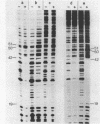
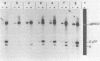
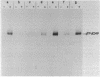
Abstract
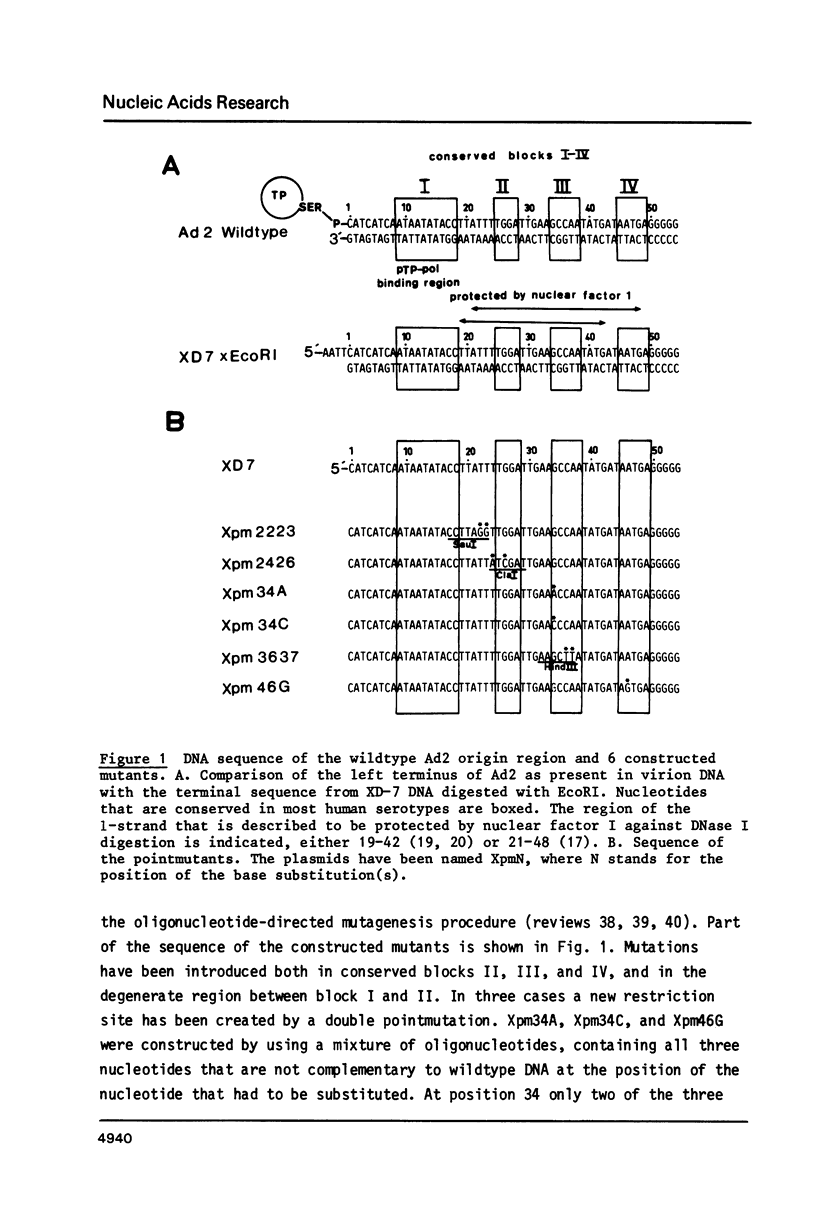
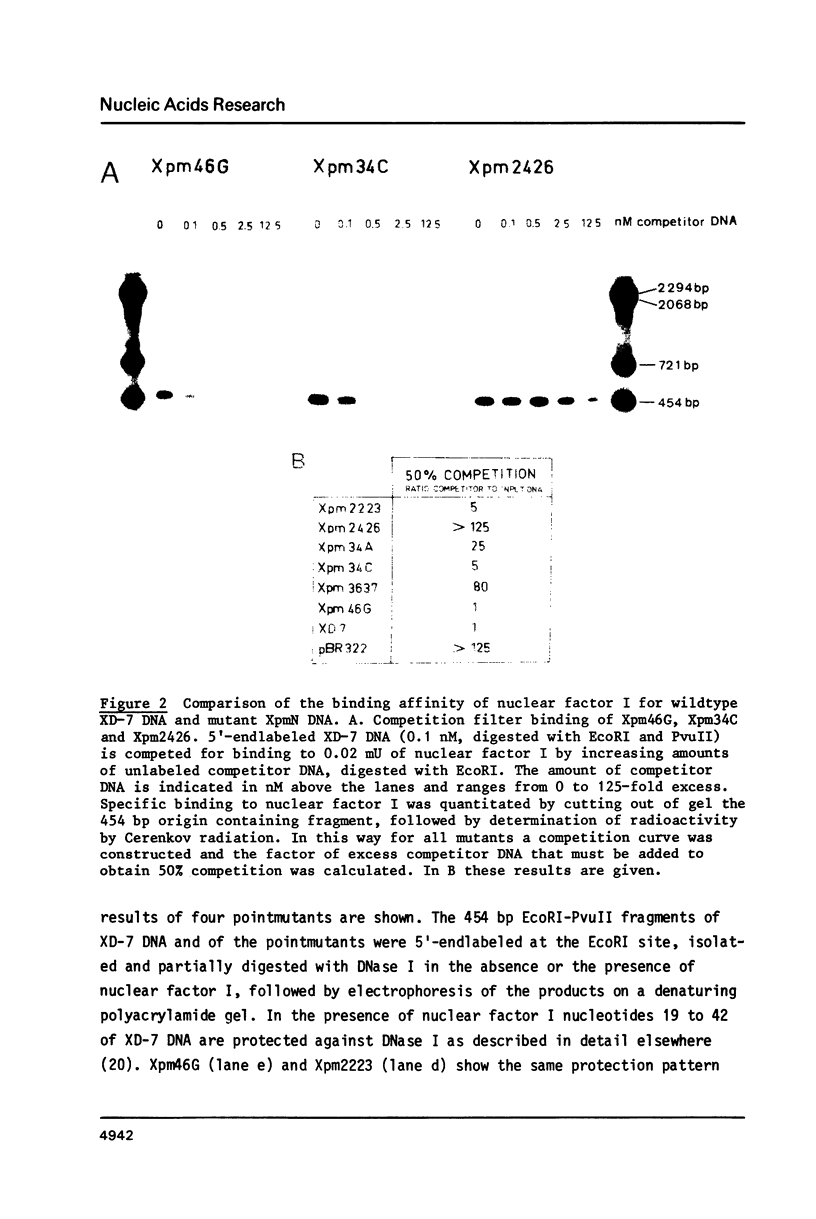
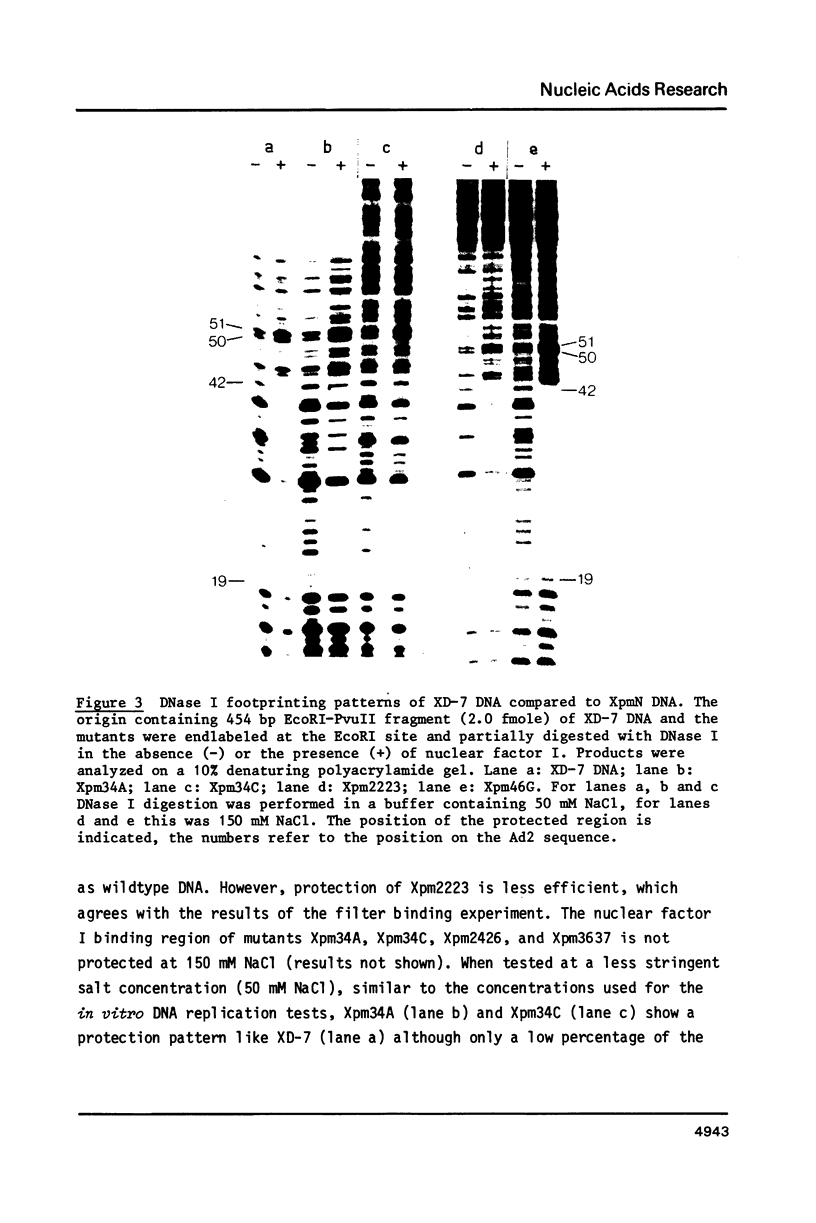
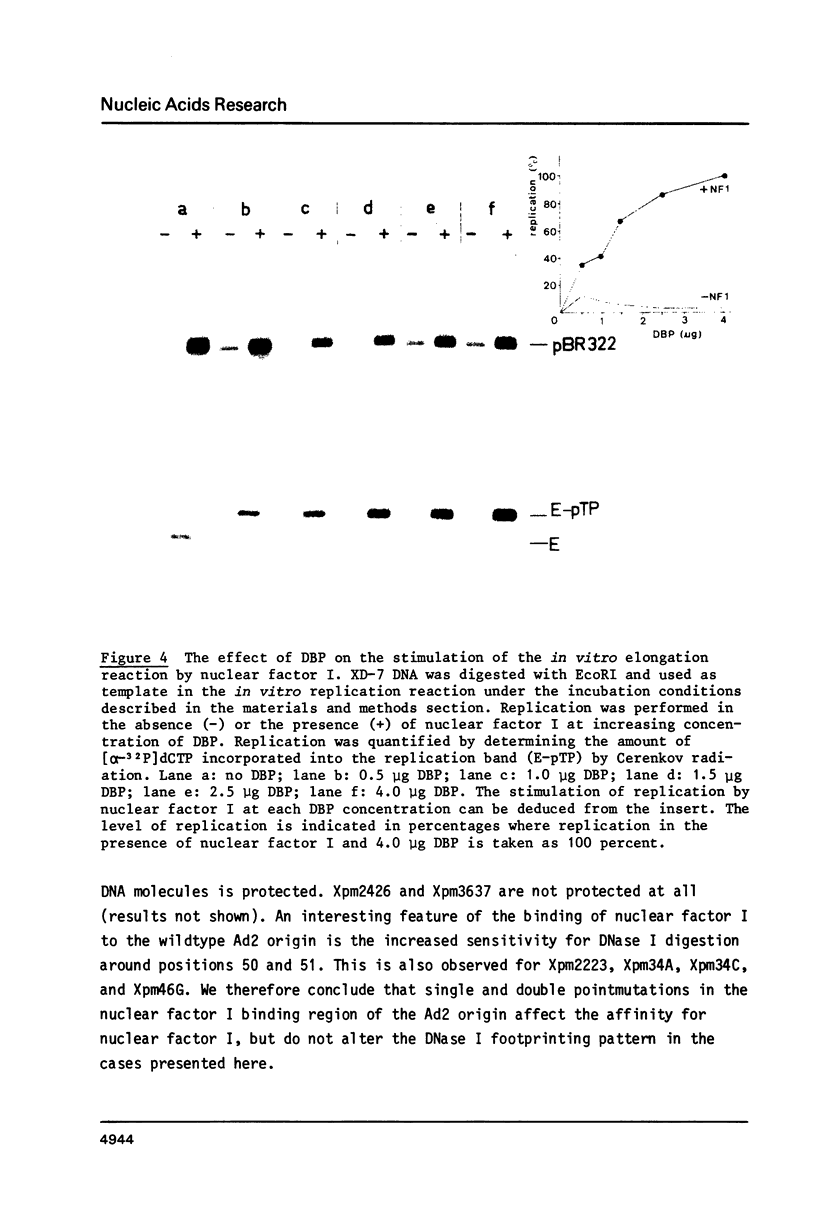
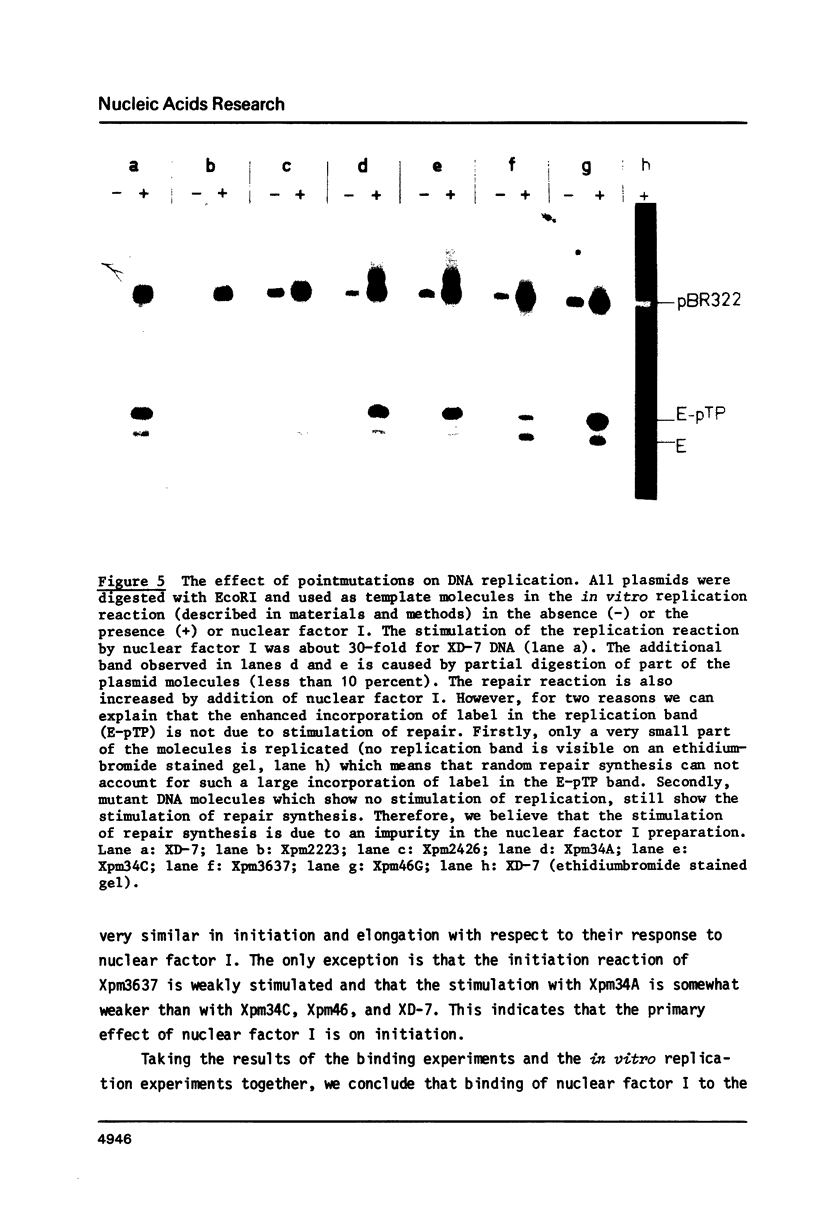
The template requirements for efficient adenovirus DNA replication were studied in vitro in a reconstituted system with cloned DNA fragments, containing the Ad2 origin region, as templates. Replication is enhanced by nuclear factor I, a cellular protein that binds specifically to the Ad2 origin. This stimulation is shown to be strongly dependent on the concentration of the adenovirus DNA binding protein. Using synthetic oligonucleotides we have constructed plasmids with base substitutions in the nuclear factor I binding region. Footprint analysis and competition filter binding studies show that two of the three small blocks of conserved nucleotides in this region are involved in the binding of nuclear factor I. The binding affinity can be influenced by the base composition of the degenerate region just outside these two blocks. In vitro initiation and DNA chain elongation experiments with the mutants demonstrate that binding of nuclear factor I to the Ad2 origin is necessary for stimulation. However, binding alone is not always sufficient since a mutation which only slightly disturbs binding is strongly impaired in stimulation of DNA replication by nuclear factor I.