitor originally identified as a RAF kinase inhibitor that also inhibits several receptor tyrosine kinases involved in tumor progression and tumor angiogenesis (17). We also investigated PLX4720, a specific B-RAF V600E inhibitor (20). Sorafenib is FDA approved for the treatment of hepatocellular carcinoma and is also a second line agent in renal cell carcinoma. Recent reports stressing the importance of C-RAF in B-RAF wild type melanomas has revived interest in the use of Sorafenib, in combination with other agents, for the treatment of melanoma. We now report that the combination of Riluzole and Sorafenib has an additive or synergistic effect in both B-RAF mutant and B-RAF wild type melanoma cells in vitro and in vivo. In addition to B-RAF inhibition, Sorafenib is a well-documented multi-kinase inhibitor of VEGF and other receptor tyrosine kinases (17). PLX4720/PLX4032 demonstrated remarkable preclinical results in in vitro and in vivo studies in suppressing melanoma cell growth. However, patients from these clinical trials were shown to become resistant to treatment with recurrence of melanoma occurring 5–9 months after start of their treatment. This stresses the need to re-examine the options in targeting melanoma effectively (48).
In cultured cell studies, Sorafenib was not very effective in suppressing C8161 cell growth while it was effective in reducing the number of viable cells in both UACC903 and 1205Lu melanoma cell lines with mutated B-RAF. Surprisingly, the combinatorial in vitro studies in C8161 cells using Riluzole and Sorafenib showed a synergistic reduction in the number of viable cells while exerting an additive effect detected in UACC903 and 1205Lu cell lines under similar conditions. These results were again observed in in vivo xenograft studies where the combination of Riluzole and Sorafenib again led to a considerable reduction in tumor progression as evident by the decrease in tumor volumes over time in all three cell lines compared to controls. It is thus possible that Sorafenib enhances the cytotoxic effects of Riluzole through suppression of downstream targets of GRM1 signaling including the MAPK pathway.
Stimulation of GRM1 was shown to modulate MAPK via the ERK mediated signaling pathway in GRM1-expressing human melanoma cells. We postulate that Riluzole decreases the levels of glutamate released from the cells disrupting the autocrine loops while Sorafenib also mediates its activities through inhibition of MAPK signaling resulting in a more profound inhibition in tumor cell growth and progression than with either agent alone in GRM1-expressing melanoma cells. It is however important to point out that Riluzole appears to suppress the MAPK pathway in a cell line dependent manner suggesting it is not the main pathway suppressing proliferation with Riluzole treatment. Recently, an alternative mode of action of Riluzole has been described with Riluzole serving as an enhancer of the Wnt-β-catenin signaling pathway which induces melanoma cells to revert to a more normal melanocytic phenotype promoting hyper-pigmentation and reducing their proliferation and metastasis (45).
PLX4720 displayed remarkable clinical responses as a single agent. Surprisingly when combined with Riluzole we did not detect further reduction in tumor cell growth in MTT or xenograft studies. This is in variance with the remarkable results observed with the combination of Riluzole and Sorafenib in vivo. We hypothesize that the encouraging results observed with the Sorafenib and Riluzole combination is likely due to Sorafenib’s role as a chemo-sensitizer by elimination of the pro-apoptotic protein, Mcl-1 resulting in enhanced cytotoxic response to Riluzole which has modest efficacy as a single agent. Elimination of Mcl-1 by Sorafenib has been shown to be through translational inhibition in a variety of cancer cell lines (29, 49). In melanoma, depletion of Mcl-1 enhances melanoma cell death by therapeutic compounds such as temozolomide and melphalan (30), sensitizes apoptosis resistance melanoma cells to Fas-mediated apoptosis (50) and renders melanoma cells susceptible to anoikis (51). Similar to other reports, we detected reduced levels of Mcl-1 only in Sorafenib treated B-RAFV600E human melanoma cells. Surprisingly, in C8161 melanoma cells with wild type BRAF, a decrease in Mcl-1 was also detected in the presence of Riluzole and Sorafenib suggesting that the reduced tumorigenicity observed in vivo may be mediated via a decline in Mcl-1. In light of these results, it is not surprising that Sorafenib but not PLX4720 sensitize the cells to Riluzole.
Considering that the majority of human melanomas harbor B-RAF mutations, agents used to treat melanoma in the clinic need to function in the presence of these mutations. Our findings suggest that the combination of Riluzole and Sorafenib would be a reasonable, combinatorial therapy for the treatment of patients with advanced melanoma and is currently undergoing clinical testing in a Phase I clinical trial in patients with advanced melanomas.
Translational Relevance.
