Abstract
Adenoid cystic carcinoma (ACC), the second most frequent malignancy of the major and minor salivary glands, comprise of approximately 15–23% of all carcinomas at these locations. ACC is uniquely formed of dual epithelial and myoepithelial cells that give rise to different phenotypic patterns. We hypothesize that the dual myoepithelial/epithelial composition of ACCs underlie their biological heterogeneity and may impact on their therapeutic management. A recurrent reciprocal translocation of t(6;9)(q22–23;p23–24) resulting in fusion gene partners comprising MYB gene the transcription factor NFIB has been reported in ACC of breast, salivary, lachrymal and ceruminal glands. In fusion positive and a subset of fusion negative ACCs, high expression of the transcript Myb was found. However, the role of Myb protein expression and the potential effect on the downstream targets have not been investigated. To investigate the biological and prognostic significance of use of elevated levels of Myb and its downstream target genes (c-kit, cox-2, bcl-2), we analyzed, by immunohistochemistry, the protein expression of these genes in 156 ACCs.
We have found that 55% of ACCs have increased Myb expression mainly confined to myoepithelial cells. We validated Myb expression on a large cohort of ACCs (156 patients). Although no significant effects of the individual Myb and downstream targets c-kit, bcl-2 and cox-2 on survival was noticed, the combinations survival curve for Myb+/c-kit+/cox-2+ showed better survival than combination Myb−/c-kit+/cox-2+. Myb may serve as a new target for the management of this disease, and future therapeutic trials of these tumors may be better based on biomarker stratification and the cellular composition of these tumors.
Key words: adenoid cystic carcinoma, c-kit, cox-2, bcl-2
Introduction
Adenoid cystic carcinoma (ACC), the second most frequent malignancy of the major and minor salivary glands, comprise of approximately 15–23% of all carcinomas at these locations.1 This tumor is uniquely formed of dual epithelial and myoepithelial cells that give rise to different phenotypic patterns. Tumors with tubular and cribriform structures typically pursue protracted and relentless course, and this behavior has been attributed to the tumor suppressive role of the myoepithelial cells. Evidence for such function has been drawn from both in-vitro and clinical studies of mammary2,3 and salivary carcinomas4,5 and is further sustained by the coincident progressive course of the solid variant upon the loss of myoepithelial cells. We hypothesize that the dual myoepithelial/epithelial composition of adenoid cystic carcinoma and other salivary carcinomas underlie, at least in part, their biological heterogeneity and may impact on their therapeutic management.6
Adenoid cystic carcinomas are characterized by frequent loss of chromosome 6q region and translocations between 6q and 9p chromosomal regions.7 Recently, Persson et al. have shown a recurrent specific translocation t(6;9)(q22–23;p23–24) in breast, salivary, lachrymal and ceruminal gland ACCs.8 The fusion gene partners in this translocation comprised MYB gene on chromosome 6q22–q23 and the transcription factor NFIB on chromosome 9p23–p24.8,9 In fusion positive tumors and a subset of fusion negative tumors, high expression of Myb was found, supporting a role for this gene in adenoid cystic carcinoma development and progression.
