Abstract
We adapted a microfluidic system used previously to generate durotactic gradients of stiffness in a 3D collagen gel, to produce haptotactic gradients of adhesive ligands through the collagen gel. Oligopeptide sequences that included bioactive peptide sequences from laminin, YIGSR, or IKVAV, were grafted separately onto type I collagen using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC). Solutions of peptide-grafted collagen and untreated collagen were then used as source and sink input solutions, respectively, in an H-shaped microfluidic network fabricated using traditional soft lithography. One-dimensional gradients of the peptide-grafted collagen solution were generated in the channel that connected the source and sink channels, and these gradients became immobilized upon self-assembly of the collagen into a 3D fibrillar gel. The slope and average concentration of the gradients were adjusted by changing the concentration of the source solutions and by changing the length of the cross-channel. A separate, underlying channel in the microfluidic construct allowed the introduction of a chick embryo dorsal root ganglion into the network. Neurites from these explants grew significantly longer up steep gradients of YIGSR, but shallow gradients of IKVAV in comparison to untreated collagen controls. When these two gradients were presented in combination, the bias in growth acceleration was the largest and most consistent. No differences were observed in the number of neurites choosing to grow up or down the gradients in any condition. These results suggest that the incorporation of distinct gradients of multiple bioactive ligands can improve directional acceleration of regenerating axons.
Key words: collagen, haptotaxis, laminin, MEMS, neural tissue engineering
Introduction
Asignificant hurdle preventing recovery from peripheral nerve or spinal cord injury is the directed regeneration of axons across an injury site (Schmidt and Leach, 2003). However, the acute and chronic tissue environment following spinal cord injury is extremely inhibitory towards axon regeneration, and though the environment following peripheral nerve injury is more conducive, regeneration without intervention occurs only for mild injuries. In peripheral tissues, the tissue proximal and distal to the injury are routinely reconnected with an autologous or bioengineered graft to provide a conduit for regenerating axons, but these techniques often fail as the gap to bridge increases. As such, significant efforts have been made to improve the environment within the graft to enhance and potentially direct axon regeneration (Schmidt and Leach, 2003). Most often, these have entailed adding factors throughout the graft that improve the growth and migration of axons and supporting cells, and introducing an internal microstructure that provides a guidance field to orient regeneration.
During development of the nervous system, growth cones are directed by a temporally-orchestrated spatial profile of gradients of soluble and insoluble molecular cues, most of which are not maintained post-development. Essentially, these gradients can produce an environment that both enhances growth and orients the growth in specific directions via attractive and/or repulsive cues. Although chemotactic gradients of diffusible soluble factors are readily produced in vitro (Guthrie and Pini, 1995; Tessier-Lavigne and Placzek, 1991), generating and maintaining these gradients in vivo is not as simple and requires well-designed and well-placed drug delivery systems (Dodla and Bellamkonda, 2008). Conversely, immobilized haptotactic gradients of adhesion molecules can be incorporated and maintained as part of an implanted biomaterial (Yu et al., 2007).
When designing biomaterials that modulate adhesion it is important to consider that the biophysical response of cells to adhesion molecules is complex and can contribute to enhanced or retarded growth or migration. The response is strongly correlated to the specific receptor and ligand (for instance, a particular integrin receptor and its partner bioactive peptide sequence), as well as the density of both the receptors and the adhesion molecules. In general, cell migration depends biphasically on the strength of cell adhesion (Palecek et al., 1997), with the highest migration speeds being at intermediate receptor/ligand concentrations. If receptors are weakly expressed and/or ligand concentration is too low, few adhesions can be made between the cell and the matrix, making cells unable to attach and exert the necessary traction to locomote. If receptors are highly expressed and/or ligand concentration is too high, adhesion strength is too great, and the cell-substrate interactions are essentially too adhesive to allow migration. Additionally, the biochemical signaling induced by ligand-adhesion receptor binding contributes by regulating expression of factors important in migration such as cytoskeletal proteins, cell adhesion receptors, extracellular matrix, and matrix metalloproteinases, and dictates the profile of the bimodal response curve with respect to ligand and receptor concentrations (Borrirukwanit et al., 2007; Lamar et al., 2008; Sameni et al., 2008; Vicente-Manzanares et al., 2009). In the context of axon regeneration, adhesion strength is important because the formation of stable adhesive contacts from the growth cone to the substrate is essential for growth cone steering and subsequent stimulation of neurite growth (Schmidt and Leach, 2003).
A plethora of extracellular matrix (ECM), cell adhesion molecules, immobilized growth factors, and bioactive fragments or specific peptide sequences have proven to stimulate neurite attachment and growth. In particular, laminin and its bioactive sequences YIGSR and IKVAV have been well studied, primarily because of the well documented role of laminin in nervous system growth and development (Chernousov et al., 2008; Luckenbill-Edds, 1997; Tucker and Mearow, 2008; Yip and Yip, 1992). Several studies have shown increased neurite outgrowth with YIGSR and IKVAV in 2D (Adams et al., 2005; Heller et al., 2005; Saneinejad and Shoichet, 1998), and some isotropic in vivo-like 3D conditions. YIGSR-grafted isotropic 3D gels have been studied with alginate, chitosan, and collagen (Dhoot et al., 2004; Duan et al., 2007; Itoh et al., 2003; Yu et al., 1999). In all cases, YIGSR grafting increased neurite outgrowth over controls, and in one case over laminin controls (Dhoot et al., 2004). However, simply increasing neurite outgrowth may not be sufficient for repair after SCI, since neurons need to be guided to their targets; therefore, directed growth of neurons is an important area of study.
Photo-immobilized gradients of nerve growth factor (NGF) within poly(2-hydroxyethylmethacylate) [p(HEMA)] gels have been used to guide neurite outgrowth from PC12 cells (Kapur and Shoichet, 2004). Pioneering experiments in Letourneau's laboratory demonstrated the importance of laminin and its peptides in growth cone turning and outgrowth (Adams et al., 2005; Condic and Letourneau, 1997; Gomez and Letourneau, 1994; Hammarback et al., 1985). In 2D, it has been shown that gradients of IKVAV direct neurite outgrowth in chick dorsal root ganglia (DRG) neurons (Adams et al., 2005). 3D gradients of laminin have been studied in vitro, and produced a distinct response with respect to the slope of the gradient. Neurites from chick DRGs were better directed and grew faster up a shallow gradient of laminin compared to a steep gradient. However, due to experimental limitations, a difference in overall growth was not demonstrated in these gradient experiments (Dodla and Bellamkonda, 2006). Recently, Bellamkonda's group has also studied 3D gradients of laminin and NGF in an in vivo model (Dodla and Bellamkonda, 2008). Regeneration only occurred in experimental conditions where there was both a gradient of NGF and laminin. If either component or both was presented uniformly, they did not obtain regeneration in a sciatic nerve regeneration model. In all cases, this regeneration was lower than in the nerve graft control. Collectively, these studies provide strong evidence for the importance of gradients in directing and enhancing neurite outgrowth.