Recently, the potent inhibitor of B-RAFV600E, PLX4720/PLX4032, was reported to induce regression of melanocytic lesions in late-stage melanoma patients. However, a significant number of these patients became refractory to the treatment, experiencing recurrence in less than one year. Previously, we identified aberrantly expressed metabotropic glutamate receptor 1 (GRM1) in ~65% of human melanoma biopsies and cell lines, suggesting the potential of developing GRM1 targeted therapies. A Phase II trial with Riluzole, an FDA approved inhibitor of glutamate release, showed mixed clinical responses with 42% of the patients exhibiting stable disease. Here, we show that Riluzole plus Sorafenib reduces the growth of wild type or B-RAFV600E human melanoma cells in vitro and in vivo. The PLX4720 and Riluzole combination has additive effects in suppressing growth of B-RAFV600E melanoma cells but less so than the Sorafenib combination in the same cells in vitro and in vivo. These results propose a therapeutic regimen using Riluzole and Sorafenib to circumvent the drug resistance arising with the use of PLX4032 in melanoma patients.
Acknowledgments
This work is supported by grants from New Jersey Commission for Cancer Research 07-1064-CCR-E0 (H.J. Lee), 09-1143-CCR-EO (S. Chen), NIH R01CA74077 (S. Chen), NIEHS ES-005022 (S. Chen), NIH R01CA124975-02S1, NIH GM66338 (J. Wangari-Talbot), and NIH R01CA124975 (J. Goydos).
Footnotes
Conflict of Interest
No conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Margolin K. Treatment of advanced melanoma with temozolomide: I. Curr Oncol Rep Sep. 2004;6:399–400. [PubMed] [Google Scholar]
- 3.Flaherty KT. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin Cancer Res. 2006;12:2366s–70s. doi: 10.1158/1078-0432.CCR-05-2505. [DOI] [PubMed] [Google Scholar]
- 4.Tarhini AA, Agarwala SS. Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther. 2006;19:19–25. doi: 10.1111/j.1529-8019.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 5.Meier F, Schittek B, Busch S, Garbe C, Smalley K, Satyamoorthy K, et al. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci. 2005;10:2986–3001. doi: 10.2741/1755. [DOI] [PubMed] [Google Scholar]
- 6.Dissanayake SK, Weeraratna AT. Detecting PKC phosphorylation as part of the Wnt/calcium pathway in cutaneous melanoma. Methods Mol Biol. 2008;468:157–72. doi: 10.1007/978-1-59745-249-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Bergami P, Huang C, Goydos JS, Yip D, Bar-Eli M, Herlyn M, et al. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11:447–60. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–12. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 9.Namkoong J, Shin SS, Lee HJ, Marin YE, Wall BA, Goydos JS, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Research. 2007;67:2298–305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 10.Ohtani Y, Harada T, Funasaka Y, Nakao K, Takahara C, Abdel-Daim M, et al. Metabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma. Oncogene. 2008;27:7162–70. doi: 10.1038/onc.2008.329. [DOI] [PubMed] [Google Scholar]
- 11.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–41. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 12.Kretschmer BD, Kratzer U, Schmidt WJ. Riluzole, a glutamate release inhibitor, and motor behavior. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:181–90. doi: 10.1007/pl00005241. [DOI] [PubMed] [Google Scholar]
- 13.Le MN, Chan JL, Rosenberg SA, Nabatian AS, Merrigan KT, Cohen-Solal KA, et al. The glutamate release inhibitor Riluzole decreases migration, invasion, and proliferation of melanoma cells. J Invest Dermatol. 2010;130:2240–9. doi: 10.1038/jid.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yip D, Le MN, Chan JL, Lee JH, Mehnert JA, Yudd A, et al. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res. 2009;15:3896–902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehnert J, Wen Y, Lee J, Li D, Pruski-Clark L, Shih W, et al. A phase II trial of riluzole, an antagonist of metabotropic glutamate receptor (GRM1) signaling in advanced melanoma. J Clin Oncol. 2010;28:15. [Google Scholar]
- 16.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 18.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–30. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–72. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nazarian RM, Prieto VG, Elder DE, Duncan LM. Melanoma biomarker expression in melanocytic tumor progression: a tissue microarray study. J Cutan Pathol. 2010;37 (Suppl 1):41–7. doi: 10.1111/j.1600-0560.2010.01505.x. [DOI] [PubMed] [Google Scholar]
- 24.Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670–81. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol. 2005;25:6225–34. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leboeuf R, Baumgartner JE, Benezra M, Malaguarnera R, Solit D, Pratilas CA, et al. BRAFV600E mutation is associated with preferential sensitivity to mitogen-activated protein kinase kinase inhibition in thyroid cancer cell lines. J Clin Endocrinol Metab. 2008;93:2194–201. doi: 10.1210/jc.2007-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Song S, Yang F, Au JL, Wientjes MG. Nontoxic doses of suramin enhance activity of doxorubicin in prostate tumors. J Pharmacol Exp Ther. 2001;299:426–33. [PubMed] [Google Scholar]
