Abstract
We compared patterns of neuropsychiatric symptom across four dementia types (AD, VAD, DLB, PDD), and two mixed groups (AD/VAD, AD/DLB) in sample of 2,963 individuals from the National Alzheimer’s Coordinating Center Uniform Data Set between September 2005 and June 2008. We used confirmatory factor analysis to compare neuropsychiatric symptom severity ratings made by collateral sources on the Neuropsychiatric Inventory (NPI-Q) for people with Clinical Dementia Rating scores of 1 or higher. A three factor model of psychiatric symptoms (mood, psychotic, and frontal) was shared across all dementia types. Between-group comparisons revealed unique neuropsychiatric profiles by dementia type. The AD group had moderate levels of mood, psychotic, and frontal symptoms while VAD exhibited the highest levels and PDD had the lowest levels. DLB and the mixed dementias had more complex symptom profiles. Depressed mood was the dominant symptom in people with mild diagnoses. Differing psychiatric symptom profiles provide useful information regarding the non-cognitive symptoms of dementia.
Declines in memory and attention are considered hallmark symptoms of Alzheimer’s Disease (AD), but non-cognitive symptoms such as changes in mood, personality, and other neuropsychiatric symptoms are also prevalent1–3 especially during later stages.4–6 Epidemiologic studies of people with incident dementia universally report mood-related symptoms7 (e.g., apathy, depression, anxiety) with psychotic features second. In specialty clinics, patients with vascular dementia (VAD) have been reported to experience changes in mood and personality including increased apathy and irritability.8–9 Patients with Dementia with Lewy Bodies (DLB) often show rigidity, poor emotional control, apathy, hallucinations, delusions, and depression.10–12 Similar symptoms are reported in Parkinson’s disease dementia (PDD).11, 13
Although neuropsychiatric symptoms have been described within a variety of dementia types, few studies have compared patterns of symptoms between dementia types using a standardized instrument8–9, 14 and those have been limited in sample size and variety of dementia diagnoses. The current study applies a factor analytic model of the Neuropsychiatric Inventory Questionnaire (NPI-Q)15–16 to individuals with AD, VAD, DLB, PDD, combined diagnoses of AD and VAD (AD/VAD), and combined AD and DLB (AD/DLB) who participated in research at one of the national Alzheimer’s Disease Centers contributing to the National Alzheimer Coordinating Center’s (NACC). Neuropsychiatric evaluation of dementia characterizes patterns of psychiatric symptoms and associates unique symptom patterns with specific neuropathological processes. The diagnostic breadth of the current sample allows us identify unique profiles of symptoms with specific dementia types, including less prevalent or rarer dementias (e.g., DLB) and blended dementias (e.g., AD/VAD). Previous studies have used exploratory factor analysis to investigate how the items of the NPI questionnaire cluster together.13–14, 17–18 Others have used latent class and mixture modeling procedures (more exploratory techniques) to identify unique patterns of symptoms in previously unidentified groups of dementia patients.19 In contrast, we used confirmatory factor analysis to validate the diagnostic groups (previously established by NACC clinicians) and compare dementia types based on their factor scores. Factor scores have the desirable property of being more sensitive symptom summary scores than observed/raw scores.
We began with an empirically validated three-factor model of neuropsychiatric symptoms that was established in patients with AD (mood, psychotic, and frontal symptom domains) by Frisoni and colleagues.17 We then tested whether this model could be extended across multiple non-AD dementia variants. Although other models of symptom clusters do exist,13–14, 17–18 we chose the three-factor model because its aggregation of symptoms is used most commonly in clinical practice. Our study characterizes unique patterns of neuropsychiatric symptoms displayed in each of these dementia types or mixed-dementias. Because this modeling procedure used a preponderant number of patients with AD, these results should be interpreted as relative to the AD-referent group.
Method
Sample
Data from the National Alzheimer Coordinating Center’s (NACC; http://www.alz.washington.edu/) Uniform Data Set consist of information from Alzheimer’s Disease Centers supported by the National Institute on Aging (NIA).16 NACC data used in the present study were collected between September 2005 and June 2008 (acquired November 2009). This data includes demographic information (Table 1) and clinical data collected for 2,963 individuals who had archival data for NPI-Q, and Clinical Dementia Rating (CDR) score of 1 or higher (to ensure presence of neuropsychiatric symptoms). Individuals met the following clinical diagnostic criteria for primary diagnosis: AD (n = 2,474), DLB (n = 151), VAD (n = 85), PDD (n = 74), AD/VAD (n=92; AD was primary diagnosis), and AD/DLB (n = 87; AD was primary diagnosis). The AD and DLB groups contain only persons without other concurrent diagnoses. For VAD and PDD, we used primary diagnosis although they include cases that are not pure, i.e. they may be of mixed diagnosis. Individuals in the AD group who were less than 60 years of age were excluded. Sample sizes for other mixed-dementia diagnoses were too small for inclusion in these analyses (n’s < 50).
