Abstract
Receptor tyrosine kinases (RTKs) have been the subject of intense investigation due to their widespread deregulation in cancer and the prospect of developing targeted therapeutics against these proteins. The Ron RTK has been implicated in tumor aggressiveness and is a developing target for therapy, but its function in tumor progression and metastasis is not fully understood. We examined Ron activity in human breast cancers and found striking predominance of an activated Ron isoform known as short-form Ron (sfRon), whose function in breast tumors has not been explored. We found that sfRon plays a significant role in aggressiveness of breast cancer in vitro and in vivo. sfRon expression was sufficient to convert slow-growing, nonmetastatic tumors into rapidly growing tumors that spontaneously metastasized to liver and bones. Mechanistic studies revealed that sfRon promotes epithelial-mesenchymal transition, invasion, tumor growth, and metastasis through interaction with p85, the regulatory subunit of phosphoinositide 3-kinase (PI3K). Inhibition of PI3K activity, or introduction of a single mutation in the p85 docking site on sfRon, completely eliminated the ability of sfRon to promote tumor growth, invasion, and metastasis. These findings reveal sfRon as an important new player in breast cancer and validate Ron and PI3K as therapeutic targets in this disease.
Keywords: breast cancer, Ron, short-form Ron, metastasis, PI3K
Introduction
Human cancers are heterogenous diseases that encompass a variety of pathological features with varied clinical outcomes. One common feature of cancers is robust activation of one or more receptor tyrosine kinases (RTKs), which allows evasion of antigrowth controls surrounding normal cell physiology in adults. RTKs activate signal transduction pathways that culminate in cellular programs regulating proliferation, apoptosis, and senescence, as well as cell migration and invasion.
The majority of cancer deaths result from dissemination of the tumor, or metastasis. Metastasis is a complicated process that requires cancer cells to escape from the tumor and enter into the vasculature, survive in the bloodstream, exit from the vasculature in distant organs, adapt to a new microenvironment, and reinitiate growth. An understanding of some of the key players in metastasis has emerged over the years, and RTKs and their pleiotropic effectors are key contributors.1 Efforts to inhibit RTKs as cancer therapy are ongoing, with limited success. One hurdle to RTK inhibition as an effective treatment in cancer is the presence of extensive RTK coactivation networks, which can contribute to drug resistance.2 Clearly, a better understanding of detailed mechanisms by which particular RTKs function in specific processes and in specific cancer types is crucial for designing new therapies and clinical trials.
Met and Ron are related RTKs that have been implicated in tumor growth and metastasis in animal models3-5 and in patients.5-7 Although the function of Met in cancer has been studied for more than 20 years, culminating in development of targeted therapies now in clinical trials,8 Ron has more recently emerged as a major player in cancer, where its expression usually correlates with more aggressive disease and poor outcomes.5,9,10 Ron inhibitors are now actively being developed in the pharmaceutical industry.
Ron is overexpressed, but not amplified or mutated, in many epithelial cancers. Often concomitant with overexpression of wild-type Ron is the expression of alternative Ron isoforms that yield constitutively active proteins.6,11 One of these variants, a 55-kD N-terminally truncated form of Ron, called “short-form” or sfRon, is generated from an alternative transcriptional start from a second promoter within exon 10 of RON.12 Translation of the shorter mRNA begins in frame, at Met913, which corresponds to a codon within exon 11 of the normal RON transcript. Thus, the sfRon protein lacks the N-terminus of Ron, including most of the extracellular domain. The transmembrane and cytoplasmic domains remain intact, however, and comprise identical amino acid sequence to the corresponding portion of full-length Ron.