We have developed a simple microfluidic-based system to evaluate the response of growing neurites to gradients of hydrogel scaffold properties. We previously used the system to generate durotactic gradients of mechanical stiffness through a 3D collagen gel (Sundararaghavan et al., 2009). The gradients were generated by controlling exposure of the fibrillar collagen network to cell-tolerated concentrations of a cross-linking agent. Herein, we modified the approach to create gradients of bioactive peptide fragments immobilized within a 3D type I collagen hydrogel. We generated gradients of immobilized YIGSR- and IKVAV-containing peptide fragments, and studied the functionality of these gradients using chick DRGs.
Methods
Collagen preparation
Type I collagen solutions were prepared as previously described (Shreiber et al., 2001), by mixing 20 μL 1 M HEPES buffer, 140 μL 0.1 N NaOH, 100 μL 10×MEM, 52 μL of M199 (Invitrogen, Carlsbad, CA), and 677 μL of a 3.0 mg/mL type I collagen solution (Elastin Products, Owensville, MO), to make a 2.0 mg/mL collagen solution. The collagen solution was kept at 4°C until use, and self-assembled into a fibrillar gel upon incubation at 37°C.
Conjugation of peptides to collagen backbone
A 17-mer peptide sequence, CRARKQASIKVAVSADR, and a 9-mer peptide sequence, CDPGYIGSR, were custom synthesized (Genscript, Piscataway, NJ), and conjugated to the backbone of collagen in suspension. These sequences were used previously for in vitro studies of bioactivity of laminin peptides (Ranieri et al., 1995). A hetero-bifunctional coupling agent, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), was used to activate the carboxylic group of the peptide by mixing 1 mL of a 1 mM solution of EDC in MES buffer (pH 2–4) with 1 mg of peptide for 10 min at 37°C, as previously described (Monteiro et al., 2009). The peptide-EDC mixture was added to 5 mL of a 3 mg/mL suspension of type I collagen (Elastin Products) in 0.02 N acetic acid. The activated peptide covalently binds to free amines on residues on the collagen backbone via nucleophilic attack. A low-pH buffer was used while coupling peptides to avoid self-assembly of collagen fibers. Peptide-EDC-collagen mixtures were incubated on a shaker overnight at 4°C, and then dialyzed against 0.02 N acetic acid for 12 h to remove any unconjugated peptide. Dialyzed peptide-grafted collagen was lyophilized at −150°C and 50 mTorr for 12 h to remove all water. Lyophilized product was re-suspended in 0.02 N acetic acid to make a 3 mg/mL solution of grafted collagen.
Grafting efficiency was evaluated by using a FITC-tagged peptide (Monteiro et al., 2009). FITC-tagged peptides were conjugated to the collagen backbone using the same method described above. Efficiency was determined with a standard curve made by admixing FITC-tagged peptide to collagen solution and comparing the fluorescence of the final product to the standard curve. The typical grafting efficiency was 55–65%, or a solution ∼37 μg peptide/mg collagen, which yields ∼74 μg peptide/mL collagen when beginning with 1 mg peptide in the 2 mg/mL collagen solutions used.
Microfluidic networks
Device fabrication
A simple, H-shaped source-sink arrangement was used to generate gradients in a cross-channel connecting source to sink. Source and sink channels were 500 μm wide×100 μm deep and were connected by a 150-μm wide and 100-μm deep cross-channel. The length of the cross-channel was either 3 mm or 5 mm, which allowed the slope of the gradient to be changed independent of the source or sink conditions. Microfluidic networks were fabricated using standard photolithography techniques (Whitesides et al., 2001) at Bell Labs/Lucent Technologies (Murray Hill, NJ). The design was drawn with AutoCAD, and a photomask was professionally printed (Cad-Art Services, Poway, CA). A silicon wafer was spin-coated with SU-8 negative photoresist (Microchem, Newton, MA) and baked for 5 min at 65°C, followed by 10 min at 100°C. The photoresist was exposed to UV light through the photomask using a Quintel 2001 CT Mask Alignment/Exposure system (Morgan Hill, CA). The coated wafer was baked again and immersed in SU-8 developer for 12 min to clear un-reacted photoresist and form the final master. A poly(dimethyl siloxane) solution (PDMS; Dow Corning, Midland, MI) was poured over the master and baked overnight at 50°C to produce a negative relief. The PDMS was removed, the design was cut out of the mold, and holes were punched for the inlet and outlet using a blunt 19-gauge syringe.
Device inoculation and assembly
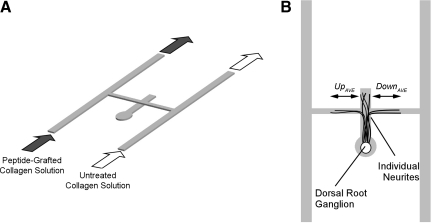
To assay neurite response to haptotactic gradients, the source-sink network was modified to allow introduction of a DRG into the system, as shown in Figure 1. A second, separate network was generated that comprised a 1-mm-diameter well connected to a straight 500-μm wide and 100-μm deep channel. This network was placed upside down (channel facing up) on a glass slide and filled with collagen solution. DRGs were isolated from E8 chick embryos (Charles River Laboratories, Wilmington, MA), and a single DRG was placed in the collagen-filled circular well. The network was transferred to a 37°C incubator to facilitate self-assembly, entrapping the DRG in the collagen gel. The source-sink network was then plasma treated and bonded to the gel-filled underlying network such that the middle of the cross-channel of the source-sink intersected with the straight channel of the underlying network approximately 1500 μm from the circular well containing the DRG.
FIG. 1.
Schematics of haptotaxis assay for neurite growth. (A) Isoparametric view of the microfluidic approach used to generate gradients. An H-shaped network is placed on an underlying network comprising a small well connected to a straight channel. The underlying network is pre-filled with collagen and a dorsal root ganglion explant is placed in the well. Peptide-grafted collagen solution is pumped into the source channel and untreated collagen solution into the sink channel to generate a gradient in the cross-channel. This gradient is immobilized upon self-assembly of the collagen into a fibrillar network. (B) Top view depicting neurite growth in the assay. A sub-population of neurites enter the cross-channel. Neurite growth is quantified by counting the number and the average length of processes that grow up or down the gradient. Modified with permission from figure originally published in Sundararaghavan et al., 2009.