Table 1.
Demographics
| VAD | AD/VAD | AD | AD/DLB | DLB | PDD | |
|---|---|---|---|---|---|---|
| (n = 85) | (n = 92) | (n = 2474) | (n = 87) | (n = 151) | (n = 74) | |
| Age Mean | 74.0 (9.8) | 74.7 (6.7) | 73.5 (7.1) | 72.2 (8.5) | 69.4 (8.7) | 69.5 (7.9) |
| % Women | 60 | 54 | 59 | 54 | 26 | 18 |
| Education | 12.4 (3.7) | 13.1 (4.0) | 13.5 (3.9) | 14.4 (3.7) | 14.1 (3.7) | 14.7 (3.8) |
| Global CDR | 1.5 (0.7) | 1.5 (0.7) | 1.5 (0.7) | 1.6 (0.7) | 1.5 (0.7) | 1.4 (0.7) |
| % CDR 1 | 61 | 59 | 62 | 51 | 60 | 70 |
| % CDR 2 | 28 | 28 | 27 | 34 | 28 | 18 |
| % CDR 3 | 11 | 13 | 11 | 15 | 12 | 12 |
Note. AD/PDD mixed diagnosis was available but the sample was too small for inclusion in these analyses (n = 45)
Procedures
Individual NACC ADC studies were approved by local human subjects committees. Written informed consent was obtained from all patients and/or collateral sources. AD diagnosis was based on NINCDS-ADRDA.20 VAD was diagnosed according to NINDS-AIREN criteria21 DLB was diagnosed according the criteria established by the DLB Consortium.22 PDD was diagnosed per the criteria established by the Movement Disorders Society Scientific Issues Committee.23
Measures
Trained clinicians administered the NPI-Q to an informant knowledgeable about the patient’s behavior. This measure assesses 10 behavioral disturbances that may occur in dementia and other neuropsychiatric disorders: delusions, hallucinations, dysphoria, anxiety, agitation/aggression, euphoria, disinhibition, irritability/lability, apathy, and aberrant motor activity. We combined the presence and severity questions for each NPI-Q item. Thus, symptom severity was rescaled from 0 to 3, and treated as a ratio scaled index of the assessed symptom, 0 indicating no symptom present and 3 indicating severe symptomatology.
Statistical Analyses
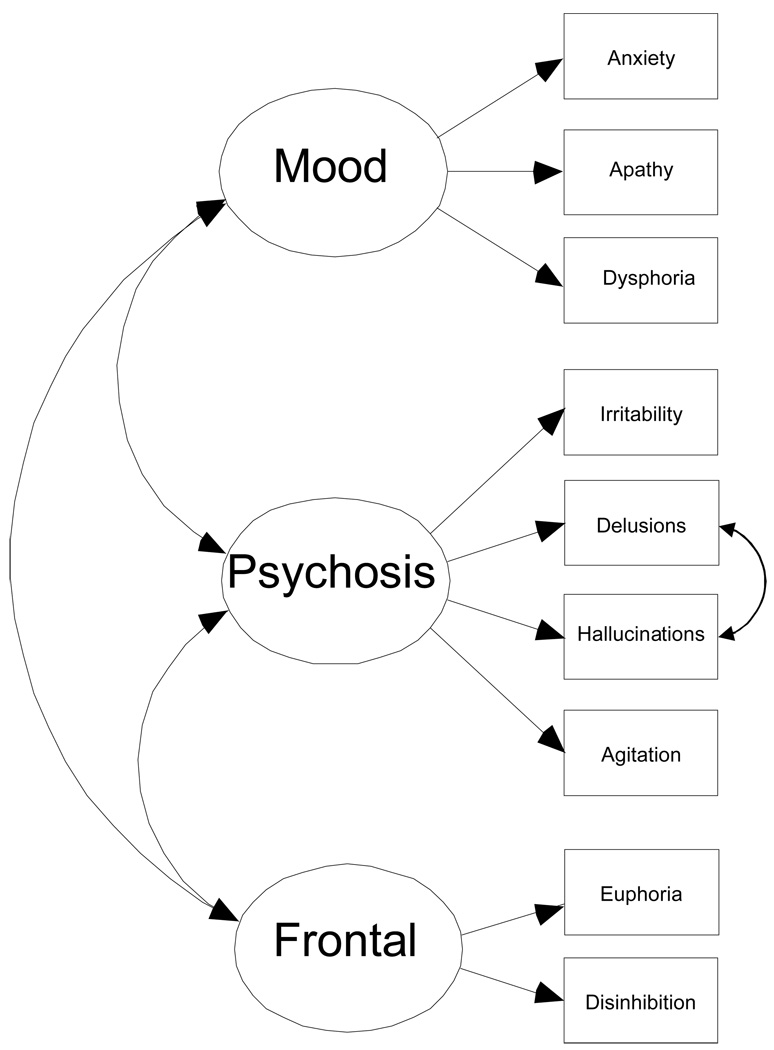
Confirmatory Factor Analysis (CFA) with full information maximum likelihood was conducted in a multi-step process.24 Step 1: to assess the relative fit of a 3-factor baseline model of psychiatric features in AD developed by Frisoni et al. 17, we collapsed across all dementia participants (n= 2,963) and examined domain content, factor loadings, and factor interrelationships (Figure 1). The three factors specified in this model are Mood (indicated by anxiety, apathy, and dysphoria items), Psychosis (irritability/lability, delusions, hallucinations, and agitation/aggression), and Frontal symptoms (euphoria and disinhibition). Step 2: The baseline model was used in a series of increasingly restrictive between group tests of invariance (TOI) comparing 4 types of dementia (AD, VAD, DLB, PDD) and 2 groups of mixed dementia (AD/VAD, and AD/DLB).Less constrained models must be accepted before subsequent higher order solutions can be interpreted. These tests indicate whether the psychometric properties of the instrument (factor structure and variance-covariance patterns) differ between the samples. The test of weak invariance is the least restrictive. Good fit indicates that the groups share the same factor