sfRon is of particular interest in cancer: the mouse ortholog of RON, stk, has been extensively studied with respect to cancer susceptibility. As with human RON, an internal promoter within the stk gene gives rise to a shortened transcript that results in production of “short-form” stk protein (sf-stk), analogous to sfRon. Mapping of resistance loci in strains of mice that are not susceptible to development of Friend virus (FV)–induced erythroleukemia led to the discovery of sf-stk as a required contributor to this cancer. A 3-nucleotide deletion polymorphism within the sf-stk promoter in resistant mouse strains results in a nonfunctional promoter, and introduction of exogenous sf-stk restores susceptibility to FV-induced erythroleukemia.13,14
In humans, sfRON mRNA is detected in both normal and malignant cells from several tissues,12 indicating that usage of the internal promoter is functionally conserved between mice and humans. sfRON proteins organize into constitutively active autophosphorylated dimers that can confer a growth advantage to cells in vitro: inactivation of sfRON using kinase-dead variants in lung cancer and erythroleukemia cells inhibited proliferation.15 Overexpression of sfRon in T47D cells caused silencing of the E-cadherin promoter via chromatin remodeling,12 which could be reversed by overexpression of unliganded estrogen receptor α.16
It is not known how sfRon functions in tumors in vivo, nor has the relevance of sfRon to breast cancer been explored. In this study, we examined the importance of sfRon in human breast cancer by utilizing primary human breast tumors and model systems in vitro and in vivo. The results were striking: we found that sfRon is the major active Ron isoform in breast tumors from patients and that it plays a significant role in the aggressiveness of breast cancer. sfRon expression was sufficient to convert slow-growing, nonmetastatic tumors into fast-growing tumors that spontaneously metastasized from the mammary gland to liver and bones: both are clinically relevant sites for breast cancer metastasis. Mechanistic studies revealed an interaction between sfRon and phosphoinositide 3-kinase (PI3K) that was required for sfRon function. Blocking the ability of sfRon to interact with PI3K thoroughly abrogated the ability of sfRon to confer aggressive tumor behavior and completely blocked metastasis. These findings provide important new information as to the function of sfRon in cancer and validate both sfRon and PI3K as potential therapeutic targets in breast cancer.
Results
sfRon is the major active Ron isoform in tumors from breast cancer patients
To assess the abundance and activity of Ron and sfRon in primary breast cancers, we first examined mRNA levels according to a method developed previously, which takes advantage of a short, unique sequence in the 5′UTR of sfRon mRNA that is derived from intron 10.12 We examined RNA from a total of 25 primary breast specimens: 5 from normal breasts following reduction mammoplasty, 10 from estrogen receptor (ER)–positive cancers, and 10 from ER–negative cancers. We detected sfRon mRNA in 76% of all breast tissues examined and found no correlation between sfRon mRNA expression, cancer status, or ER status (Suppl. Table S1). This is in agreement with Bardella et al., who reported sfRon mRNA expression in a variety of normal and cancerous tissue types.12 We found that in both normal breast and breast cancers, sfRon mRNA was detected in a greater proportion of samples than Ron mRNA. With the number of tissues we examined, the expression of Ron mRNA did not significantly predict expression of sfRon mRNA or vice versa (Fisher exact test); however, our data are consistent with a report that hypermethylation/silencing of the region surrounding the Ron promoter is associated with increased transcription from the sfRon promoter in non–small cell lung cancer.15 Our data indicated that, at least in the human breast, the sfRon promoter is functional and leads to production of sfRon mRNA in the majority of breast cancers and normal breast tissue.
To determine the relative expression and activation levels of Ron and sfRon proteins in breast cancers, we carried out analysis using several different antibodies that are specific for the C-terminus of the protein and therefore are able to recognize both Ron and sfRon. One of these antibodies, anti-Ron C-20, recognizes both phosphorylated and nonphosphorylated Ron proteins (pRon and Ron, respectively) but has higher affinity for the nonphosphorylated protein (Suppl. Figs. S1 and S2A). The other antibodies used were anti-pRon Y1238/1239 (specific for phosphorylation in the kinase domain) and anti-pRon Y1353/1360 (specific for phosphorylation in the docking site), which both recognize activated Ron and sfRon in normal and cancerous tissues.
Western analysis of breast cancers from 29 different patients using anti-Ron C-20 revealed high expression of Ron protein in 31% of tumors and low levels of expression in 20% of tumors (Fig. 1 shows a representative blot with 6 samples), which is consistent with previous reports.6 sfRon was detected in 69% of all tumors examined (Fig. 1 and data not shown) and existed as both an unmodified 55-kDa protein and as 2 posttranslationally modified higher molecular weight forms that were previously noted but not described.15 The higher molecular weight sfRon bands (HMW sfRon) are specific to sfRon protein because they appear in breast cancer cells only when the sfRon cDNA is introduced (Suppl. Fig. S2A and S2B). The migration of HMW sfRon bands (~10-kDa shift for each) is consistent with the fact that the C-terminus of Ron is ubiquitylated at multiple sites through direct interaction with the E3 ubiquitin ligase Cbl following its activation17 and our own data that phosphorylated sfRon can be ubiquitylated in MCF7 cells (Suppl. Fig. S2C).