c-Myb transcription factor was identified as the mammalian homolog of v-myb, a transforming retroviral oncogene linked to avian leukemia. High expression of this gene has been associated with oncogenic activity and poor prognosis in several human cancers including T-cell leukemia, acute myelogenous leukemia,10 colorectal tumors,11 and most recently in adenoid cystic carcinomas.8,12 In addition, c-Myb has been implicated in progenitor cell maintenance and is required for proper cellular differentiation in the hematopoietic system, neuronal cells, skin cells and colonic crypts. c-Myb high expression is frequently associated with a variety of immature cell lineages, and expression levels decrease as cells differentiate. Myb is highly expressed in almost all estrogen receptor (ER)-positive breast tumors and is a direct target of estrogen/ER signaling.13,14 Estrogen has a protective effect from sustained colon cancer cell growth at least partly through suppression of c-myb and bcl-2.12
As a transcription factor, MYB has been shown to affect downstream target genes involved in several neoplastic processes. Among these are cyclooxygenase-2 (COX-2), Bcl-2, BclX(L) and c-kit. In colorectal carcinoma promoter studies indicated that c-Myb can activate COX-2 transcription, whereas dominant-negative Myb mediated repression.11 The expression of COX-2 is correlated with VEGF-C and lymphatic vessel density in prostate and breast cancer, and ACC of uterine cervix indicating COX-2 is associated with tumor growth and metastasis.15
C-MYB proto-oncogene also serves as a transactivator of important cellular genes such as kit receptor and CD34. Several studies have shown that c-kit (CD117) protein, a member of class III receptor tyrosine kinase family, is expressed in the majority of ACC. Products of this gene are required for fundamental cellular processes and tumorigenesis. Elevated c-kit expression has been detected in a variety of other tumors especially gastrointestinal stromal tumor (GIST), seminoma and malignant melanoma. C-kit is a target of the tyrosine kinase inhibitor imatinib mesylate (GleevecTM), to which significant treatment response has been achieved in chronic myelogenous leukemia (CML) and advanced c-kit-positive GIST. Whereas gain-of-function mutations in exon 9 and 11 constitute a critical molecular event for c-kit overexpression in GIST and seminoma, no such point mutation has been found in ACC. Evidence of c-kit amplification of the subcentromeric region of chromosome 4q in a subset of ACC has been reported by our group.
A key regulator of apoptosis is bcl-2. Previous work has shown that bcl-2 expression is regulated by c-myb binding. A decrease in bcl-2 has also been observed with a decrease in c-myb associated with colon cell differentiation. Experiments in human leukemia cell lines showed that forced expression of bcl-2 rescues the cells from apoptosis, without preventing either their withdrawal from the cell cycle or their differentiation. v-Myb has been linked to the activation of bcl-2 through promoter-driven reporter. c-Myb directly transactivates the endogenous c-myc and Bcl-2 genes, which explains at least in part how c-Myb regulates proliferation and survival. Immunohistochemical gene Bcl-2 protein is expressed in virtually all benign and malignant salivary gland tumors. This observation suggests an important role of this protein in the development of these tumors.
Because of the limited number of ACC studies, however, biological role of MYBas an oncogene and/or transcriptional regulator of other genes in ACC tumorigenesis remains unknown.8,12 To investigate the biological and prognostic significance of elevated levels of Myb and its downstream target genes, we analyzed, by immunohistochemistry, the protein expression of these genes in 156 adenoid cystic carcinomas.
Results
Demographic and pathologic findings.
The study cohort comprised of 82 women and 74 men, who ranged in age from 15.9–81.9 y, with the median age 51.4 y. One-hundred thirty-eight patients (91.4%) were white or Hispanic and 18 patients (8.6%) were black. Tumors sites included 18 in the parotid gland, 20 in the hard palate, 18 in the maxillary sinus, 15 in the submandibular and sublingual gland and 85 in various minor salivary glands sites.
Histopathologic and clinical findings.
Histopathologically, tissue cores show at least two distinctive patterns within and between cores of the same case. A predominant pattern was determined based on the presence of more than 50% of a given pattern in tumor. Among the 156 tumors for which a predominant type could be ascertained, 41 were tubular (26.1%), 98 (62.4%) had predominantly cribriform patterns (composed of epithelial and myoepithelial neoplastic cells) and 18 (11.5%) had the solid pattern (predominantly devoid of myoepithelial cells). Sixty-seven patients (43%) developed distant metastasis, 89 patients had no evidence of metastasis; 30 patients (20.4%) died in less than 3 y, and 117 patients survived more than 3 y.
Myb, c-kit, cox-2 and bcl-2 expression.
Normal salivary gland: C-kit, cox-2 and bcl-2 were expressed in membrane and/ or cytoplasm of salivary ductal cells while faint cytoplasmic expression was noted in epithelial cells of the intercalated and striated ducts. Immunoreactivity for c-kit, cox-2 and bcl-2 was absent in peripheral myoepithelial cells of the intercalated ducts. Myb expression was not detected in non-tumoral salivary gland parenchyma.