structure and the same factor loadings. The test of strong invariance requires all assumptions for weak as well as the same test item intercepts across groups. No differences at strong invariance (equal factor loadings and item intercepts) indicate that the groups share similar psychometric configurations for the NPI-Q. Differences at strong invariance indicate the groups differ in the amount of the trait measured. This provides the analytic reasoning for Step 3, a between group comparison on latent means using a test of latent mean invariance. Failure of a test of latent mean invariance suggests that groups differ in their mean levels of mood, psychotic, and frontal symptoms. Step 4: If step 3 fails to fit, we estimate how much these groups differ by constraining as many between group coefficients to be equal as theoretically feasible and statistically warranted (model optimization). This yields a parsimonious model of between group similarity and difference.25 To evaluate model changes at each step of the analyses, we used goodness-of-fit indices as prescribed by Cheung & Rensvold.26 In the first 2 steps, the psychometric properties of the model were evaluated using change in the Root Mean Square Error of Approximation (ΔRMSEA < − .02). In the final 2 steps (between group comparisons) we used change in −2 Log Likelihoods (ΔX2 (df=15) > 7.3) to determine model fit.
Figure 1.
Caption: Accepted Baseline Three-Factor Model of Neuropsychiatric Symptoms on the NPI-Q. Adjustment for CDR as a covariate is not represented in the figure.
Results
Sample characteristics
Dementia severity (CDR) did not differ between the groups (F = 1.11, p = .35; note all participants CDR ≥ 1). Omnibus tests for group differences in Age, Gender, and Education were all significant (all F’s > 4.58, p < .001). Bonferroni corrected pairwise comparisons revealed that the PDD and DLB groups were younger than all other groups (p<.001) and contained far fewer women (Χ2 (5) = 107.5, p < .001); however, all other group comparisons were nonsignificant. Importantly, AD participants were statistically equivalent to all other dementia groups on Age, Gender, and Education and thus were not controlled for in the modeling process. There was modest variation in level of education across the groups (Range 12.4 to 14.7), but only the two most extreme groups differed significantly (VAD vs. PDD).
Because dementia severity (CDR) affected symptom presentation (Table 2), all models reported here are adjusted for CDR status. Participants with more progressed AD reported more psychotic symptoms (β = .23, p < .001) and more frontal symptoms (β = .10, p < .01). For DLB, higher dementia severity predicted an increase in psychotic symptoms (β = .30, p < .01). For AD/VAD, higher dementia severity predicted increased mood symptoms (β = .42, p < .05) and psychotic symptoms (β = .55, p < .001). For AD/DLB, higher dementia severity predicted increased frontal symptoms (β = .42, p < .05). Disease severity did not predict the levels of any type of symptoms on the NPI in the PDD or VAD groups.
Table 2.