Haptotactic gradient generation
A gradient through the cross-channel was formed by pumping two different collagen solutions in the inlet channels. The inlet solutions used in the various experimental conditions are summarized in Table 1. Grafted peptide gradients were generated using the 37 mg peptide/mg collagen at full strength (100%) or half strength (50%) as the source condition, and untreated collagen (0%) as the sink. Controls were performed with 100% strength as both the source and sink conditions where peptide-grafted collagen was uniformly presented, and with untreated collagen (0% strength) as the source and sink where no peptide-grafted collagen was presented. These conditions were assayed in networks with a 5-mm cross-channel. The gradient conditions and no-peptide controls were assayed in networks with a 3-mm cross-channel. Based on results from these experiments, a final condition was assayed, where a combination of 100% YIGSR and 50% IKVAV was used as the source, and 0% as the sink in a 5-mm network. For this assay, YIGSR-grafted collagen was prepared with 2× the initial peptide concentration, which was then diluted 1:1 with IKVAV-grafted collagen.
Table 1.
Table of All Conditions Tested for Adhesion Experiments
| |
IKVAV/YIGSR |
|||
|---|---|---|---|---|
| Source | Sink | Gradient concentration (μg peptide/mL collagen/mm) | Average concentration (μg peptide/mg collagen) | |
| 3 mm | 100% | 0% | 24.7 | 18.5 |
| 50% | 0% | 12.4 | 9.25 | |
| 0% | 0% | Uniform | 0 | |
| 5 mm | 100% | 0% | 14.9 | 18.5 |
| 50% | 0% | 7.44 | 9.25 | |
| 100% | 100% | Uniform | 37 | |
| 0% | 0% | Uniform | 0 | |
| 100% YIGSR, 50% IKVAV | 0% | 14.9 (YIGSR), 7.44 (IKVAV) | 18.5 (YIGSR), 9.25 (IKVAV) | |
Gradient generation was visualized by spiking either the peptide-grafted collagen or the native collagen (selected at random) with a small amount (<1%) of FITC-tagged collagen solution (Fig. 2). After visual confirmation of the gradient, the networks were transferred to a humidified 37°C 5% CO2 incubator to allow self-assembly and immobilization of the gradient.
FIG. 2.
Representative gradient formed by mixing collagen solutions in the microfluidic network and allowing self-assembly. A solution of FITC-grafted collagen was used as the source solution and untreated collagen as the sink solution, which produced a gradient of fluorescence in the cross-channel. The gradient profile can be changed by altering the concentration of the source and/or sink solutions, and by changing the length of the cross-channel.
Networks were then perfused with fresh medium (DMEM supplemented with 10% FBS and 100 ng/mL NGF; R&D Systems, Minneapolis, MN) through both inlets via gravity flow. For the gradients formed in 5-mm-long cross-channels, DRGs were cultured in the networks for 5 days to allow neurites to grow through the collagen gel and extend up and into the cross-channel a significant distance, potentially in either direction. For gradients formed in the 3-mm cross-channel, neurites grew for 4 days instead of 5 days to prevent neurites from entering the source/sink channel.
Immunohistochemistry and imaging
To visualize neurite growth, after the culture period, neurites were stained immunohistochemically in the networks for neurofilament proteins. Inlet solutions were changed to 4% paraformaldehyde for 3 h to fix the collagen and cells, then changed to a rinse buffer comprising 1% BSA+0.5% Triton in PBS for 3 h. Inlet solutions were changed to a 10% goat serum blocking solution in rinse buffer for 4 h, and then to an anti-neurofilament antibody cocktail of 1:200 α-NF 200 and 1:1000 α-NF 68 (Sigma-Aldrich, St. Louis, MO) overnight. The networks were rinsed for 4 h, and inlets were switched to a 1:400 dilution of goat anti-mouse Alexa 568 secondary antibody (Molecular Probes/Invitrogen, Eugene, OR) and incubated overnight. The devices were rinsed a final time for 4 h and then transferred to an inverted epifluorescence microscope for imaging.
Outgrowth metrics
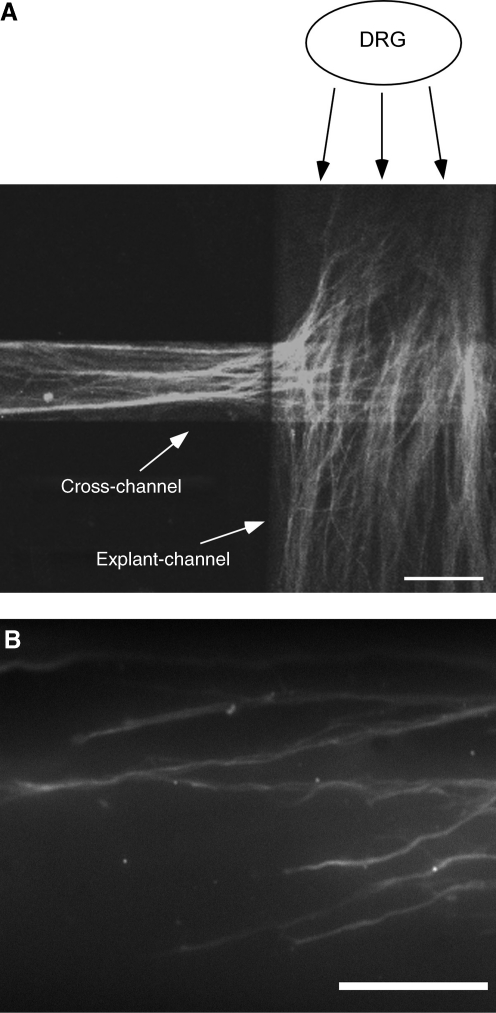
Neurite growth was quantified as the number and length of neurite segments projecting up the adhesion gradient versus down the adhesion gradient, or in opposite directions for control experiments without peptide-grafted collagen, or with uniform presentation of peptide-grafted collagen, where no gradient was present. For each device, digital images were taken with a 40× objective at the intersection of the explant channel and cross-channel, and at the end of the individual growth cones while recording the motorized stage position (Fig. 3). Using Olympus Microsuite Image Analysis Software, the (x,y) coordinates were recorded for each growth cone and for the channel intersection to determine the distance of growth in the gradient channel. For each experiment, at least one experimental treatment (an adhesion gradient or full adhesion condition), and one control with untreated collagen was performed with DRG explants from the same chick embryo.
FIG. 3.