ACCs: C-kit, cox-2 and bcl-2 expression was predominantly limited to the inner ductal cells and was negative in the myoepithelial cells in both tubular and cribriform patterns of ACCs. C-kit expression was found in 93% of the cribriform, 98% of the tubular patterns and 100% of the solid form (Table 1). Cox-2 expression was found in 60% of the cribriform, 71% of the tubular patterns and 72% of the solid form (Table 1). Bcl-2 expression was found in 86% of the cribriform, 83% of the tubular patterns and 83% of the solid form (Table 1).
Table 1.
Prevalence of selected markers among histological types in adenoid cystic carcinoma patients
| Characteristic | MYB, n = 86 (54.8%) | C-KIT, n = 149 (94.9%) | COX-2, n = 101 (64.3%) | BCL-2, n = 133 (84.7%) | ||||||||||
| Overall count | Overall % | Count | % | p* | Count | % | p* | Count | % | p* | Count | % | p* | |
| Histological Type | ||||||||||||||
| Cribriform | 98 | 62.4% | 49 | 50.0% | 0.1382 | 91 | 92.9% | 0.2599 | 59 | 60.2% | 0.1740 | 84 | 85.7% | 0.6540 |
| Tubular | 41 | 26.1% | 25 | 61.0% | 0.3687 | 40 | 97.6% | 0.6814 | 29 | 70.7% | 0.3492 | 34 | 82.9% | 0.8011 |
| Solid | 18 | 11.5% | 12 | 66.7% | 0.3232 | 18 | 100.0% | 0.5979 | 13 | 72.2% | 0.6034 | 15 | 83.3% | 0.7406 |
p*, probability of null hypothesis from 2-tailed fisher exact test.
Myb expression was mainly restricted to myoepithelial cells and was negative in ductal cells of both the tubular and cribriform patterns. Myb expression was found in 55% (86/156): 50% of the cribriform, 61% of the tubular patterns and 12% of the solid form (in these, scattered rare peripheral myoepithelial cells were present) (Table 1).
Clinicopathologic correlations.
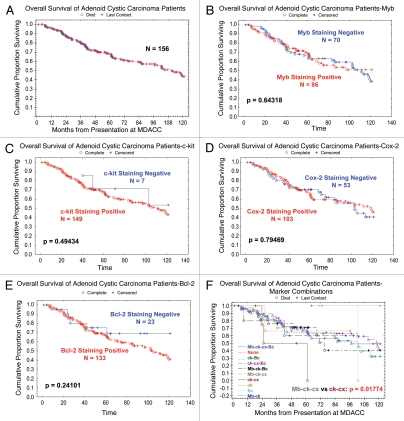
No significant correlation between individual markers (c-kit, cox-2, bcl-2, Myb) and survival was noticed. Also, there was no significant correlation between different combinations of markers and survival, with the exception of Myb/c-kit/cox-2. None of the five patients (0/5) who had Myb/c-kit/cox-2 positivity died, but three of the five patients (3/5) who had c-kit/cox-2 positivity and were negative for Myb died (p = 0.01744). The Kaplan-Meier plots of the overall survival of the population are shown in Figure 1.
Figure 1.
(A) The Kaplan-Meier plots of the overall survival of the population. (B–E) No significant correlation between individual markers (Myb, c-kit, cox-2, bcl-2) and survival was noticed. (F) Also, there was no significant correlation between different combinations of markers and survival, with the exception of Myb/c-kit/cox-2.