Sample Means (Standard Error), Rates of Occurrence (%), Mean Severity (Standard Error), and Observed Severity (Standard Error) among individuals reporting symptom presence for NPI-Q Items and Symptom Domains
| VAD | AD/VAD | AD | AD/DLB | DLB | PDD | |
|---|---|---|---|---|---|---|
| (n = 85) | (n = 92) | (n = 2,474) | (n = 87) | (n = 151) | (n = 74) | |
| Mood Domain (0–3) | 0.90 a (0.08) | 0.74 b (0.06) | 0.78 b (0.01) | 0.97 a (0.07) | 1.06 a (0.06) | 0.67 b (0.06) |
| Anxiety | ||||||
| Occurrence (0/1) | 42% a | 40% a | 46% a | 60% b | 64% b | 28% c |
| Mean Severity (0–3) | 0.68 (0.10) a | 0.58 (0.09) a | 0.70 (0.02) a | 0.95 (0.10) b | 1.09 (0.07) b | 0.46 (0.10) a |
| Observed Severity (1–3) | 1.61 (0.11) a | 1.43 (0.11) a | 1.53 (0.02) a | 1.60 (0.09) a | 1.72 (0.07) a | 1.62 (015) a |
| Dysphoria | ||||||
| Occurrence (0/1) | 56% a | 50% a | 46% b | 57% a | 53% a | 53% a |
| Mean Severity (0–3) | 0.94 (0.09) a | 0.69 (0.09) a | 0.67 (0.02) a | 0.81 (0.09) a | 0.79 (0.07) a | 0.65 (0.10) a |
| Observed Severity (1–3) | 1.67 (0.09) a | 1.37 (0.10) a | 1.48 (0.02) a | 1.40 (0.09) a | 1.49 (0.07) a | 1.23 (0.10) b |
| Apathy | ||||||
| Occurrence (0/1) | 66% a | 63% a | 57% b | 66% a | 73% c | 57% b |
| Mean Severity (0–3) | 1.29 (0.11) a | 1.09 (0.10) a | 0.94 (0.02) b | 1.16 (0.11) a | 1.39 (0.08) a | 0.85 (0.12) b |
| Observed Severity (1–3) | 1.96 (0.10) a | 1.72 (0.10) b | 1.65 (0.02) b | 1.77 (0.10) b | 1.91 (0.07) a | 1.50 (0.11) b |
| Psychosis Domain (0–3) | 0.60 a (0.06) | 0.42 b (0.05) | 0.52 c (0.01) | 0.52 c (0.06) | 0.55 c (0.04) | 0.46 b (0.05) |
| Agitation | ||||||
| Occurrence (0/1) | 52% a | 37% a | 47% a | 48% a | 47% a | 51% a |
| Mean Severity (0–3) | 0.86 (0.10) a | 0.61 (0.10) a | 0.73 (0.02) a | 0.70 (0.10) a | 0.72 (0.07) a | 0.62 (0.11) a |
| Observed Severity (1–3) | 1.66 (0.10) a | 1.65 (0.12) a | 1.56 (0.02) a | 1.45 (0.11) b | 1.52 (0.08) b | 1.21 (0.11) b |
| Hallucinations | ||||||
| Occurrence (0/1) | 14% a | 12% a | 13% a | 39% b | 55% c | 46% b |
| Mean Severity (0–3) | 0.24 (0.07) a | 0.15 (0.06) a | 0.19 (0.01) a | 0.62 (0.07) b | 0.99 (0.05) b | 0.60 (0.07) c |
| Observed Severity (1–3) | 1.67 (0.20) a | 1.27 (0.21) a | 1.50 (0.04) a | 1.59 (0.12) a | 1.80 (0.08) b | 1.29 (0.12) a |
| Delusions | ||||||
| Occurrence (0/1) | 27% a | 22% a | 28% b | 41% c | 40% c | 32% b |
| Mean Severity (0–3) | 0.44 (0.09) a | 0.29 (0.08) a | 0.43 (0.02) a | 0.75 (0.09) b | 0.68 (0.07) b | 0.50 (0.09) a |
| Observed Severity (1–3) | 1.61 (0.15) a | 1.35 (0.16) a | 1.56 (0.03) a | 1.81 (0.12) a | 1.69 (0.09) a | 1.54 (0.15) a |
| Irritability | ||||||
| Occurrence (0/1) | 54% a | 42% a | 48% a | 51% a | 52% a | 47% a |
| Mean Severity (0–3) | 0.86 (0.09) a | 0.64 (0.09) a | 0.71 (0.02) a | 0.75 (0.09) a | 0.84 (0.07) a | 0.65 (0.10) a |
| Observed Severity (1–3) | 1.59 (0.10) a | 1.51 (0.10) a | 1.48 (0.02) a | 1.48 (0.02) a | 1.61 (0.07) a | 1.37 (0.11) a |
| Frontal Domain (0–3) | 0.36 a (0.06) | 0.28 b (0.05) | 0.25 b (0.01) | 0.30 b (0.05) | 0.24 b (0.04) | 0.11 c (0.03) |
| Disinhibition | ||||||
| Occurrence (0/1) | 31% a | 32% a | 28% a | 34% a | 26% a | 18% a |
| Mean Severity (0–3) | 0.61 (0.09) a | 0.50 (0.08) a | 0.43 (0.02) a | 0.55 (0.08) a | 0.44 (0.06) a | 0.26 (0.09) a |
| Observed Severity (1–3) | 2.00 (0.14) a | 1.59 (0.13) a | 1.52 (0.03) a | 1.60 (0.13) a | 1.72 (0.11) a | 1.46 (0.19) a |