Images of neurite growth in the collagen gel-filled network. A dorsal root ganglion (DRG) was placed within a collagen gel in the underlying explant channel and cultured for 4–5 days, at which time the networks were perfused with paraformaldehyde and then stained immunohistochemically for neurofilament proteins. (A) As shown in this confocal micrograph, neurites grew from the DRG and either continued in the explant channel or grew up and into the cross-channel of the overlying H-shaped network. (B) To measure the length of neurites in either direction, the growing end of neurites was identified from epifluorescence images taken with an inverted microscope with a 40× objective, and the length back to the intersection of the explant and cross-channels was determined based on the stage position and location within the image (scale bars: A=150 μm and B=75 μm). Reproduced with permission from Sundararaghavan et al., 2009.
Results
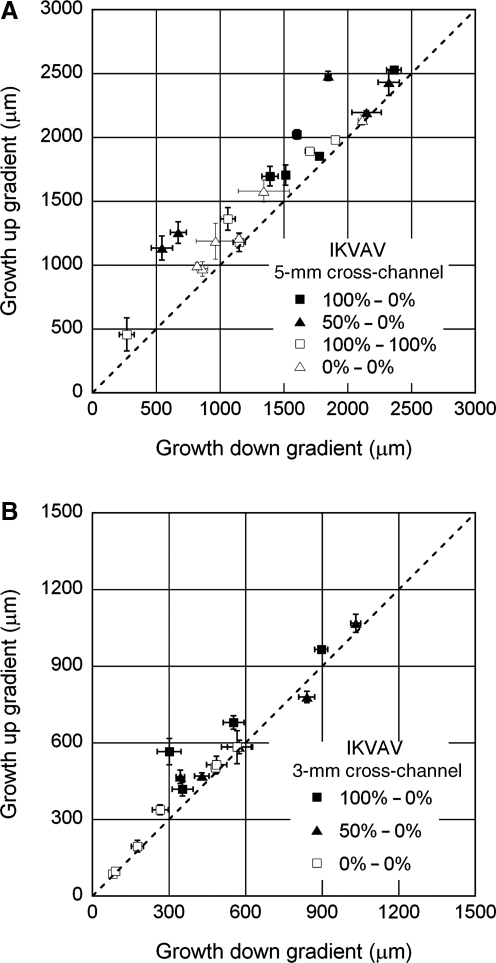
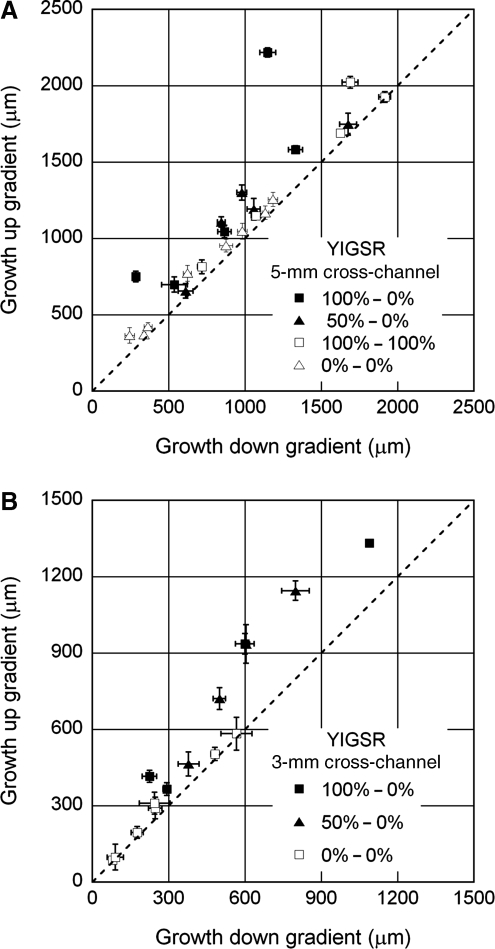
In all conditions, though most of the neurites remained in the underlying culture channel, many (∼15–40) neurites extending from the DRG entered the overlying cross-channel and experienced the adhesion gradient or appropriate control condition (Fig. 3). The length and number of these neurites projecting up and down the adhesion gradient (or in opposite directions for control cases) were quantified and compared. Average neurite segment lengths within the gradient channel were plotted per device for a given condition on the same set of axes, where growth up the gradient was plotted on the ordinate and down the gradient on the abscissa. For control conditions where no gradient was present, the longer growth was plotted on the ordinate. For completely uniform growth, the average lengths should be equal in the two directions, and the data should fall along a line with a slope of one. Points demonstrating biased growth up the gradient fall above this line. The data are presented in Figure 4 (IKVAV), Figure 5 (YIGSR), and Figure 6 (IKVAV+YIGSR). Although never completely equal, growth in conditions where uniform collagen was presented (either untreated or uniformly grafted) was clustered near the line that indicated perfectly symmetric growth. For IKVAV experiments, only the gradients formed with 50% grafted solution over the 5-mm distance appeared to consistently bias neurite growth up the gradient. This represents the shallowest gradient formed. Experiments from the remainder of the IKVAV gradient conditions (100–0% over 5 mm, 100–0% over 3 mm, and 50–0% over 3 mm) demonstrated growth with similar divergence from uniform growth as the control conditions. Conversely, for YIGSR gradient experiments, steeper gradients appeared to boost neurite bias, and the shallow 50–0% gradient formed over 5 mm produced the only growth that was overtly uniform. To compare growth anisotropy across all conditions, the average relative growth up the gradient versus down the gradient was calculated for each condition. After the omnibus analysis of variance (ANOVA) proved significant (p<0.0001), this growth ratio for each condition was compared to controls with no grafted collagen (0–0%) using Fisher's Least Significant Difference (LSD) Test. As shown in Table 2, steep gradients of YIGSR produced significantly different growth ratios, whereas only the shallowest gradient of IKVAV produced significant differences.
FIG. 4.
Neurite growth anisotropy in experiments with IKVAV-grafted collagen with a 5-mm cross-channel (A), and a 3-mm cross-channel (B). Each point represents the average length of neurites extending up the gradient and down the gradient from a single network±standard error of the mean (SEM). For conditions where no gradient was present, the direction with longer average growth was plotted as the ordinate value. The dashed line represents perfectly symmetric growth. In no case was growth perfectly uniform, though in control conditions, the points fell close to the dashed line. In gradient conditions, only the shallow gradients produced with 50% IKVAV-grafted collagen as the source across a 5-mm channel appeared to consistently bias growth up the gradient of adhesion. The experiments were further quantified to determine the average relative growth bias (Table 2) and average total length (Fig. 7).
FIG. 5.