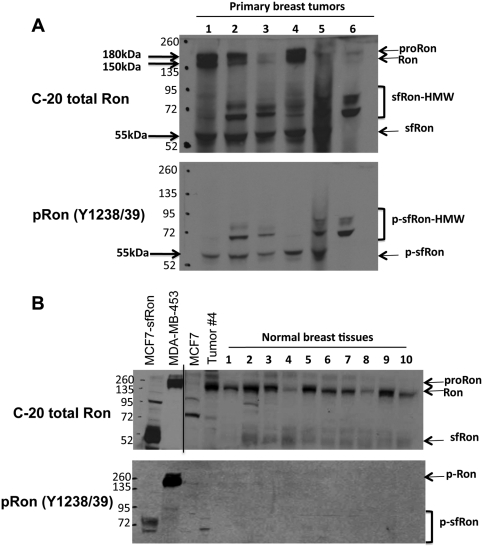
Figure 1.
sfRon is the major active Ron isoform in tumors from breast cancer patients. (A) Representative Western blot of breast tumor lysates from 6 different patients using antibodies specific for the C-terminus of Ron (C-20; upper blot) or those specific for active, phosphorylated Ron (pRon Y1238/1239; lower blot). (B) Representative Western blot of breast tissue lysates from 10 different patients following reduction mammoplasty using antibodies specific for the C-terminus of Ron (C-20; upper blot) or those specific for active, phosphorylated Ron (pRon Y1238/1239; lower blot). Tumor 4 is the same sample as that shown in A. The line on the top gel denotes separation of 2 different film exposures from the same blot. The proform of Ron (proRon), Ron β chain (Ron), and sfRon (or phosphorylated sfRon [p-sfRon]) are indicated. The putative ubiquitylated sfRon (sfRon-HMW) is also noted.
Examination of sfRon and Ron proteins in 10 normal breast tissues isolated from reduction mammoplasty patients revealed that Ron is the main isoform expressed in normal breast. In contrast to tumor tissue, no phosphorylated Ron or sfRon protein was detected in normal breast tissue (Fig. 1B). Taken together, these data showed that sfRon protein and mRNA levels do not correlate well in breast tissues and that sfRon protein becomes specifically upregulated in breast cancers. Furthermore, sfRon and HMW sfRon are the major isoforms of active (phosphorylated) Ron in breast tumors from patients, which suggests that sfRon could play an important role in breast cancer.
sfRon confers EMT and invasive capabilities in breast cancer cells
Despite its abundance in primary patient samples, sfRon protein is not detectable in most breast cancer cell lines (Suppl. Fig. S3), and its function is not understood. We asked whether introduction of sfRon into breast cancer cells would alter their behavior in vitro or in vivo. sfRon was introduced into human MCF7 breast cancer cells, and protein expression was verified by Western blotting with anti-Ron C-20 and anti-pRon antibodies (Suppl. Fig. S2A). It is important to note that although our lines were engineered to express exogeneous Ron and sfRon, the level of phosphorylated Ron is very similar to that observed in actual patient tumors, indicating that our model system exhibits biologically relevant levels of activated sfRon (Suppl. Fig. S2B).
To determine whether expression of sfRon affected cell proliferation or survival in culture, we assayed mitochondrial dehydrogenase activity as a surrogate for cell number, as a function of time. While cells expressing sfRon proliferated slower at low density, there was no significant difference in the overall rate of growth over 6 days compared to parental MCF7 cells. This was in contrast to overexpression of Ron, which did significantly increase proliferation (Fig. 2A, left panel). We also found no difference in the ability of sfRon cells to survive in serum-free conditions for the same period of time, whereas Ron expression improved survival (Fig. 2A, right panel).
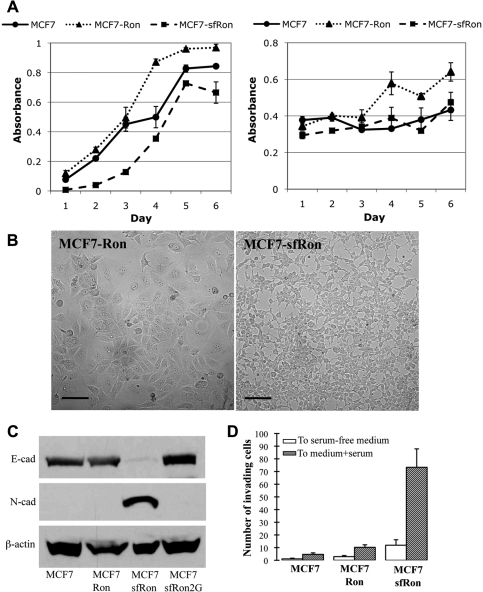
Figure 2.
