Abstract
A novel, to our knowledge, in situ photoirradiation system for solid-state NMR measurements is improved and demonstrated to successfully identify the M-photointermediate of pharaonis phoborhodopsin (ppR or sensory rhodopsin II), that of the complex with transducer (ppR/pHtrII), and T204A mutant embedded in a model membrane. The 13C NMR signals from [20-13C]retinal-ppR and ppR/pHtrII revealed that multiple M-intermediates with 13-cis, 15-anti retinal configuration coexisted under the continuously photoirradiated condition. NMR signals observed from the photoactivated retinal provide insights into the process of photocycle in the ppR/pHtrII complex.
Pharaonis phoborhodopsin (ppR or sensory rhodopsin II) is a negative phototaxis receptor of Natronomonas pharaonis, and forms a 2:2 complex with the cognate transducer (pHtrII), which transmits the photosignal into cytoplasm (1, 2). Light absorption of ppR initiates trans-cis photoisomerization of the retinal chromophore followed by cyclic chemical reaction consisting of several intermediates (K, L, M, and O) (3) as shown in Fig. 1.
Figure 1.
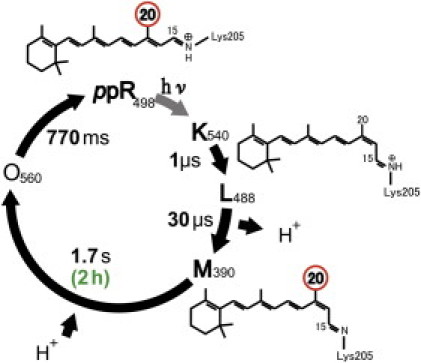
Photochemical reaction cycle of pharaonis phoborhodopsin (ppR). ppR absorbs blue light and forms K(540)-, L(488)-, M(390)-, and O(560)-intermediates. The M-intermediate has a long lifetime of 1.7 s in a membrane (3), whereas it has a much longer lifetime of 2 h in a membrane mimetic containing OG (4).
K(540) state is an intermediate with the half-lifetime of 1 μs and has 13-cis, 15-anti retinal configuration. The L(488)-state can be transformed from the K-state with a half-lifetime of 30 μs and has 13-cis, 15-anti retinal configuration. After the L-state, proton is removed from the Schiff base group to form the M(390)-state with a long half-lifetime of 1.7 s and has 13-cis, 15-anti retinal configuration. Interestingly, a very long lifetime of the M-state has been reported with a half-lifetime of 2 h in a membrane mimetic containing n-octyl-β-D-glucoside (OG) (4). After the M-state, it transforms to the O(560)-state with a half-lifetime of 770 ms by taking protons and has 13-trans, 15-syn retinal configuration (5). Here, 540 nm, 488 nm, 390 nm, and 560 nm denote wavelengths of the maximum absorption, λmax for K-, L-, M-, and O-intermediates, respectively. The M- and O-intermediates are thought to be active states for signal transduction. The crystal structure of the ppR/pHtrII complex suggests the formation of two specific hydrogen bonds between Tyr199ppR and Asn74pHtrII, and between Thr189ppR and Glu43pHtrII/Ser62pHtrII (6). Thr204 is an important residue for color tuning and photocycle kinetics of ppR (7), and these observations provide additional important roles of Thr204 in ppR for the negative phototaxis function of the complex (8). Thus, T204A mutant does not show signal transduction activity despite forming M-intermediate.
Steric hindrance between C14-H of retinal and Thr204 occurred upon the formation of K-intermediate (9). At the same time, a specific hydrogen-bonding alteration occurred between Thr204 and Tyr174 in a pHtrII-dependent manner (10). Helix movement of ppR, outward tilting of the helix F, during the photocycle is suggested by various groups (11, 12, 13), and it is thought to be an essential step for the activation of pHtrII. However, no helix-tilting was observed in the crystal structure of the M-intermediate of the ppR/pHtrII complex (14). Thus, the structural changes upon the formation of the active M-intermediate continue to be an exciting topic of discussion. To this end, solid-state NMR techniques can be applied to elucidate site-specific positions for such membrane-embedded systems (15, 16).
In this study, we successfully trapped the M-intermediate using the well-improved in situ photoirradiated solid-state NMR spectroscopy and photoirradiation efficiency was dramatically increased as compared with the previous system (17) (see Materials and Method in the Supporting Material). This system allowed us to observe the NMR signals of photointermediates to gain what we believe to be novel insights into the mechanism of signal transduction in the ppR/pHtrII complex.