Neurite growth anisotropy in experiments with YIGSR-grafted collagen with a 5-mm cross-channel (A), and a 3-mm cross-channel (B). Each point represents the average length of neurites extending up the gradient and down the gradient from a single network±standard error of the mean (SEM). For conditions where no gradient was present, the direction with longer average growth was plotted as the ordinate value. The dashed line represents perfectly symmetric growth. In no case was growth perfectly uniform, though in control conditions, the points fell close to the dashed line. In gradient conditions, neurite growth appeared to be consistently biased up the gradient of adhesion for all conditions except the shallowest gradient produced with 50% YIGSR-grafted collagen as the source across a 5-mm channel. The experiments were further quantified to determine the average relative growth bias (Table 2) and average total length (Fig. 7).
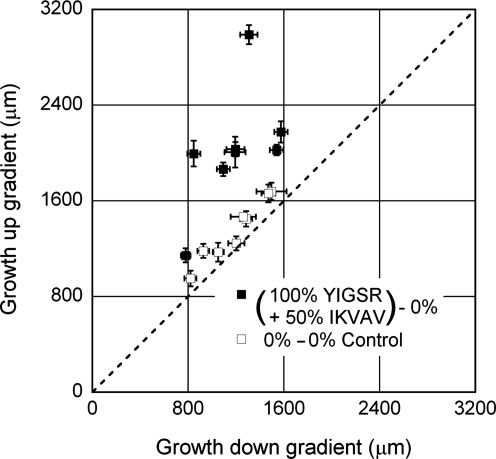
FIG. 6.
Neurite growth anisotropy in experiments with a combination of a relatively steep YIGSR-grafted collagen gradient, and shallow IKVAV-grafted collagen with a 5-mm cross-channel. Each point represents the average length of neurites extending up the gradient and down the gradient from a single network±standard error of the mean (SEM). For the control condition, the direction with longer average growth was plotted as the ordinate value. The dashed line represents perfectly symmetric growth. In no case was growth perfectly uniform, though in control conditions, the points fell close to the dashed line. In the combined gradient condition, neurite growth was substantially and consistently biased up the gradient of adhesion. The experiments were further quantified to determine the average relative growth bias (Table 2) and average total length (Fig. 7).
Table 2.
Summary of Results from Adhesion Gradient Experiments
| |
|
|
|
Average number of neurites per device (standard error) |
|
|
|
|---|---|---|---|---|---|---|---|
| Channel width | Peptide | Condition | Number of devices (n) | Down | Up | Growth ratio (standard error) | p Value for growth ratio (versus controls) |
| 3 mm | IKVAV | Control | 7 | 13 (2) | 14 (2) | 1.10 (0.03) | N/A |
| 0–100 | 4 | 14 (1) | 17 (2) | 1.34 (0.18) | 0.144 | ||
| 0–50 | 5 | 17 (2) | 18 (4) | 1.08 (0.07) | 0.687 | ||
| YIGSR | Control | 8 | 13 (2) | 13 (2) | 1.11 (0.03) | N/A | |
| 0–100 | 4 | 14 (2) | 17 (2) | 1.47 (0.15) | 0.019* | ||
| 0–50 | 4 | 15 (2) | 14 (2) | 1.41 (0.07) | 0.051 | ||
| 5 mm | IKVAV | Control | 6 | 13 (2) | 14 (2) | 1.14 (0.04) | N/A |
| 0–100 | 5 | 16 (2) | 15 (1) | 1.14 (0.04) | 0.954 | ||
| 0–50 | 5 | 12 (1) | 12 (2) | 1.47 (0.21) | 0.010* | ||
| 100–100 | 5 | 11 (1) | 11 (2) | 1.23 (0.13) | 0.455 | ||
| YIGSR | Control | 7 | 12 (1) | 12 (1) | 1.16 (0.05) | N/A | |
| 0–100 | 5 | 12 (2 | 15 (2) | 1.65 (0.28) | 1.1e-4* | ||
| 0–50 | 5 | 15 (2) | 14 (1) | 1.18 (0.06) | 0.754 | ||
| 100–100 | 5 | 13 (2) | 14 (1) | 1.09 (0.03) | 0.708 | ||
| YIGSR+IKVAV | Control | 8 | 12 (4) | 11 (4) | 1.14 (0.02) | N/A | |
| 0–100Y+50I | 8 | 12 (4) | 11 (4) | 1.74 (0.14) | 1.6e-7* | ||
p<0.05.
Results are presented as the average value, with standard error of the mean in parentheses. For each condition, the number of neurites growing up the gradient versus down the gradient was compared with a paired t-test, and no significant differences were identified. The growth ratio was calculated as the average growth up the gradient (or in the longer direction for controls), divided by growth down the gradient (or in the shorter direction for controls). These values were compared with analysis of variance, followed by pair-wise comparisons against the appropriate controls with Fisher's LSD test.
Based on these results, a final condition was examined, where a steep YIGSR gradient and a shallow IKVAV gradient were combined in the 5-mm-wide networks. As shown in Figure 6, this combination produced consistent, biased growth up the gradient of the peptide-grafted collagen. The growth ratio for the combined condition was the largest and was significantly different than controls with the smallest p value (Table 2; p=1.6e-7).
The peptide-grafted collagen could enhance growth regardless of providing an effective directional cue. The average length of neurite segments for each of the conditions are shown in Figure 7A for 5-mm experiments, and Figure 7B for 3-mm experiments. Consistent with the spread of the points for each separate condition seen in Figures 4–6, there was substantial error associated with the average lengths. Previous experience suggested that day-to-day variations in the growth were linked systematically to a variable held constant within each experiment—most likely differences in the viability of explants from a particular chick embryo (Sundararaghavan et al., 2009). These day-to-day variations were accounted for in the statistical analysis of the length of neurites in gradient and uniform conditions by comparing the length of neurite growth among different conditions that shared control experiments (i.e., each subplot shown in Figs. 4–6), with a two-way ANOVA using type III sum of squares, where the gradient condition was a fixed effect and the day of the experiment was a random effect (Shreiber et al., 2001), followed by Fisher's LSD test for pair-wise comparisons when the omnibus ANOVA was significant.
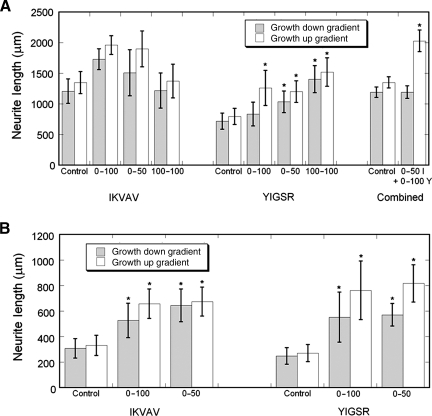
FIG. 7.