| Euphoria | ||||||
| Occurrence (0/1) | 5% a | 9% a | 5% a | 7% a | 3% a | 0% a |
| Mean Severity (0–3) | 0.11 (0.04) a | 0.14 (0.03) a | 0.07 (0.01) a | 0.11 (0.04) a | 0.05 (0.03) a | 0.00 (0.00) a |
| Observed Severity (1–3) | 2.25 (0.28) a | 1.63 (0.20) a | 1.29 (0.05) a | 1.67 (0.23) a | 1.40 (0.25) a | 0.00 (0.00) a |
| Correlation Mood & Psychosis† | 0.32 | 0.80 | 0.62 | 0.80 | 0.80 | 0.32 |
| Correlation Mood & Frontal† | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 |
| Correlation Psychosis & Frontal† | 0.52 | 0.52 | 0.52 | 0.52 | 0.52 | 0.52 |
Note. Entries with the same superscript letter (a, b, c) are not significantly different from each other. Latent means derived using effects coding that yields interpretable mean estimates in the same scale as observed indicators.34
Correlations estimated with phantom variable technique which standardizes covariance coefficients yielding interpretable correlations.35
Step 1
We examined domain content, factor loadings, and factor interrelationships of the three factor model originally proposed by Frisoni and colleagues (Figure 1).17 The best fitting model was both similar to and different from the Frisoni model. Like Frisoni, we found that the aberrant motor activity item loaded highly on all three factors (betas ranged from 0.34 to 0.51) and resulted increased model misfit (Δ RMSEA = +.01). Thus, it was excluded from the baseline model. Unlike Frisoni, we found that the apathy item fit well in the baseline, thus it was included. Finally, delusions and hallucinations items covaried significantly (Δ RMSEA < −.02).
Step 2
Multiple-group TOI’s were used to compare the different types of dementia (Table 3). The tests of weak invariance (equivalent factor loadings) and strong invariance (equivalent factor loadings and item intercepts) indicated that the groups were statistically equivalent at these levels (neither Δ RMSEA > −.02).
Table 3.
Tests of Invariance Across Groups
| Model | x2 | df | RMSEA | RMSEA 90% CI |
Constraint Tenable? |
|---|---|---|---|---|---|
| Weak Invariance (Factor Loadings)† | 565.5 | 204 | 0.060 | 0.054–0.066 | Yes |
| Strong Invariance (Item Intercepts)† | 700.7 | 234 | 0.064 | 0.058–0.069 | Yes |
| Latent Mean Invariance† | 779.5 | 249 | 0.066 | 0.061–0.071 | No |
| Latent Mean Invariance Optimized†† | 652.2 | 240 | 0.059 | 0.054–0.065 | Yes |
Change in RMSEA is used to evaluate psychometric properties of the model (weak and strong invariance). Change in X2/df is used to evaluate between group differences (latent mean invariance).26
The optimized model equates all between-group factor means and correlations that are statistically warranted. Figure 1
Step 3
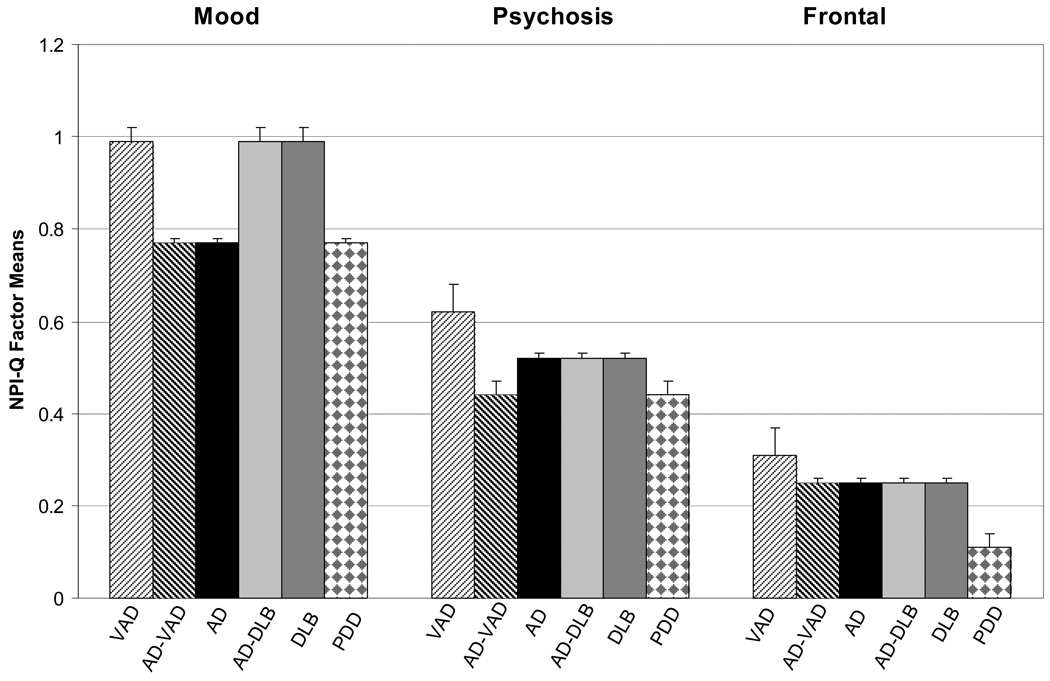
The test for equal latent means failed (Δχ2(15) = 79.0, p<.001) indicating that although the groups shared the same model configuration (3 latent domains made up by Mood, Psychosis, and Frontal symptom clusters), neuropsychiatric profiles varied by dementia type (Table 2). Compared to the other four patient groups, AD participants scored in the moderate range across the symptom domains (Mood = .78, Psychosis = .52, and Frontal = .25). Patients with VAD reported the highest symptom levels (Mood = .90, Psychosis = .60, and Frontal = .36) and PDD reported the lowest (Mood = .67, Psychosis = .46, and Frontal = .11).