sfRon confers EMT and invasive capability in breast cancer cells. (A) Left panel: proliferation of MCF7 and MCF7-sfRon cells based on mitochondrial dehydrogenase activity, read as absorbance over time. Right panel: survival of MCF7 and MCF7-sfRon cells in serum-free conditions based on mitochondrial dehydrogenase activity. (B) Phase-contrast micrograph illustrating altered morphology of MCF7 cells expressing sfRon (right panel) compared to those expressing full-length Ron (left panel). Photographs were taken at the same magnification; scale bars represent 100 µm. (C) Western blot with antibodies specific for E-cadherin, N-cadherin, and β-actin showed that sfRon decreased expression of E-cadherin and increased expression of N-cadherin. (D) sfRon induced invasion, as quantified by determining the number of cells that passed through Matrigel-coated Boyden chambers. No significant change was observed in MCF7-Ron cells. White bars: random invasion toward serum-free medium; gray bars: invasion toward medium containing 10% serum. Error bars reflect standard deviation from at least 3 experimental replicates.
Approximately 2 to 3 weeks following introduction of sfRon, the cells underwent a distinct morphological change resembling epithelial-to-mesenchymal transition (EMT): conversion from large cuboidal cells with prominent cell-cell contacts to smaller cells with reduced cell-cell contact and spindle-like morphology (Fig. 2B). The EMT was verified by assessing the molecular features that define the transition: decreased expression of the epithelial marker E-cadherin and increased expression of the mesenchymal markers N-cadherin and vimentin (Fig. 2C and Suppl. Fig. S3). Other defining characteristics of EMT were also noted, including enrichment of β-catenin in nuclei, increased activity of matrix metalloproteinase 2, and increased expression of the EMT master regulators Twist, SLUG, and SIP1 (Suppl. Fig. S3).
The EMT often correlates with increased proliferation, survival, migration, and the ability to invade through extracellular matrices in vitro and in vivo.18,19 Although we found no change in MCF7-sfRon cell proliferation or survival in our assays, we noted a marked increase in the ability of MCF7-sfRon cells to invade through a mixture of extracellular matrix (ECM) proteins known as Matrigel. MCF7-sfRon cells exhibited up to 14-fold increased invasion activity compared to parental MCF7 cells (Fig. 2D). This occurred despite no change in basic cell migration (data not shown) and did not occur with overexpression of full-length Ron, indicating that sfRon specifically confers invasive ability to breast cancer cells. Notably, sfRon induced EMT, invasion, and tumor growth in another breast cancer cell line, MDA-MB-361 (Suppl. Fig. S4), indicating that the activity of sfRon is not limited to MCF7 cells.
PI3K signaling is required for the invasive activity of sfRon
Ron is known to activate several signaling routes, including the mitogen-activated protein kinase (MAPK), PI3K, Src, and phospholipase C (PLC) pathways, in part via direct binding of SH2 domain containing adaptor proteins to the C-terminal docking site.9 As discussed above, sfRon is identical to the last 488 amino acids of Ron but can exist in a phosphorylated state despite its inability to bind the MSP ligand.12 However, very little is known about which signaling pathways are activated by sfRon. We observed that MCF7-sfRon cells exhibited strong activation of the PI3K pathway, as assessed by phosphorylation of the PI3K target Akt (Fig. 3A). In contrast, sfRon expression blocked the MAPK pathway, which was reflected in loss of Erk1/2 phosphorylation (Fig. 3A). Full-length Ron did not have this effect, indicating that sfRon elicits yet another function(s) that is distinct from that of Ron.
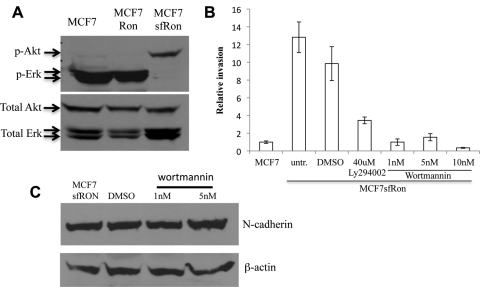
Figure 3.
PI3K signaling is activated by sfRon and is required for the invasive activity of sfRon. (A) Western blot on whole-cell lysates from parental MCF7 cells or those expressing Ron or sfRon, using antibodies specific for Akt or Erk (phosphorylated or total). (B) Addition of PI3K inhibitors Ly294002 (40 uM) or wortmannin (1, 5, or 10 nM) blocked sfRon-induced invasion. Error bars reflect standard deviation from at least 3 experimental replicates. (C) Western analysis of N-cadherin on wortmannin-treated MCF7-sfRon cells. The results revealed that blocking PI3K signaling could not reverse sfRon-induced EMT.