Discussion
Our results show c-kit/cox-2/bcl-2 expression to be limited mainly to epithelial cells while Myb is identified in myoepithelial cells of ACCs. These findings lend credence to the presumed biological differences between myoepithelial and epithelial cells in this entity and may play a major contributing factor in the biological heterogeneity and therapy response of these tumors. In this large cohort c-kit immunoreactivity was predominantly restricted to in the inner ductal cells of the tubular and cribriform patterns, and was homogeneously expressed in the solid form, in keeping with our prior studies. Cox-2 and bcl-2 although limited to the inner epithelial cells showed variable degree of immunoreactivity within the ductal cells (Table 1). Our results also show the expression of Myb to be mainly limited to the peripheral myoepithelial cells in the majority of the tubular and cribriform patterns of these tumors,in more than half of the cases (55%). Individually, no significant effects of the markers on survival was noticed. However, co-expression of Myb/c-kit/cox-2 showed an association with poor patients outcome. None of the five patients who had Myb/c-kit/cox-2 positivity died, but three of the five patients who had c-kit/cox-2 positivity and negative for Myb died (p = 0.01) Figure 1. In a recent reported study on 37 patients, there was a trend toward increased PNI and higher rate with local relapse in patients with balanced MYB-NFIB translocation.16
Prior genetic, molecular and phenotypic investigations of ACC yielded minimal results.
The finding of a recurrent gene fusion and subsequent aberrant expression involving MYB provides an opportunity for developing a clinically useful biomarker for diagnosis and prognosis. In our previous study the MYB-NFIB fusion was detected in 28% primary and 35% metastatic ACCs, but not in any of the non-ACC salivary carcinomas analyzed.12 Different exons in both the MYB and NFIB genes were involved in the fusions, resulting in expression of multiple chimeric variants. MYB was overexpressed in the majority of the ACCs; MYB expression was significantly higher in tumors carrying the MYB-NFIB fusion.12 No correlation with other clinicopathologic markers, factors, and survival was found.12 It was concluded that the MYB-NFIB fusion characterizes a subset of ACCs and contributes to MYB overexpression.12 Additional mechanisms may be involved in MYB overexpression in ACCs lacking the MYB-NFIB fusion.12 These findings indicate that MYB overexpression, as a result of the MYB-NFIB fusion or through alternative mechanisms, is a significant feature of most salivary ACCs, suggesting that MYB may be involved in ACC development and could be a potential target to develop novel therapeutic strategies for ACC treatment.12 A subsequent study16 on a series of 37 adenoid cystic carcinoma cases, 112 other salivary gland neoplasms, and 409 nonsalivary gland tumors found that the balanced translocation, involving MYB and NFIB, was present in approximately one half of adenoid cystic carcinoma cases (18 of 37, 49%).16 An additional subset of cases (6 of 37, 16%) had an abnormal MYB FISH pattern suggestive of an unusual translocation of MYB but not involving NFIB, and that this might be in part due to a local duplication of MYB, as has been reported in human T-ALL cells.16 As seen in other salivary gland tumor translocations, a significant subset of cases did not have apparent MYB involvement, suggesting an alternative mechanism for oncogenesis.16 Overall, 65% of adenoid cystic carcinoma cases have a FISH pattern suggestive of MYB translocation.16 This is consistent with earlier published chromosomal analyses, suggesting that only a subset of adenoid cystic carcinomas harbor recurrent chromosomal rearrangements.12,16 Outcome studies provide some evidence to suggest that there is clinical prognostic significance to the presence of the MYB translocation. In the recent study of 37 adenoid cystic carcinoma cases, there is a trend toward an increase in local relapse with cases that have a balanced MYB-NFIB translocation; there is also a suggestion that these cases are more likely to show PNI.16
c-Kit expression has been reported to be limited to the inner ductal epithelial cells, whereas EGFR expression was limited mainly to the outer myoepithelial cells in the majority of ACCs with tubular and cribriform patterns.6 In solid ACCs, c-Kit was positive uniformly, whereas EGFR consistently was negative.6 A significant statistical correlation was observed between c-Kit expression and a poor 3-y outcome, and EGFR expression was correlated with a better 3-y outcome.6 These findings suggest the importance of cellular subtype localization of biomarkers in the clinical and therapeutic stratification of patients with ACC.6
In ACC of uterine cervix the expression of cox-2 induces the expression of VEGF, increase angiogenesis and enhance tumor growth and invasion.15 A prior study on malignant salivary tumors noted negligible cox-2 staining in six cases of ACC.17 Another isolated study on malignant salivary tumors described increased bcl-2 expression in adenoid cystic carcinoma (five cases) compared with other salivary neoplasms.18
In summary, we validated Myb expression on a large cohort of adenoid cystic carcinoma (156 patients). We have found that 55% of adenoid cystic carcinoma tumors have increased Myb expression, a feature that is unique compared with other salivary gland cancers, and provides a useful diagnostic tool. Although no significant effects of the individual Myb and downstream targets c-kit, bcl-2 and cox on survival was noticed, the combination Myb+/c-kit+/cox-2+ showed better survival than combination Myb−/c-kit+/cox-2+ (p = 0.01744). However, this is a subset analysis on a small number of patients and larger multi-institutional studies are needed. If ongoing bench work will support and translate into clinical, MYB may serve as a new target for the management of this disease, and future therapeutic trials of these tumors may be better based on biomarker stratification and the cellular composition of these tumors.