Step 4
In the final optimized model (Figure 2), diagnoses clustered in two levels on the Mood symptom domain (VAD = AD/DLB = DLB > AD =AD/VAD = PDD), in three levels on the Psychosis symptom domain (VAD > AD = AD/DLB = DLB > AD/VAD = PDD), and in three levels on the Frontal symptom domain (VAD > AD/VAD = AD = AD/DLB = DLB > PDD). Notably DLB and AD/DLB had identical patterns of factor means across all domains. AD/VAD had an identical pattern to AD on Mood and Frontal symptoms, but AD/VAD was lower than AD on Psychosis. Dementia types also shared similar correlation structures (Table 2) between Mood and Frontal symptoms (r = .38) and between Psychotic and Frontal symptoms (r = .52). Thus the groups shared similar relationships among the least frequently occurring neuropsychiatric symptoms. The dementia types did differ significantly in how Mood and Psychotic symptoms correlated. In AD they correlated in the moderate range (r = .62) and could not be not equated to any of the other diagnosis groups. In the two mixed diagnoses and in DLB correlations were higher (r = .80). Finally, VAD and PDD shared similar low correlations (r = .32).
Figure 2.
Clinical Profiles by Dementia Type: Optimized Factor Means on NPI-Q
Discussion
Using a large sample of mild to moderate staged people with dementia from the NACC database, this study compared the neuropsychiatric profiles of 4 common dementia types (AD, VAD, DLB, PDD) and 2 mixed dementias (AD/VAD and AD/DLB). We found that dementia types shared many common symptoms and could be compared to one another along three psychiatric symptom domains (Mood, Psychosis, and Frontal). The group profiles differed in the severity and inter-relatedness of the symptom domains. Participants with AD consistently reported moderate symptom severities, VAD the highest, and PDD the lowest (probably due to their younger age and relative milder stage of disease). DLB and the mixed dementias reported more complex symptom profiles. Given that these DLB patients probably have concomitant AD-type changes it appears the multiple co-occurring neuropathologies are interactive in some way, resulting in more extensive psychiatric symptom complexes.
Across dementia types, caregivers reported that the person with dementia experienced psychiatric symptoms like Anxiety, Dysphoria, and Apathy (items that make up the Mood symptom domain) more frequently than symptoms like Irritability, Delusions, Hallucinations, and Agitation (Psychosis symptom domain). Note that visual hallucinations are part of the diagnostic criteria for DLB and were expected to be higher in this group. Euphoria and Disinhibition (Frontal symptom domain) were the least likely to be endorsed.
These data indicate a consistent neuropsychiatric symptom profile (Mood > Psychosis > Frontal) across dementia types wherein depressed mood is a prominent feature in all.1–2, 7–9, 11, 13 Although each disease is the result of different underlying pathologies (or blends of pathologic processes), they all reflect the presence of major neurologic disease resulting in extensive cell death and brain atrophy. The trauma associated with these processes appears to yield a depressive tone similar to what is witnessed in post-ictal stroke patients and other brain trauma.27 This is likely why the VAD and AD/VAD blend report such high rates of mood symptoms.