The pleckstrin homology domain containing kinase PDK1 is recruited to membranes in response to PI3K activation, where it is responsible for phosphorylating Akt and other signaling molecules.20 To further verify increased PI3K activity in cells expressing sfRon, we performed Western blotting for 2 downstream targets of PI3K (Akt and PDK1) in the presence and absence of PI3K inhibitors (LY294002 or wortmannin). As expected, phosphorylation of Akt, but not PDK1, was blocked in response to PI3K inhibitors (Suppl. Fig. S5A and S5B); PI3K activity does not specifically affect phosphorylation status of PDK1 but promotes its localization to the membrane.20,21 To determine the subcellular localization of PDK1 in response to sfRon in the presence or absence of PI3K inhibitors, we also carried out immunofluorescence for PDK1 and captured images using confocal microscopy. We found that PDK1 was recruited to the plasma membrane in the presence of sfRon, and this effect was eliminated by treatment with PI3K inhibitors (Suppl. Fig. S5C-L). Thus, our data indicate that sfRon induces PI3K activity, resulting in localization of PDK1 to the plasma membrane and activation of Akt.
The robust signaling of sfRon through PI3K prompted us to determine whether this pathway was required for sfRon function in breast cancer cells. Invasion of MCF7-sfRon cells was inhibited by addition of either of the PI3K inhibitors (Fig. 3B). Blockade of invasion by both PI3K inhibitors was dose dependent: nearly complete inhibition of invasion was achieved with concentrations as low as 1 nM wortmannin (Fig. 3B).
It is notable that although inhibition of PI3K activity was sufficient to block invasion of MCF7-sfRon cells, it was not sufficient to reverse the EMT. The cells retained their N-cadherinhigh phenotype (Fig. 3C) and retained expression of EMT transcription factors and mesenchymal morphology (Suppl. Fig. S6A and S6B) in the presence of wortmannin, even over long-term culture with this inhibitor. These data suggested that the EMT phenotype, even in the presence of sfRon, is not sufficient to endow invasive capabilities to these cells. We then asked whether sfRon-induced EMT could be inhibited by treating with wortmannin before introduction of sfRon into the cells. Inhibition of PI3K prior to expression of sfRon blocked sfRon-induced EMT and cell invasion (Suppl. Fig. S6C and S6D). Thus, PI3K activity is required for sfRon to orchestrate the EMT and is necessary for invasion in all scenarios tested (before and after EMT). However, the EMT phenotype is not sufficient to promote invasion in the absence of PI3K signaling.
To validate our observation that PI3K activity is required for sfRon to induce EMT and invasion, we applied a genetic approach to manipulate sfRon signaling, similar to an approach previously used for Met.22 The C-terminal docking site of Ron contains 2 tandem tyrosines in a degenerate motif (Y1353VQLPATY1360MNLGP), which contains a predicted binding site for the SH2 domain of the PI3K regulatory subunit, p85 (Fig. 4A). To determine whether sfRon binds p85, we carried out GST pull-down experiments and found that sfRon does indeed interact with PI3K through the SH2 domain of p85 (Fig. 4B).
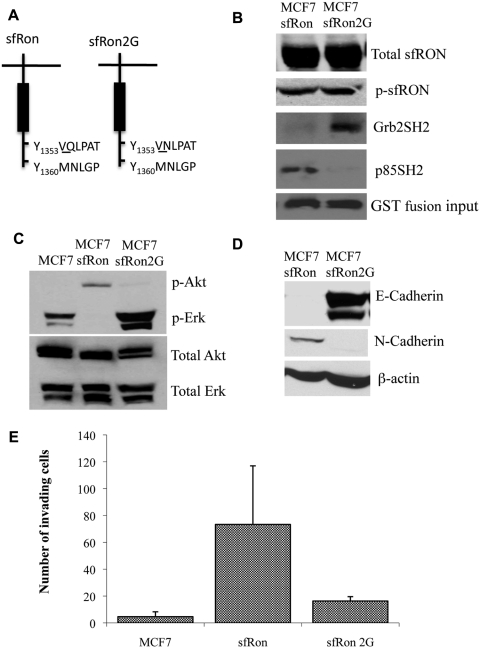
Figure 4.