[15-13C, 20-13C]retinal-ppR and pHtrII(1-159) with a His-Tag (6×His) at the C-terminal were expressed in the Escherichia coli BL21(DE3) strain in M9 medium. Purified proteins were incorporated into a lipid film of egg phosphatidylcholine (PC) (ppR/eggPC molar ratio of 1:30).
First, to investigate the trapping of an active intermediate of photoreceptor membrane proteins, we photoirradiated isomerization from the ground-state (G-state) to the M-intermediate of ppR, which was performed on [20-13C]retinal-ppR dissolved in OG at 20°C. This is because the lifetime of the M-intermediate (2 h) is much longer than those of the other intermediates. The sample was light-irradiated outside the magnet and quickly inserted in the magnet to observe the 13C directly detected magic-angle spinning (MAS) NMR spectrum of the M-intermediate (see Fig. S2 in the Supporting Material). The 13C directly detected MAS NMR spectrum obtained from the G-state of ppR in OG showed a peak at 13.4 ppm. A new peak from the M-intermediate appeared at 21.6 ppm and the peak at 13.4 ppm completely disappeared. This observed chemical shift value is consistent with that of the M-intermediate of bacteriorhodopsin (BR) (18). This observation clearly showed that all-trans retinal in the G-state was efficiently transformed to the 13-cis, 15-anti configuration of retinal in the M-intermediate under the strong light-irradiation.
Second, in situ photoirradiated cross-polarization (CP) MAS solid-state NMR experiments were performed on a sample under near-physiological condition (in the lipid bilayer systems) such as [15-13C, 20-13C]retinal-ppR in egg PC at 0°C and −20°C (Fig. 2). In addition to the long lifetime for the M(390)-intermediate (1.7 s), the relatively long-lived O(560)-intermediate (0.77 s) can be irradiated by the light (532 nm). In this case, O-state is excited to G-state (3) and hence only M-intermediate can be trapped under the continuous photoirradiation condition. In the G-state, a peak of 20-13C in retinal appeared at 13.3 ppm and the signal shifted to 22.3 ppm for the M-intermediate at 0°C (Fig. 2 A). The observation of peaks at 24.1, 22.5, and 21.7 ppm in ppR indicates the existence of at least three distinct M-intermediates under the photoirradiated condition at −20°C (Fig. 2 C). 15-13C signals were also observed for light and dark conditions. It is noted that the signal position did not change between light and dark conditions, whereas intensity was reduced in the M-state (see Fig. S3). This observation is surprising because the shift of 5 ppm for 15-C from G (all-trans)- to M-state has been observed in BR (see Table S1 in the Supporting Material).
Figure 2.
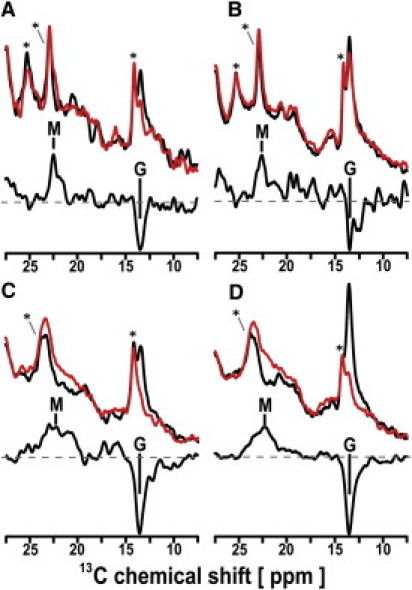
13C CP-MAS NMR spectra of [20-13C, 15-13C]retinal-ppR (A and C) and ppR/pHtrII complex (B and D) taken at 0°C (A and B) and −20°C (C and D). (Top spectra) Data obtained from the light (red) and dark (black) states of ppR (A and C) and ppR/pHtrII complex (B and D). (Bottom spectra) Difference between those of light (red) and dark (black) spectra. The peaks M and G indicate the 13C NMR signals of [20-13C]retinal in the M-intermediate and the G-state, respectively. (Asterisk) Natural abundant lipid signals.