Lower levels of endorsement of Psychosis and Frontal symptom domains is likely due to the mild and moderate stages of dementia patients tested. In this sample, people in the later stages of dementia reported more of the symptoms that make up the Frontal and Psychosis domains; increasing dementia severity (CDR) was related to more severe Frontal symptoms (AD) and more Psychosis symptoms in AD, DLB, AD/DLB, and AD/VAD. Dementia status (CDR) did not correlate with symptom severity in PDD and VAD. The PDD group did not have as many later stage individuals, were slightly younger, and did not suffer as many neuropsychiatric symptoms. This may have attenuated the correlations of disease severity with neuropsychiatric symptoms in the PDD group. There were adequate numbers of later stage VAD participants and this group endorsed the greatest symptom severity. Thus, dementia severity (CDR) had less impact on symptom presentation in VAD.
Profiles of the mixed dementias indicated that when AD is concomitant with VAD, the AD-like psychiatric features tend to dominate but that when AD is concomitant with DLB, DLB-like psychiatric features tend to dominate. Across the 3 domains measured, AD/VAD participants reported less severe symptoms than VAD alone and this was similar to AD participant reports. The profile differences between VAD and AD/VAD may account for mixed reports of symptoms in the literature, some reporting more severe depression & anxiety,9, 28 others reporting equivalent symptoms in AD and VAD.29 Both sets of observations may both be true depending on the makeup of the patient group studied. DLB and AD/DLB had identical patterns of factor means, most starkly characterized by high levels of mood symptoms. The presence of simultaneous AD and DLB pathology may represent an additive effect where the two diseases together result in more significant manifestation of mood symptoms.30 This is consistent with research reports that patients with DLB often experience apathy and depression.11–12
NACC participants are recruited and enrolled in a variety of ways across participating ADCs, thus this large dataset likely suffers from diagnostic inconsistencies. Specifically, NACC is a clinic-based convenience sample and its generalizability may be limited. This clinic-based sample likely overestimates true prevalence of AD in the geriatric population. Participating ADCs focus on AD diagnosis and recruitment, thus biasing the sample toward over inclusion of AD variants. For example, the PDD participants reported mild psychiatric symptoms given their dementia status. These participants differ from previous reports about the NPI-Q used in PDD.13 Differences in when and how these participants present for evaluation may explain why this PDD group was unexpectedly low in reporting psychiatric symptoms. As a result, these PDD data may not generalize well to movement disorder clinics. Others have noted that caregivers’ appreciation of psychiatric symptoms may outstrip their ability to judge accurately symptom presence and severity, resulting in relatively higher levels of variability witnessed in these types of data.31 In spite of these modest limitations the results of this study show that the NPI-Q can be used effectively to assess many types of dementia, their neuropsychiatric symptom profiles have unique features, and these results are consistent with several other studies.2, 11, 13, 32–33
Although AD was over-represented in these data because it is the most prevalent dementia type worldwide and the primary target of NACC participating ADC’s, this pattern of results is not dependent on the large AD sample size. When analyses were repeated with a randomly selected AD subsample (N = 300) and tested using bootstrapped confidence intervals, results were identical. Further, the other dementias reported here are of great clinical value because of their relative rarity and that they were tested with a standardized battery. Due to the large sample size of the NACC database, the present study offers unique advantages because it aggregates relatively understudied dementias. Thus, we could demonstrate that the Frisoni and colleagues’ 3-factor model of the NPI-Q can be applied in a wide range of geriatric clinical settings to investigate patterns of neuropsychiatric symptom severity across many dementia types.
ACKNOWLEDGEMENTS
The study was supported by U01 AG016976, NS058252, AG029615, AG09009 and AG033673 from the National Institute on Aging, Bethesda, MD.