PI3K activation through interaction with sfRon is required for EMT and invasion. (A) Diagram showing mutation of the sfRon docking site (2G), designed to disrupt PI3K binding and facilitate Grb2 binding. (B) Western blot demonstrating that the sfRon2G mutation did not change sfRon and phosphorylated sfRon (p-sfRon, detected with anti-pY1238/39 Ron) expression in MCF7 cells (top 2 panels). Lower panels: Western blot for sfRon following GST pull-down assays using the SH2 domain of Grb2 (Grb2SH2) or the SH2 domain of the p85 subunit of PI3K (p85SH2). The amount of GST protein used in the assays is shown in the lowest panel. (C) Western blot showing that sfRon2G promotes MAPK signaling (shown by p-Erk) instead of PI3K signaling (shown by p-Akt). (D) The sfRon2G mutant failed to induce EMT in MCF7 cells, as assessed by Western blotting for E-cadherin and N-cadherin proteins. (E) The sfRon2G mutant was defective for invasion ability, as measured by quantification of cells that crossed the Matrigel-coated membrane in a Boyden chamber assay.
We mutated a single amino acid in the putative p85 binding site to convert it to an optimal predicted binding site for Grb2 (Q1355N); we refer to this mutant as sfRon2G (Fig. 4A). Grb2 binding facilitates activation of the Ras/Raf/MAPK pathway downstream of Ron.23,24 We then generated an MCF7 cell line expressing sfRon2G and verified that the expression and phosphorylation levels of each sfRon protein were identical in these cell lines (Fig. 4B). GST pull-down experiments showed that the sfRon2G mutation eliminated binding of p85 to sfRon and instead promoted Grb2 binding (Fig. 4B). Indeed, sfRon2G expression led to phosphorylation of Erk1/2 (Fig. 4C), whereas wild-type sfRon blocks Erk activation (Figs. 3A and 4C). In addition, presumably because p85 can no longer bind to the sfRon2G protein, which uncouples PI3K activity from sfRon, Akt was no longer phosphorylated (Fig. 4C).
Next, we examined whether sfRon2G could promote invasion to a similar extent as that induced by sfRon. The single mutation that switches sfRon signaling from the PI3K pathway to the Ras/Raf/MAPK pathway blocked both EMT and invasion (Fig. 4D and 4E). This was not due to restoration of MAPK activity because the MEK inhibitor U0126 did not rescue the decreased invasion of cells expressing sfRon2G (data not shown). These data are consistent with our results using pharmacological inhibitors of PI3K and verify that PI3K activity, through interaction of p85 with the C-terminus of sfRon, is required for sfRon-mediated invasion of breast cancer cells.
sfRon promotes tumor growth and metastasis
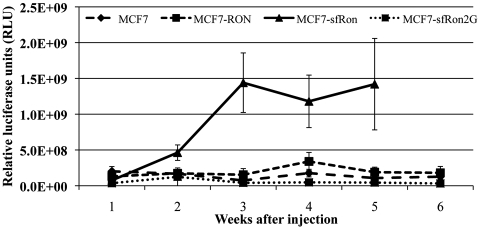
In vitro EMT and invasion assays are often correlated with metastatic behavior in vivo but are nevertheless a surrogate measure for aggressiveness. Metastasis is a complicated, multistep process that requires invasion of tumor cells through the ECM and into the local environment, entry into the vasculature, extravasation and colonization in other organs, and continued growth to form metastatic tumors. Our in vitro data indicate that sfRon promotes EMT and endows cells with the ability to invade through ECM. We next asked whether sfRon might promote metastasis in vivo. As a first indication of potential metastatic activity, we performed experimental metastasis assays by carrying out intracardiac injection of luciferase-labeled tumor cells. We found that cells expressing either Ron or sfRon colonized metastases more efficiently and earlier than control cells (Suppl. Table S1), and mice injected with MCF7-sfRon tumors had to be euthanized earlier than control mice due to tumor burden (Suppl. Fig. S7). Therefore, in addition to increasing the invasive ability of tumor cells, sfRon provided potential metastatic function in vivo, albeit with an experimental metastasis assay. These data prompted us to investigate whether sfRon promotes metastasis in a more physiologically relevant spontaneous metastasis assay and whether this function depends on interaction with PI3K.
To determine if sfRon is sufficient to promote tumor progression and/or spontaneous metastasis from actual mammary tumors, we performed orthotopic transplantation of the various lines into mammary glands of NOD/SCID mice. Expression of sfRon had profound effects on tumor growth beginning just 2 weeks after injection of cells, which also required its ability to bind PI3K (Fig. 5). Tumors expressing sfRon reached the maximum tumor size by 5 to 7 weeks, whereas tumors containing the vector control, full-length Ron, or the sfRon2G mutant did not reach the same endpoint until at least 20 weeks (Fig. 5 and data not shown). Therefore, despite no detectable effect on cell proliferation or survival in vitro, sfRon significantly promoted tumor growth in vivo, which depended on its ability to bind and activate PI3K.