Materials and Methods
Archival formalin-fixed paraffin blocks of adenoid cystic carcinomas from 156 patients accessioned at the University of Texas, MD Anderson Cancer Center between 1988 and 2006 formed this study. The material for tissue microarray was created using two 1.0 mm diameter cores of spatially different areas of representative tumor from each paraffin block. Pathologic patterns and the phenotypic expression of the Myb, c-kit, cox and bcl-2 were recorded independently and were compared with clinical factors including gender, age and stage along with clinical outcomes.
Immunohistochemistry and immunoreactivity analysis.
Immunohistochemical analysis for Myb, c-kit, cox and bcl-2 were performed using the BOND MAX IHC staining protocol by Vision Biosystems (Norwell, MA) on 4 µm paraffin sections of the tissue microarray material. In brief, following dewaxing, washing and rehydration of the slides through xylene and graded alcohols, Tris-EDTA buffer was used for antigen retrieval. Slides were subsequently treated with 3% hydrogen peroxide to block endogenous peroxidase. Following incubation with the primary antibodies, myb, c-kit, cox and bcl-2 (Dako, 1:100 dilution), the secondary conjugate antibody was applied. Finally, each specimen-containing slide was developed using the chromogen DAB and counterstained with hematoxylin.
Myb, c-kit, cox and bcl-2 immunohistochemistry was independently evaluated by twp pathologists. For Myb, only nuclear staining was considered positive. Lesional tissues with strong staining in > 50% of the neoplastic cells were scored as strongly positive. Weak or strong staining in < 50% of the cells was scored as weakly positive. Less than 5% staining was scored as negative.
Membranous and cytoplasmic staining were scored for c-kit expression whereas a complete lack of any staining were considered negative and staining was recorded as high (3+), intermediate (2+), low (1+). The cox-2 and bcl-2 staining pattern was granular and localized in the cytoplasm. Each case was rated according to a score that added a scale of intensity to the number of tumor cells that stained for cox-2. The intensity of staining was on the following scale: 0, no staining; 1+, mild staining; 2+, moderate staining; 3+, intense staining. The number of neoplastic cells stained was evaluated as follows: 0, <10% of cells stained in the microscopic fields; 1+, < 25% of cells stained positive; 2+ between 25 and 50% stained positive; 3+, between 50 and 75% stained positive; 4+, > 75% stained positive.
Statistical analysis.
Descriptive statistics for scaled values and frequencies of study patients within the categories for each of the parameters of interest were enumerated with the assistance of commercial statistical software. Correlations between biomarkers and between biomarkers and endpoints were assessed by Pearson's Chi-square or, where there are fewer than ten subjects in any cell of a 2 × 2 grid, by the two-tailed Fisher exact test. Curves describing overall were generated by the Kaplan-Meier product limit method. The statistical significance of differences between the actuarial curves was tested by the log rank test. Follow-up time was the time from first appointment at the University of Texas MD Anderson Cancer Center for the primary tumor of concern until the date of last contact or death. Calculated p values < 0.05 were considered significant. These statistical tests were performed with the assistance of the Statistica (StatSoft, Inc., Tulsa, OK) and SPSS (SPSS for Windows, SPSS Inc., Chicago IL) statistical software applications.