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmeyer U., Nowock J., Sippel A. E. The TGGCA-binding protein: a eukaryotic nuclear protein recognizing a symmetrical sequence on double-stranded linear DNA. Nucleic Acids Res. 1984 May 25;12(10):4295–4311. doi: 10.1093/nar/12.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Eukaryotic DNA replication: viral and plasmid model systems. Annu Rev Biochem. 1982;51:901–934. doi: 10.1146/annurev.bi.51.070182.004345. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Ostrove J. M., Kelly T. J., Jr Initiation of adenovirus DNA replication: detection of covalent complexes between nucleotide and the 80-kilodalton terminal protein. J Virol. 1982 Jan;41(1):265–270. doi: 10.1128/jvi.41.1.265-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Rawlins D. R. Template requirements for the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1984 Jan;81(1):100–104. doi: 10.1073/pnas.81.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam S., Waterman K., Smith M. The base-pairing specificity of cellulose-pdT9. Nucleic Acids Res. 1975 May;2(5):625–634. doi: 10.1093/nar/2.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M., Nagata K., Hurwitz J. Isolation of human DNA sequences that bind to nuclear factor I, a host protein involved in adenovirus DNA replication. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4013–4017. doi: 10.1073/pnas.81.13.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheimer R. A., Nagata K., Lindenbaum J., Hurwitz J. Protein-primed replication of plasmids containing the terminus of the adenovirus genome. I. Characterization of an in vitro DNA replication system dependent on adenoviral DNA sequences. J Biol Chem. 1984 Jun 25;259(12):7807–7814. [PubMed] [Google Scholar]
- Guggenheimer R. A., Stillman B. W., Nagata K., Tamanoi F., Hurwitz J. DNA sequences required for the in vitro replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3069–3073. doi: 10.1073/pnas.81.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T. The origin of adenovirus DNA replication: minimal DNA sequence requirement in vivo. EMBO J. 1985 Feb;4(2):421–426. doi: 10.1002/j.1460-2075.1985.tb03645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lally C., Dörper T., Gröger W., Antoine G., Winnacker E. L. A size analysis of the adenovirus replicon. EMBO J. 1984 Feb;3(2):333–337. doi: 10.1002/j.1460-2075.1984.tb01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Field J., Horwitz M. S., Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5225–5229. doi: 10.1073/pnas.79.