Figure 5.
sfRon expression increases tumor growth through activation of PI3K. Orthotopic tumor growth rate for MCF7, MCF7-Ron, MCF7-sfRon, and MCF-sfRon2G cells upon orthotopic injection into mammary fat pads of NOD/SCID mice (n = 5-7 per group; error bars reflect standard deviations).
We noted no obvious differences in tumor histology including the appearance of microvessels or necrosis between the experimental groups (Suppl. Fig. S8). The histology of sfRon-expressing tumors, however, appeared more poorly differentiated as judged by lack of any glandular features and consisted of smaller cells. The sfRon2G mutant-expressing tumors appeared similar to control tumors and tumors expressing Ron. These data are consistent with our findings that sfRon drives an EMT, which we also confirmed in vivo by loss of E-cadherin staining in sfRon-expressing but not sfRon2G-expressing tumors (Suppl. Fig. S8).
MCF7 cells are tumorigenic but generally nonmetastatic.25 To determine whether sfRon contributes to spontaneous metastasis, we performed ex vivo luciferase imaging on organs isolated from each mouse at necropsy. While control MCF7 tumors did not metastasize at all in our studies, we observed spontaneous metastasis to bone, liver, and spleen from primary tumors expressing Ron or sfRon (Table 1). On the other hand, the sfRon2G mutant failed to increase metastasis even over a 27-week period (Table 1; to avoid the confounding factor of tumor size on metastasis, the endpoint for all groups was based on the tumor size rather than time). These data indicate that p85 binding is required for sfRon to promote both tumor growth and metastasis.
Table 1.
Summary of Orthotopic Tumor Growth and Spontaneous Metastasis in NOD/SCID Mice Injected with MCF7, MCF7-Ron, MCF7-sfRon, or MCF7-sfRon2G Cells
| MCF7 | MCF7-Ron | MCF7-sfRon | MCF7-sfRon2G | |
|---|---|---|---|---|
| Tumor formation (time to endpoint) | 5/5 (20+ weeks) | 6/6 (20+ weeks) | 8/8 (5-7 weeks) | 5/5 (27 weeks) |
| Metastasis (any site) | 0/5 | 5/6 (83%) | 6/8 (75%) | 0/5 |
| Bone | 0/5 | 1/6 (17%) | 5/8 (67.5%) | 0/5 |
| Lung | 0/5 | 2/6 (33%) | 0/8 | 0/5 |
| Liver | 0/5 | 2/6 (33%) | 1/8 (12.5%) | 0/5 |
| Spleen | 0/5 | 0/6 | 1/8 (12.5%) | 0/5 |
Discussion
Ron has recently emerged as an important mediator of tumor progression in human cancers. Based on its association with aggressive disease, as well as its increased expression in tumor versus normal cells, Ron is an exciting new therapeutic target in cancer. We and others have previously shown that Ron or its ligand promotes breast tumor growth and metastasis in various cell lines and in mouse models4,5,26 and that expression of critical components of the Ron signaling pathway in tumors is prognostic for poor outcome in breast cancer patients.5 However, due to common limitations on tissue availability, previous studies using primary tumors have mainly utilized gene expression microarray data rather than examining active Ron proteins. We utilized several antibodies to assess the relative abundance of Ron isoforms from primary breast cancers and found a striking result: most of the active, phosphorylated Ron exists as sfRon, not as full-length Ron.
Our studies show that engineered expression of sfRon in breast cancer cells, which led to the same activation levels as those seen in primary tumors, confers a more aggressive phenotype: loss of epithelial features, increased invasive capability, increased tumor growth, and robust metastasis. These data could explain the increased incidence of sfRon expression in patient tumors compared to Ron expression; sfRon might provide a selective advantage to the tumors that express it. It is intriguing that sfRon expression is not retained in most breast cancer cell lines, and exogenously expressed sfRon did not increase cell proliferation or survival in our studies in vitro. Ron, on the other hand, is expressed in many breast cancer cell lines and facilitates both proliferation and survival in that setting. Our data show a prominent effect of sfRon on tumor growth in vivo but not in vitro, suggesting that environmental context plays a key role in sfRon-mediated tumor progression.
It has been reported that sfRon can increase growth of T47D cells in vitro in addition to promoting an EMT-like state12; it is possible that the effects of sfRon on proliferation may differ with cell context, culture conditions, and/or sfRon expression levels. We did note that the proliferation rate of sfRon-expressing MCF7 cells was greater than controls between days 3.5 to 5 (slope of the graph in Fig. 2A), but we did not find a significant effect over the course of the entire assay.