Average lengths of neurite growth (±standard error of the mean; SEM) for experiments with a 5-mm cross-channel (A) and 3-mm cross-channel (B). The large error bars are consistent with the spread of the individual points shown in Figs. 4–6, which was linked to day-to-day variations in the growth from explants from a particular chick embryo. These variations were accounted for statistically with a two-way analysis of variance (ANOVA), using the gradient condition as a fixed effect, and the day of the experiment as a random effect, followed by post-hoc testing against the untreated control with Fisher's Least Significant Difference Test to identify which conditions produced accelerated growth (*p<0.05). It is noted that the omnibus ANOVA for IKVAV in 5-mm cross-channels failed to be significant (p=0.110), which precluded subsequent testing of individual conditions against the control. Most conditions with any grafted IKVAV or YIGSR produced growth that was significantly greater than controls, except down the gradient of 0–100% YIGSR in 5-mm channels, and down the gradient of combined peptides in 5-mm channels. It is noteworthy that growth in IKVAV-grafted conditions followed a bi-modal response with respect to average concentration.
For all sets of experiments, there were significant differences among the neurite segment lengths (max p=0.02), except IKVAV experiments with the 5-mm cross-channel (p=0.110). In that condition, the growth in uniform, IKVAV-grafted collagen was markedly lower than the growth in either direction in the gradient conditions, and was approximately equal to growth in ungrafted controls. In both the IKVAV and YIGSR experiments with the 3-mm cross-channel, neurite growth up and down the 100–0% and 50–0% gradients was significantly different than in the untreated controls (all p<0.001). In YIGSR experiments with a 5-mm cross-channel, all conditions were different than untreated controls (p<0.001), except growth down the 100–0% gradient (p=0.091). In experiments with YIGSR and IKVAV combined, growth up the gradient was significantly different than the control (p=0.007), but growth down the gradient was not (p=0.825)
Neurite number
In addition to neurite length, the number of neurites projecting in a given direction within the cross-channel was also measured. Average neurite counts are listed in Table 2. Paired t-tests were performed within each condition to compare the number of neurites projecting up and down the gradients. For control experiments, the pairs were grouped according to longer and shorter growth. No significant differences were detected in neurite number between any of the paired groups (min p=0.090). The total number of neurites was also compared across the conditions with two-way ANOVAs as performed with neurite lengths. In no cases were there significant differences between experimental conditions and untreated controls (min p=0.258).
Discussion
The potential to stimulate and direct the growth of neuronal processes with gradients of extracellular cues has clear implications in developing strategies to repair and regenerate injured tissue in the central and peripheral nervous systems. Chemotactic gradients of soluble, attractive cues (such as nerve growth factor), or repulsive cues (such as semaphorins) are the most studied and are readily generated in vitro to examine growth on 2D substrates (Snow and Letourneau, 1992), or in a 3D culture system (Moore et al., 2006). However, although in vivo cells can differentially synthesize factors to provide gradients of these soluble chemical cues, to incorporate these gradients into an implanted biomaterial would require inclusion of devices for the sustained and controlled release of the factors as well as specific spatial localization of the devices (Dodla and Bellamkonda, 2008). Alternatively, immobilized gradients can be introduced to the biomaterial and characterized ex vivo, and then implanted to present a sustained gradient to enhance and potentially direct growth.
Using a simple microfluidic network to generate stable gradients of cross-links, we previously showed that gradients of mechanical properties in a 3D collagen gel accelerate growth of regenerating neurites towards regions of greater compliance (Sundararaghavan et al., 2009). After modifying the system to generate gradients of different collagen solutions, we found that regenerating neurites are differentially sensitive to immobilized gradients of different bioactive peptide sequences from laminin, and that the growth response also depends on the steepness of the gradient. We found that neurite growth through type I collagen grafted with YIGSR was enhanced compared to untreated collagen, and that the growth was accelerated up steep gradients of the immobilized peptide.
In contrast, growth in IKVAV-grafted collagen appeared accelerated up shallow gradients. In general, growth was enhanced in the IKVAV-grafted gels—gels with IKVAV produced longer neurite growth than the untreated control gels. Importantly, however, growth was equal to controls in the condition where IKVAV-grafted collagen was uniformly presented (i.e., 100–100% IKVAV). In general, cell migration depends biphasically on the strength of cell adhesion (Palecek et al., 1997), with the highest migration speeds being at intermediate receptor/ligand concentrations. The migration of fibroblasts and smooth muscle cells in and/or on our peptide-grafted gels was consistent with this phenomenon (Monteiro et al., 2009). It is quite likely that the IKVAV ligand concentration in the 100–100% case generated an environment that was too sticky for the growth cones to effectively release. We also note that the molecular weight of the IKVAV-containing peptide was approximately double that of the YIGSR-containing peptide. As such, the gradients of IKVAV were shallower than the gradients of YIGSR in terms of molarity, which further distinguishes the response of neurites to the two bioactive ligands.
We did not see any differences in the turning behavior of neurites in our system as a function of the collagen condition or gradient. In no case was there a bias for the number of neurites projecting up or down a gradient, and the total numbers of neurites were statistically similar among all of the conditions. As such, as a biomaterial, the peptide-grafted collagen did not direct growth as much as directionally accelerate growth. For IKVAV in particular, these results are contrary to previous reports that demonstrate the ligand as important in turning behavior (Adams et al., 2005). In our system, the outgrowth of neurites through a fibrillar collagen gel without grafted ligands is substantial, and others have used collagen-based systems in vitro and in vivo (Carbonetto et al., 1983; Labrador et al., 1998; O'Connor et al., 2001). Growth cones express integrins, such as β1 (Grabham and Goldberg, 1997), that bind ligands on collagen, and our system naturally presents a superposition of collagen and laminin bioactive motifs. Studies of turning behavior have generally been performed where the background adhesive signal is less specific, such as with poly-lysine (Adams et al., 2005), or non-existent, such as with agarose (Dodla and Bellamkonda, 2006).