- 28.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 29.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–9. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 30.Augustine CK, Toshimitsu H, Jung SH, Zipfel PA, Yoo JS, Yoshimoto Y, et al. Sorafenib, a multikinase inhibitor, enhances the response of melanoma to regional chemotherapy. Mol Cancer Ther. 2010;9:2090–101. doi: 10.1158/1535-7163.MCT-10-0073. [DOI] [PubMed] [Google Scholar]
- 31.Rzeski W, Ikonomidou C, Turski L. Glutamate antagonists limit tumor growth. Biochem Pharmacol. 2002;64:1195–200. doi: 10.1016/s0006-2952(02)01218-2. [DOI] [PubMed] [Google Scholar]
- 32.Lee HJ, Wall B, Chen S. G-protein-coupled receptors and melanoma. Pigment Cell Melanoma Res. 2008;21:415–28. doi: 10.1111/j.1755-148X.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baade PD, Fritschi L, Freedman DM. Mortality due to amyotrophic lateral sclerosis and Parkinson’s disease among melanoma patients. Neuroepidemiology. 2007;28:16–20. doi: 10.1159/000097851. [DOI] [PubMed] [Google Scholar]
- 34.Corona JC, Tovar-y-Romo LB, Tapia R. Glutamate excitotoxicity and therapeutic targets for amyotrophic lateral sclerosis. Expert Opin Ther Targets. 2007;11:1415–28. doi: 10.1517/14728222.11.11.1415. [DOI] [PubMed] [Google Scholar]
- 35.Sarchielli P, Di Filippo M, Candeliere A, Chiasserini D, Mattioni A, Tenaglia S, et al. Expression of ionotropic glutamate receptor GLUR3 and effects of glutamate on MBP- and MOG-specific lymphocyte activation and chemotactic migration in multiple sclerosis patients. J Neuroimmunol. 2007;188:146–58. doi: 10.1016/j.jneuroim.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Pin J-P, Duvoisin R. The metabotropic glutamate receptors: Structure and functions. Neuropharmcology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 37.Mao L, Wang JQ. Group I metabotropic glutamate receptor-mediated calcium signalling and immediate early gene expression in cultured rat striatal neurons. Eur J Neurosci. 2003;17:741–50. doi: 10.1046/j.1460-9568.2003.02495.x. [DOI] [PubMed] [Google Scholar]
- 38.Hofer AM. Another dimension to calcium signaling: a look at extracellular calcium. J Cell Sci. 2005;118:855–62. doi: 10.1242/jcs.01705. [DOI] [PubMed] [Google Scholar]
- 39.Marin YE, Namkoong J, Cohen-Solal K, Shin SS, Martino JJ, Oka M, et al. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signaling. 2006;18:1279–86. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Yan W, Fu Y, Tian D, Liao J, Liu M, Wang B, et al. PI3 kinase/Akt signaling mediates epithelial-mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;382:631–6. doi: 10.1016/j.bbrc.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 41.Julius D, Livelli TJ, Jessell TM, Axel R. Extopic expresion of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989;244:1057–62. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- 42.Gutkind JS, Novotny EA, Brann MR, Robbins KC. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc Natl Acad Sci. 1991;88:4703–7. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen LF, Lefkowitz RJ, Caron MG, Cotecchia S. G-protein-coupled receptor genes as protooncogenes: constitutively activating mutation of the alpha 1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc Natl Acad Sci U S A. 1991;88:11354–8. doi: 10.1073/pnas.88.24.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, et al. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3:23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- 45.Biechele TL, Camp ND, Fass DM, Kulikauskas RM, Robin NC, White BD, et al. Chemical-genetic screen identifies riluzole as an enhancer of Wnt/beta-catenin signaling in melanoma. Chem Biol. 2010;17:1177–82. doi: 10.1016/j.chembiol.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin SS, Namkoong J, Wall BA, Gleason R, Lee HJ, Chen S. Oncogenic activities of metabotropic glutamate receptor 1 (Grm1) in melanocyte transformation. Pigment Cell Melanoma Res. 2008a;21:368–78. doi: 10.1111/j.1755-148X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin SS, Wall B, Goydos JS, Chen S. AKT2 is a downstream target of metabotropic glutamate receptor 1 (mGlu1) Pigment Cell Melanoma Res. 2010;23:103–11. doi: 10.1111/j.1755-148X.2009.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smalley KS. PLX-4032, a small-molecule B-Raf inhibitor for the potential treatment of malignant melanoma. Curr Opin Investig Drugs. 2010;11:699–706. [PubMed] [Google Scholar]
- 49.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–27. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 50.Chetoui N, Sylla K, Gagnon-Houde JV, Alcaide-Loridan C, Charron D, Al-Daccak R, et al. Down-regulation of mcl-1 by small interfering RNA sensitizes resistant melanoma cells to fas-mediated apoptosis. Mol Cancer Res. 2008;6:42–52. doi: 10.1158/1541-7786.MCR-07-0080. [DOI] [PubMed] [Google Scholar]
- 51.Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–56. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]