Acknowledgments
This study was supported in part by the National Institutes of Health National Institute of Dental and Craniofacial Research and Rare Disease Research grant number U01DE019765 (A.K.E.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or National Institutes of Health.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125:149–152. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 2.Bissell MJ, Kenny PA, Radisky DC. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AJ, DiGiovanna MP, Ross DA, Sasaki CT, Carter D, Son YH, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96:149–158. doi: 10.1002/ijc.1013. [DOI] [PubMed] [Google Scholar]
- 5.Kokemueller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the head and neck—a 20 years experience. Int J Oral Maxillofac Surg. 2004;33:25–31. doi: 10.1054/ ijom.2003.0448. [DOI] [PubMed] [Google Scholar]
- 6.Bell D, Roberts D, Kies M, Rao P, Weber RS, El-Naggar AK. Cell type-dependent biomarker expression in adenoid cystic carcinoma: biologic and therapeutic implications. Cancer. 2010;116:5749–5756. doi: 10.1002/cncr.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenman G, Sandros J, Mark J, Edstrom S. Partial 6q deletion in a human salivary gland adenocarcinoma. Cancer Genet Cytogenet. 1989;39:153–156. doi: 10.1016/0165-4608(89)90180-5. [DOI] [PubMed] [Google Scholar]
- 8.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA. 2009;106:18740–18744. doi: 10.1073/ pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenman G, Andersson MK, Andren Y. New tricks from an old oncogene: gene fusion and copy number alterations of MYB in human cancer. Cell Cycle. 2010;9:2986–2995. doi: 10.4161/ cc.9.15.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slamon DJ, Boone TC, Murdock DC, Keith DE, Press MF, Larson RA, et al. Studies of the human c-myb gene and its product in human acute leukemias. Science. 1986;233:347–351. doi: 10.1126/science.3014652. [DOI] [PubMed] [Google Scholar]
- 11.Wilkins HR, Doucet K, Duke V, Morra A, Johnson N. Estrogen prevents sustained COLO-205 human colon cancer cell growth by inducing apoptosis, decreasing c-myb protein and decreasing transcription of the antiapoptotic protein bcl-2. Tumour Biol. 2010;31:16–22. doi: 10.1007/s13277-009-0003-2. [DOI] [PubMed] [Google Scholar]
- 12.Mitani Y, Li J, Rao PH, Zhao YJ, Bell D, Lippman SM, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability and clinicopathologic significance. Clin Cancer Res. 2010;16:4722–4731. doi: 10.1158/1078-0432.CCR-10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drabsch Y, Robert RG, Gonda TJ. MYB suppresses differentiation and apoptosis of human breast cancer cells. Breast Cancer Res. 2010;12:55. doi: 10.1186/bcr2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonda TJ, Leo P, Ramsay RG. Estrogen and MYB in breast cancer: potential for new therapies. Expert Opin Biol Ther. 2008;8:713–717. doi: 10.1517/14712598.8.6.713. [DOI] [PubMed] [Google Scholar]
- 15.Kim JY, Lim SJ, Park K, Lee CM, Kim J. Cyclooxygenase-2 and c-erbB-2 expression in uterine cervical neoplasm assessed using tissue microarrays. Gynecol Oncol. 2005;97:337–341. doi: 10.1016/j.ygyno.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.West RB, Kong C, Clarke N, Gilks T, Lipsick JS, Cao H, et al. MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Pathol. 2011;35:92–99. doi: 10.1097/PAS.0b013e3182002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akrish S, Peled M, Ben-Izhak O, Nagler RM. Malignant salivary gland tumors and cyclo-oxygenase-2: a histopathological and immunohistochemical analysis with implications on histogenesis. Oral Oncol. 2009;45:1044–1050. doi: 10.1016/j.oraloncology.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Al-Rawi NH, Omer H, Al Kawas S. Immunohistochemical analysis of P(53) and bcl-2 in benign and malignant salivary glands tumors. J Oral Pathol Med. 2010;39:48–55. doi: 10.1111/j.1600-0714.2009.00816.x. [DOI] [PubMed] [Google Scholar]