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Nagata K., Friefeld B. R., Enomoto T., Field J., Guggenheimer R. A., Ikeda J. E., Horwitz M. S., Hurwitz J. Isolation of proteins involved in the replication of adenoviral DNA in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):731–740. doi: 10.1101/sqb.1983.047.01.084. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Adenovirus DNA replication in vitro: synthesis of full-length DNA with purified proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4266–4270. doi: 10.1073/pnas.80.14.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Specific binding of a cellular DNA replication protein to the origin of replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6177–6181. doi: 10.1073/pnas.80.20.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga K. A., Van der Bliek A. M., Van der Horst G., Groot Koerkamp M. J., Tabak H. F., Veeneman G. H., Van Boom J. H. In vitro site-directed mutagenesis with synthetic DNA oligonucleotides yields unexpected deletions and insertions at high frequency. Nucleic Acids Res. 1983 Dec 20;11(24):8595–8608. doi: 10.1093/nar/11.24.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Chow K. C., Enns R. E., Ahern K. G., Corden J. L., Harpst J. A. In vitro replication directed by a cloned adenovirus origin. Gene. 1983 Sep;23(3):293–305. doi: 10.1016/0378-1119(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Rawlins D. R., Rosenfeld P. J., Wides R. J., Challberg M. D., Kelly T. J., Jr Structure and function of the adenovirus origin of replication. Cell. 1984 May;37(1):309–319. doi: 10.1016/0092-8674(84)90327-1. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Rijnders A. W., van Bergen B. G., van der Vliet P. C., Sussenbach J. S. Specific binding of the adenovirus terminal protein precursor-DNA polymerase complex to the origin of DNA replication. Nucleic Acids Res. 1983 Dec 20;11(24):8777–8789. doi: 10.1093/nar/11.24.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Stillman B. W. Adenovirus terminal protein precursor. Partial amino acid sequence and the site of covalent linkage to virus DNA. J Biol Chem. 1982 Nov 25;257(22):13499–13506. [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F., Mathews M. B. Purification of an adenovirus-coded DNA polymerase that is required for initiation of DNA replication. Cell. 1982 Dec;31(3 Pt 2):613–623. doi: 10.1016/0092-8674(82)90317-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. W. The replication of adenovirus DNA with purified proteins. Cell. 1983 Nov;35(1):7–9. doi: 10.1016/0092-8674(83)90201-5. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. The mechanism of adenovirus DNA replication and the characterization of replication proteins. Curr Top Microbiol Immunol. 1984;109:53–73. doi: 10.1007/978-3-642-69460-8_2. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Function of adenovirus terminal protein in the initiation of DNA replication. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2221–2225. doi: 10.1073/pnas.79.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Initiation of adenovirus DNA replication in vitro requires a specific DNA sequence. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6446–6450. doi: 10.1073/pnas.80.21.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsernoglou D., Tucker A. D., Van der Vliet P. C. Crystallization of a fragment of the adenovirus DNA binding protein. J Mol Biol. 1984 Jan 15;172(2):237–239. doi: 10.1016/s0022-2836(84)80042-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen B. G., van der Ley P. A., van Driel W., van Mansfeld A. D., van der Vliet P. C. Replication of origin containing adenovirus DNA fragments that do not carry the terminal protein. Nucleic Acids Res. 1983 Apr 11;11(7):1975–1989. doi: 10.1093/nar/11.7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet P. C., van Dam D., Kwant M. M. Adenovirus DNA replication in vitro is stimulated by RNA from uninfected HeLa cells. FEBS Lett. 1984 Jun 4;171(1):5–8. doi: 10.1016/0014-5793(84)80449-4. [DOI] [PubMed] [Google Scholar]