The yields of overall M-intermediates in ppR were evaluated to be 45% and 80% at 0 and −20°C, respectively, by inspecting the peak areas of the G-state. This difference in their yields can be attributed to a longer lifetime of the M-intermediate at a lower temperature. It is important to point out the single peak with a shoulder observed for the M-intermediate at 0°C, whereas multiplet lines were observed at −20°C. These multiplet signals can be attributed to the coexistence of several different interactions between the retinal and protein. The M-intermediates were also trapped for the [15-13C, 20-13C]retinal-ppR/pHtrII complex that has signal transduction function as revealed by the appearance of a CP MAS 13C NMR peak at 22.6 ppm at 0°C (Fig. 2 B) and three distinct peaks at 23.5, 22.3, and 21.3 ppm at −20°C (see Table S1). Thus, multiple M-intermediates were also observed in the ppR/pHtrII complex at −20°C (Fig. 2 D). It was noted that the signal in the M-intermediate for ppR was slightly different from that in the ppR/pHtrII complex. The chemical shift values of [20-13C]retinal in the M-intermediate of ppR distribute more widely and in a lower field than those of the ppR/pHtrII complex.
Finally, the 20-13C signal of T204A was observed as shown in Fig. 3. The signal in ground state at 14.0 ppm was converted to that in M-state at 21.3 ppm at −20°C. It is noted that the signal at 21 ppm did not show multiplet lines, but did show a singlet line. In addition, the chemical shift value of 20-13C in the M-state is similar to that of BR rather than ppR. This result indicates that the M-state of T204A has 13-cis, 15-anti configuration and takes a single state.
Figure 3.
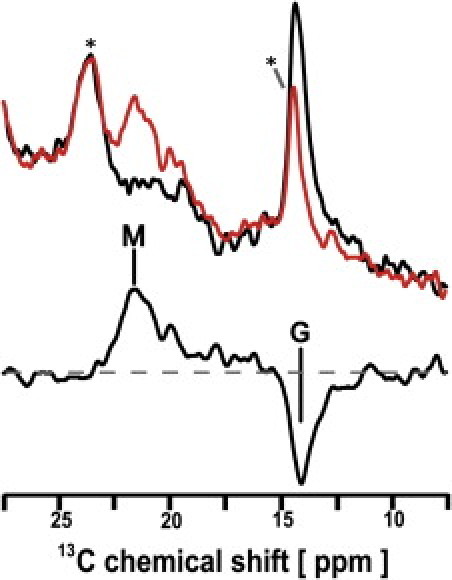
13C CP-MAS NMR spectra of [20-13C] retinal-T204A taken at −20°C. (Top spectra) Data obtained from the light (red) and dark (black) states of T204A. (Bottom spectrum) Difference between those of light (red) and dark (black) spectra. The peaks M and G indicate the 13C NMR signals of [20-13C]retinal in the M-intermediate and the G-state, respectively. (Asterisk) Natural abundant lipid signals.
In conclusion, we have successfully observed the M-intermediate from ppR, ppR/pHtrII complex, and T204A mutant embedded in a model membrane using in situ photoirradiated solid-state MAS NMR spectroscopy. Our results show that the multiple M-intermediates coexist at −20°C due to different retinal-protein interactions in ppR and also in the ppR/pHtrII complex. Multiple M-intermediates have been observed in BR as Mo and Mn states whose chemical shift difference was 1.8 ppm (19). Because only M-intermediate can be trapped, it is now possible to observe the structural changes of the protein side in the photoactivated state using in situ photoirradiated solid-state NMR experiments.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Priority Area (21017002) and a Grant-in-Aid for Young Scientists (B) (22770101) from the Ministry of Culture, Sports, Science and Technology of Japan and a SUNBOR Grant from Suntory Institute for Bioorganic Research. A.R. was a fellow of the Japan Society for the Promotion of Science during this research in Japan. This research was partly supported by the National Institutes of Health (GM084018 and GM095640 to A.R.).
Editor: Marc Baldus.
Footnotes
Materials and method, three figures, and one table are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(11)01242-2.