The authors thank all NACC participants, the NACC staff for help in procuring data, and the individual ADCs that contributed to this database.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strauss ME, Pasupathi M. Primary caregivers' descriptions of Alzheimer patients' personality traits: temporal stability and sensitivity to change. Alzheimer Dis Assoc Disord. 1994 Fall;8(3):166–176. doi: 10.1097/00002093-199408030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JL, Victoroff JI. Noncognitive neuropsychiatric syndromes in Alzheimer's disease. Neuropsychiatry Neuropsychology and Behavioral Neurology. 1990;3:140–158. [Google Scholar]
- 3.Balsis S, Carpenter BD, Storandt M. Personality change precedes clinical diagnosis of dementia of the Alzheimer type. J Gerontol B Psychol Sci Soc Sci. 2005 Mar;60(2):P98–P101. doi: 10.1093/geronb/60.2.p98. [DOI] [PubMed] [Google Scholar]
- 4.Aalten P, de Vugt ME, Jaspers N, Jolles J, Verhey FR. The course of neuropsychiatric symptoms in dementia. Part I: findings from the two-year longitudinal Maasbed study. Int J Geriatr Psychiatry. 2005 Jun;20(6):523–530. doi: 10.1002/gps.1316. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg M, Tschanz JT, Corcoran C, et al. The persistence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2004 Jan;19(1):19–26. doi: 10.1002/gps.1025. [DOI] [PubMed] [Google Scholar]
- 6.Tariot PN, Mack JL, Patterson MB, et al. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer' Disease. The Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer's Disease. Am J Psychiatry. 1995 Sep;152(9):1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008 Feb;23(2):170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srikanth S, Nagaraja AV, Ratnavalli E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison on Alzheimer disease, vascular dementia and frontotemporal dementia. Journal of the Neurological Sciences. 2005;236:43–48. doi: 10.1016/j.jns.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Fuh JL, Wang SJ, Cummings JL. Neuropsychiatric profiles in patients with Alzheimer’s disease and vascular dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:1337–1341. doi: 10.1136/jnnp.2004.056408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64:1397–1403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 11.McKeith I, Mintzer J, Aarsland D, et al. Dementia with Lewy bodies. The Lancet Neurology. 2004;3(1):19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- 12.Galvin JE, Malcom H, Johnson D, Morris JC. Personality traits distinguishing dementia with Lewy bodies from Alzheimer disease. Neurology. 2007 May 29;68(22):1895–1901. doi: 10.1212/01.wnl.0000263131.80945.ad. [DOI] [PubMed] [Google Scholar]
- 13.Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with Parkinson' disease. Journal of Neurology Neurosurgery and Psychiatry. 1999;67(4):492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aalten P, de Vugt ME, Lousberg R, et al. Behavioral Problems in Dementia: A Factor Analysis of the Neuropsychiatric Inventory. Dementia and Geriatric Cognitive Disorders. 2003;15(2):99–105. doi: 10.1159/000067972. [DOI] [PubMed] [Google Scholar]
- 15.Cummings JL, Mega M, Gray K. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 16.Beekly DL, Pfeiffer EM, van Belle G, et al. The National Alzheimer's Coordinating Center (NACC) Database: An Alzheimer's Disease Database. Alz.Dis.Assoc.Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 17.Frisoni B, Rozzini L, Gozetti A, et al. Behavioral syndromes in Alzheimer's disease: description and correlates. Dementia and Geriatric Cognitive Disorders. 1999;10(2):130–138. doi: 10.1159/000017113. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier S, Wirth Y, Mobius HJ. Effects of memantine on behavioural symptoms in Alzheimer's disease patients: an analysis of the Neuropsychiatric Inventory (NPI) data of two randomised, controlled studies. Int J Geriatr Psychiatry. 2005 May;20(5):459–464. doi: 10.1002/gps.1341. [DOI] [PubMed] [Google Scholar]
- 19.Lyketsos CG, Sheppard JM, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer's disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry. 2001 Nov;16(11):1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular Dementia - Diagnostic-Criteria for Research Studies - Report of the Ninds-Airen International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 22.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies; third report of the DLB consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 23.Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee Report. SIC Task Force Appraisal of Clinical Diagnostic Criteria for Parkinsonian Disorders. Movement Disorders. 2003;18:467–486. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 24.Joreskog KG, Sorbom D, editors. LISREL 8: User's Reference Guide. Chicago: Scientific Software International, Incorporated; 1998. [Google Scholar]
- 25.Kaplan D. Structural Equation Modeling: Foundations and Extensions. Thousand Oaks, CA: Sage; 2000. [Google Scholar]
- 26.Cheung G, Rensvold R. Evaluating Goodness-of-Fit Indexes for Testing Measurement Invariance. Structural equation modeling. 2002;9(2):233–255. [Google Scholar]
- 27.Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004 Jan;61(1):42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- 28.Sulzer DL, Levin HS, Mahler ME, High WM, Cummings JL. A comparison of psychiatric symptoms in vascular dementia and Alzheimer's disease. American Journal of Psychiatry. 1993;150:1806–1812. doi: 10.1176/ajp.150.12.1806. [DOI] [PubMed] [Google Scholar]
- 29.Verhey FRJ, Ponds RWHM, Rozendaal N, Jolles J. Depression, insight, and personality changes in Alzheimer's disease and vascular dementia. Journal of Geriatric Psychiatry and Neurology. 1995;8:23–27. [PubMed] [Google Scholar]
- 30.Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65:1232–1238. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- 31.de Medeiros K, Robert P, Gauthier S, et al. The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. International Psychogeriatrics. 2010;22(Special Issue 06):984–994. doi: 10.1017/S1041610210000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. Journal of the American Medical Association. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 33.Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 34.Little T, Slegers DW, Card NA. A non-arbitrary method of identifying and scaling latent variables in SEM and MACS models. Structural Equation Modeling. 2006;13(1):59–72. [Google Scholar]
- 35.Rindskopf D. Using phantom and imaginary latent variables to parameterize constraints in linear structural models. Psychometrika. 1984;49:37–47. [Google Scholar]