There appeared to be an additive effect of the YIGSR- and IKVAV-grafted collagens presented as gradients. When a steep gradient of YIGSR was combined with a shallow gradient of IKVAV, the directed acceleration of neurite growth was the most consistent and the most substantial (Figs. 6 and 7). Since both of the peptides are present on laminin, the potential synergy is not surprising. Indeed, Tong and Shoichet previously demonstrated that combined presentation of YIGSR and IKVAV peptides immobilized to a fluoropolymer film encouraged neurite outgrowth equal to that of laminin (Tong and Shoichet, 2001). However, on an individual laminin molecule, there is one of each of the bioactive peptides—IKVAV on the α-1 chain (Tashiro et al., 1989), and YIGSR on the β-1 chain (Iwamoto et al., 1987). Accordingly, when a gradient of laminin is generated, the gradients of IKVAV and YIGSR are equal. As such, steep gradients of laminin may optimally present YIGSR, but sub-optimally present IKVAV. By preparing separate solutions of IKVAV- and YIGSR-grafted collagen, and then combining these two at different source and sink combinations, optimal concentrations and gradients of the pair can be achieved that are not possible with the full-length molecule. Dodla and Bellamkonda evaluated neurite outgrowth from chick DRGs within agarose gels presenting a range of laminin-1 gradients (Dodla and Bellamkonda, 2006). All of the laminin-1 gradients in that study were considerably shallower than any of the gradients used in this report, and the concentrations were significantly less. The substantial differences in approaches and in the potential presentation of the bioactive ligands preclude a direct comparison of concentrations and gradients. Nevertheless, it is noteworthy that Dodla and Bellamkonda found that the greatest directional enhancement of outgrowth occurred up the shallowest gradients tested. In their optimal shallow gradient, growth down the gradient of laminin was superior to that in the optimal isotropic controls. We also observed that the growth rate down a gradient could be superior to controls. Finally, they observed a bimodal response of neurite growth rate with respect to laminin concentration, similar to our observations of neurite length on IKVAV-grafted collagen.
The response of the regenerating neurites to the 3D fibrillar environment is complex, and several factors may have contributed to the observed response that would not be at play if the immobilized gradients were prepared on a two-dimensional substrate. For example, in our previous work, we demonstrated that neurites are stimulated to grow down a gradient of stiffness. If, upon self-assembly the peptide-grafted gel presents a different stiffness than the untreated gel, then a confounding variable that can dictate neurite response solely or in combination with the grafted ligands would be introduced. Similarly, if the peptide-collagen generates a gel with different microstructure, such as fiber density, fiber thickness, and/or pore size, then gradients of steric hindrance and possibly gradients of diffusible nutrients could be established. However, we previously showed that the peptide-grafted collagen has rheological properties equivalent to the untreated collagen (Monteiro et al., 2009). Furthermore, no differences in fiber size or number were detected from high-resolution confocal images of fluorescence-spiked peptide-grafted and untreated gels. Accordingly, we attribute the observed results to the primary change in grafted, bioactive ligands on the collagen across the gradient channel.
This study further demonstrates the versatility of the simple approach to assay axon growth in gradients of cues, particularly when viewed in combination with our previous report on durotactic gradients. The steepness of the gradient and the average concentration can be controlled independently by changing source and sink concentrations and the width of the cross-channel. The underlying channel can also be placed at defined locations along the cross-channel to interrogate different entry concentrations within the gradient. Separate solutions of collagen can be prepared with different grafted ligands to assay combinatorial gradients, with gradients acting co-directionally or in opposition. Moreover, the durotactic gradients can be superposed onto the haptotactic gradients by introducing a solution of a cell-tolerated cross-linker, such as genipin, into the source channel, and saline into the sink channel for a defined period of time. Furthermore, since the gradient of the cross-linker is effectively a gradient of a diffusible factor, chemotactic gradients can be superimposed on the haptotactic and/or durotactic gradients to capture the response to multiple directional modalities. Of course, many of the advantages of BioMEMS-based assays of cell behavior, such as small reagent volumes, are also retained.
Acknowledgments
Funding for these studies was provided by the National Science Foundation (grant NSF-ARRA-CBET 0846328), National Institutes of Health (NIH grant 1R21EB009245-01A1), and the New Jersey Commission on Spinal Cord Research (03-3028-SCR-E-0). H.G.S. was supported by a NSF Training Fellowship (NSF-IGERT on Integratively Designed Biointerfaces, DGE 033196). S.N.M. was supported by the Rutgers-UMDNJ Biotechnology Training Program (NIH grant 5T32GM008339-20). Silicon masters were fabricated at Bell Labs/Lucent Technologies through a grant from the New Jersey Nanotechnology Consortium.
Author Disclosure Statement
No competing financial interests exist.
References
- Adams D.N. Kao E.Y. Hypolite C.L. Distefano M.D. Hu W.S. Letourneau P.C. Growth cones turn and migrate up an immobilized gradient of the laminin IKVAV peptide. J. Neurobiol. 2005;62:134–147. doi: 10.1002/neu.20075. [DOI] [PubMed] [Google Scholar]
- Borrirukwanit K. Lafleur M.A. Mercuri F.A. Blick T. Price J.T. Fridman R. Pereira J.J. Leardkamonkarn V. Thompson E.W. The type I collagen induction of MT1-MMP-mediated MMP-2 activation is repressed by alphaVbeta3 integrin in human breast cancer cells. Matrix Biol. 2007;26:291–305. doi: 10.1016/j.matbio.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Carbonetto S. Gruver M.M. Turner D.C. Nerve fiber growth in culture on fibronectin, collagen, and glycosaminoglycan substrates. J. Neurosci. 1983;3:2324–2335. doi: 10.1523/JNEUROSCI.03-11-02324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernousov M.A. Yu W.M. Chen Z.L. Carey D.J. Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56:1498–1507. doi: 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- Condic M.L. Letourneau P.C. Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature. 1997;389:852–856. doi: 10.1038/39878. [DOI] [PubMed] [Google Scholar]
- Dhoot N.O. Tobias C.A. Fischer I. Wheatley M.A. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. A. 2004;71:191–200. doi: 10.1002/jbm.a.30103. [DOI] [PubMed] [Google Scholar]