Supporting Material
References and Footnotes
- 1.Spudich J.L., Luecke H. Sensory rhodopsin II: functional insights from structure. Curr. Opin. Struct. Biol. 2002;12:540–546. doi: 10.1016/s0959-440x(02)00359-7. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki D., Irieda H., et al. Sudo Y. Phototactic and chemotactic signal transduction by transmembrane receptors and transducers in microorganisms. Sensors (Basel Switzerland) 2010;10:4010–4039. doi: 10.3390/s100404010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy S., Kikukawa T., et al. Kamo N. All-optical switching in pharaonis phoborhodopsin protein molecules. IEEE Trans. Nanobioscience. 2006;5:178–187. doi: 10.1109/tnb.2006.880828. [DOI] [PubMed] [Google Scholar]
- 4.Sudo Y., Nishihori T., et al. Kamo N. A long-lived M-like state of phoborhodopsin that mimics the active state. Biophys. J. 2008;95:753–760. doi: 10.1529/biophysj.107.125294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imamoto Y., Shichida Y., et al. Yoshizawa T. Chromophore configuration of pharaonis phoborhodopsin and its isomerization on photon absorption. Biochemistry. 1992;31:2523–2528. doi: 10.1021/bi00124a012. [DOI] [PubMed] [Google Scholar]
- 6.Gordeliy V.I., Labahn J., et al. Engelhard M. Molecular basis of transmembrane signaling by sensory rhodopsin II-transducer complex. Nature. 2002;419:484–487. doi: 10.1038/nature01109. [DOI] [PubMed] [Google Scholar]
- 7.Shimono K., Hayashi T., et al. Kamo N. Importance of the broad regional interaction for spectral tuning in Natronobacterium pharaonis phoborhodopsin (sensory rhodopsin II) J. Biol. Chem. 2003;278:23882–23889. doi: 10.1074/jbc.M301200200. [DOI] [PubMed] [Google Scholar]
- 8.Sudo Y., Furutani Y., et al. Spudich J.L. Functional importance of the interhelical hydrogen bond between Thr204 and Tyr174 of sensory rhodopsin II and its alteration during the signaling process. J. Biol. Chem. 2006;281:34239–34245. doi: 10.1074/jbc.M605907200. [DOI] [PubMed] [Google Scholar]
- 9.Sudo Y., Furutani Y., et al. Kandori H. Steric constraint in the primary photoproduct of an archaeal rhodopsin from regiospecific perturbation of C-D stretching vibration of the retinyl chromophore. J. Am. Chem. Soc. 2005;127:16036–16037. doi: 10.1021/ja056203a. [DOI] [PubMed] [Google Scholar]
- 10.Furutani Y., Kamada K., et al. Kandori H. Structural changes of the complex between pharaonis phoborhodopsin and its cognate transducer upon formation of the M photointermediate. Biochemistry. 2005;44:2909–2915. doi: 10.1021/bi047893i. [DOI] [PubMed] [Google Scholar]
- 11.Wegener A.A., Chizhov I., et al. Steinhoff H.J. Time-resolved detection of transient movement of helix F in spin-labeled pharaonis sensory rhodopsin II. J. Mol. Biol. 2000;30:881–891. doi: 10.1006/jmbi.2000.4008. [DOI] [PubMed] [Google Scholar]
- 12.Spudich J.L. Variations on a molecular switch: transport and sensory signaling by archaeal rhodopsins. Mol. Microbiol. 1998;28:1051–1058. doi: 10.1046/j.1365-2958.1998.00859.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida H., Sudo Y., et al. Kamo N. Transient movement of helix F revealed by photo-induced inactivation by reaction of a bulky SH-reagent to cysteine-introduced pharaonis phoborhodopsin (sensory rhodopsin II) Photochem. Photobiol. Sci. 2004;3:537–542. doi: 10.1039/b315454h. [DOI] [PubMed] [Google Scholar]
- 14.Moukhametzianov R., Klare J.P., et al. Gordeliy V.I. Development of the signal in sensory rhodopsin and its transfer to the cognate transducer. Nature. 2006;440:115–119. doi: 10.1038/nature04520. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura I., Yoshida H., et al. Naito A. Dynamics change of phoborhodopsin and transducer by activation: study using D75N mutant of the receptor by site-directed solid-state 13C NMR. Photochem. Photobiol. 2008;84:921–930. doi: 10.1111/j.1751-1097.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 16.Etzkorn M., Seidel K., et al. Baldus M. Complex formation and light activation in membrane-embedded sensory rhodopsin II as seen by solid-state NMR spectroscopy. Structure. 2010;18:293–300. doi: 10.1016/j.str.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura I., Kihara N., et al. Naito A. Solid-state NMR studies of two backbone conformations at Tyr185 as a function of retinal configurations in the dark, light, and pressure adapted bacteriorhodopsins. J. Am. Chem. Soc. 2007;129:1016–1017. doi: 10.1021/ja0664887. [DOI] [PubMed] [Google Scholar]
- 18.Bajaj V.S., Mak-Jurkauskas M.L., et al. Griffin R.G. Functional and shunt states of bacteriorhodopsin resolved by 250 GHz dynamic nuclear polarization-enhanced solid-state NMR. Proc. Natl. Acad. Sci. USA. 2009;106:9244–9249. doi: 10.1073/pnas.0900908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petkova A.T., Hatanaka M., et al. Herzfeld J. Tryptophan interactions in bacteriorhodopsin: a heteronuclear solid-state NMR study. Biochemistry. 2002;41:2429–2437. doi: 10.1021/bi012127m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