- Dodla M.C. Bellamkonda R.V. Anisotropic scaffolds facilitate enhanced neurite extension in vitro. J. Biomed. Mater. Res. A. 2006;78:213–221. doi: 10.1002/jbm.a.30747. [DOI] [PubMed] [Google Scholar]
- Dodla M.C. Bellamkonda R.V. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33–46. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X. Mclaughlin C. Griffith M. Sheardown H. Biofunctionalization of collagen for improved biological response: scaffolds for corneal tissue engineering. Biomaterials. 2007;28:78–88. doi: 10.1016/j.biomaterials.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Gomez T.M. Letourneau P.C. Filopodia initiate choices made by sensory neuron growth cones at laminin/fibronectin borders in vitro. J. Neurosci. 1994;14:5959–5972. doi: 10.1523/JNEUROSCI.14-10-05959.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabham P.W. Goldberg D.J. Nerve growth factor stimulates the accumulation of beta1 integrin at the tips of filopodia in the growth cones of sympathetic neurons. J. Neurosci. 1997;17:5455–5465. doi: 10.1523/JNEUROSCI.17-14-05455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie S. Pini A. Chemorepulsion of developing motor axons by the floor plate. Neuron. 1995;14:1117–1130. doi: 10.1016/0896-6273(95)90260-0. [DOI] [PubMed] [Google Scholar]
- Hammarback J.A. Palm S.L. Furcht L.T. Letourneau P.C. Guidance of neurite outgrowth by pathways of substratum-adsorbed laminin. J. Neurosci. Res. 1985;13:213–220. doi: 10.1002/jnr.490130115. [DOI] [PubMed] [Google Scholar]
- Heller D.A. Garga V. Kelleher K.J. Lee T.C. Mahbubani S. Sigworth L.A. Lee T.R. Rea M.A. Patterned networks of mouse hippocampal neurons on peptide-coated gold surfaces. Biomaterials. 2005;26:883–889. doi: 10.1016/j.biomaterials.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Itoh S. Yamaguchi I. Suzuki M. Ichinose S. Takakuda K. Kobayashi H. Shinomiya K. Tanaka J. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003;993:111–123. doi: 10.1016/j.brainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y. Robey F.A. Graf J. Sasaki M. Kleinman H.K. Yamada Y. Martin G.R. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Kapur T.A. Shoichet M.S. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J. Biomed. Mater. Res. A. 2004;68:235–243. doi: 10.1002/jbm.a.10168. [DOI] [PubMed] [Google Scholar]
- Labrador R.O. Buti M. Navarro X. Influence of collagen and laminin gels concentration on nerve regeneration after resection and tube repair. Exp. Neurol. 1998;149:243–252. doi: 10.1006/exnr.1997.6650. [DOI] [PubMed] [Google Scholar]
- Lamar J.M. Iyer V. Dipersio C.M. Integrin alpha3beta1 potentiates TGFbeta-mediated induction of MMP-9 in immortalized keratinocytes. J. Invest. Dermatol. 2008;128:575–586. doi: 10.1038/sj.jid.5701042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbill-Edds L. Laminin and the mechanism of neuronal outgrowth. Brain Res. Brain Res. Rev. 1997;23:1–27. doi: 10.1016/s0165-0173(96)00013-6. [DOI] [PubMed] [Google Scholar]
- Monteiro G.A. Fernandes A.V. Sundararaghavan H.G. Shreiber D.I. Positively and negatively modulating cell adhesion to type I collagen via peptide grafting. Tissue Eng. 2009. Part A [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Moore K. Macsween M. Shoichet M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 2006;12:267–278. doi: 10.1089/ten.2006.12.267. [DOI] [PubMed] [Google Scholar]
- O'Connor S.M. Stenger D.A. Shaffer K.M. Ma W. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci. Lett. 2001;304:189–193. doi: 10.1016/s0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- Palecek S.P. Loftus J.C. Ginsberg M.H. Lauffenburger D.A. Horwitz A.F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Ranieri J.P. Bellamkonda R. Bekos E.J. Vargo T.G. Gardella J.A., Jr. Aebischer P. Neuronal cell attachment to fluorinated ethylene propylene films with covalently immobilized laminin oligopeptides YIGSR and IKVAV. II. J. Biomed. Mater. Res. 1995;29:779–785. doi: 10.1002/jbm.820290614. [DOI] [PubMed] [Google Scholar]
- Sameni M. Dosescu J. Yamada K.M. Sloane B.F. Cavallo-Medved D. Functional live-cell imaging demonstrates that beta1-integrin promotes type IV collagen degradation by breast and prostate cancer cells. Mol. Imaging. 2008;7:199–213. [PMC free article] [PubMed] [Google Scholar]
- Saneinejad S. Shoichet M.S. Patterned glass surfaces direct cell adhesion and process outgrowth of primary neurons of the central nervous system. J. Biomed. Mater. Res. 1998;42:13–19. doi: 10.1002/(sici)1097-4636(199810)42:1<13::aid-jbm3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Schmidt C.E. Leach J.B. Neural tissue engineering: strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- Shreiber D.I. Enever P.A. Tranquillo R.T. Effects of pdgf-bb on rat dermal fibroblast behavior in mechanically stressed and unstressed collagen and fibrin gels. Exp. Cell Res. 2001;266:155–166. doi: 10.1006/excr.2001.5208. [DOI] [PubMed] [Google Scholar]
- Snow D.M. Letourneau P.C. Neurite outgrowth on a step gradient of chondroitin sulfate proteoglycan (CS-PG) J. Neurobiol. 1992;23:322–336. doi: 10.1002/neu.480230311. [DOI] [PubMed] [Google Scholar]
- Sundararaghavan H.G. Monteiro G.A. Firestein B.L. Shreiber D.I. Neurite growth in 3D collagen gels with gradients of mechanical properties. Biotechnol. Bioeng. 2009;102:632–643. doi: 10.1002/bit.22074. [DOI] [PubMed] [Google Scholar]
- Tashiro K. Sephel G.C. Weeks B. Sasaki M. Martin G.R. Kleinman H.K. Yamada Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J. Biol. Chem. 1989;264:16174–16182. [PubMed] [Google Scholar]
- Tessier-Lavigne M. Placzek M. Target attraction: are developing axons guided by chemotropism? Trends Neurosci. 1991;14:303–310. doi: 10.1016/0166-2236(91)90142-h. [DOI] [PubMed] [Google Scholar]
- Tong Y.W. Shoichet M.S. Enhancing the neuronal interaction on fluoropolymer surfaces with mixed peptides or spacer group linkers. Biomaterials. 2001;22:1029–1034. doi: 10.1016/s0142-9612(00)00338-0. [DOI] [PubMed] [Google Scholar]
- Tucker B.A. Mearow K.M. Peripheral sensory axon growth: from receptor binding to cellular signaling. Can. J. Neurol. Sci. 2008;35:551–566. doi: 10.1017/s0317167100009331. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M. Choi C.K. Horwitz A.R. Integrins in cell migration—the actin connection. J. Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides G.M. Ostuni E. Takayama S. Jiang X. Ingber D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- Yip J.W. Yip Y.P. Laminin—developmental expression and role in axonal outgrowth in the peripheral nervous system of the chick. Brain Res. Dev. Brain Res. 1992;68:23–33. doi: 10.1016/0165-3806(92)90244-q. [DOI] [PubMed] [Google Scholar]
- Yu L.M. Kazazian K. Shoichet M.S. Peptide surface modification of methacrylamide chitosan for neural tissue engineering applications. J. Biomed. Mater. Res. A. 2007;82:243–255. doi: 10.1002/jbm.a.31069. [DOI] [PubMed] [Google Scholar]
- Yu X. Dillon G.P. Bellamkonda R.B. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999;5:291–304. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]