We also found that sfRon and Ron signals appear to be divergent: while Ron activates both MAPK and PI3K pathways,24,27,28 sfRon turns off the MAPK pathway and signals strongly through PI3K in the breast cancer cells we examined. Mutation of the PI3K binding site in sfRon, or treatment with PI3K inhibitors, was sufficient to completely inhibit the tumor-promoting functions of sfRon. The role for PI3K signaling in sfRon-driven tumor progression is currently not known but does not appear to be through the Akt/mTOR pathway because inhibition of Akt or mTOR was not able to suppress invasion (Suppl. Fig. S8), and prolonged growth of sfRon-expressing breast cancer cells in the presence of wortmannin eventually led to alternative activation of Akt without restoring invasive and metastatic capabilities to the cells (data not shown). Akt-independent signaling downstream of PI3K, through activation of PDK1 and SGK3, has been recently described in various cancer cell lines, including breast cancer,21 suggesting that this arm of signaling should be explored as a potential downstream effector pathway for sfRon.
Lack of knowledge about critical mechanisms of metastasis has precluded design of therapies that specifically prevent or treat metastatic disease. Rigorous studies of metastasis require whole animal systems, and models in which metastasis spontaneously occurs from primary tumors are rare. We have recently developed mouse model systems in which breast cancers spontaneously metastasize from the orthotopic tumor, so the entire process can be studied.5,29 Expression of sfRon provides another example in which metastasis occurs readily from the primary site and therefore holds promise as a model to better understand metastasis. It is interesting that Ron also promoted metastasis, despite having no significant effect on tumor growth. The mechanisms by which Ron promotes metastasis, and whether it also depends on PI3K, are currently underway.
Occult metastatic disease is thought to be present in some patients at the time of diagnosis of primary breast and other cancers,30,31 and adjuvant therapy is an attempt to prevent growth of any disseminated cells. In the case of breast cancer, where the primary tumor can usually be excised, prevention of early steps of metastasis such as tumor migration and invasion may be less clinically relevant than preventing growth of cells that have already disseminated. Our data indicate that sfRon may play a role in growth of tumor cells even after they have spread throughout the body. This is a promising result, suggesting that sfRon, and perhaps PI3K, might be a viable potential target in the adjuvant setting. Our data warrant future studies examining whether the presence of active sfRon correlates with clinical outcomes and whether Ron kinase inhibition would prove useful as new therapy for breast cancer.
Materials and Methods
Tissues
All tissue samples were collected from patients who provided informed consent at Huntsman Cancer Hospital/University of Utah under an approved institutional review board protocol.
Cell lines and reagents
MCF7 cells used in these studies were generously provided by Heide Ford (University of Colorado, Denver, CO) and were previously described.32 MCF7 cells were maintained in DMEM/F-12 supplemented with 10% FBS, 10 µg/mL insulin, and 100 U/mL penicillin. All cells were incubated at 37°C in humidified air containing 5% CO2 and routinely passaged with trypsin-EDTA.
qRT-PCR
Total RNA was extracted with the Qiagen RNeasy Mini kit (Qiagen, Valencia, CA), according to the manufacturer’s protocol. cDNA was synthesized with M-MuLV reverse transcriptase (Fermentas Life Sciences, Glen Burnie, MD), using 1 µg total RNA per 20 µL reaction. Quantitative real-time PCR was performed on cDNA samples (details and primer sequences are in the supplementary material).
Western blotting and immunoprecipitation
Western blotting was carried out on 50- to 100-µg tumor or tumor cell lysates, or immunoprecipitates from 1-mg lysate, following separation by 12% SDS-polyacrylamide gel electrophoresis and transfer to PVDF membranes (Millipore Corporation, Billerica, MA). Details on preparation of protein lysates and antibodies used are in the supplementary material.
Cell proliferation and survival assays
For proliferation assays, cells were seeded at 2,000 cells per well and allowed to grow in complete medium for 6 days. Relative cell numbers were assayed each day using a Quick Cell Proliferation assay (BioVision, Mountain View, CA), which measures cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases, according to the manufacturer’s instructions. For survival assays, cells were seeded at 100,000 cells per well in serum-free medium and relative cell number assayed each day using the same mitochondrial dehydrogenase assay.
Invasion assays
Growth factor–reduced Matrigel invasion chambers (24-well; 8.0-µm pore) were used according to the manufacturer’s instructions (BD Biosciences, Franklin Lakes, NJ). The Matrigel in each chamber was rehydrated with serum-free medium for 2 hours at 37°C in a humidified incubator. To block cellular proliferation during the assay, cells were treated with mitomycin C (10 µg/mL, Sigma, St. Louis, MO) just prior to the assay. For some experiments, inhibitors of PI3K (wortmannin [Sigma] or LY294002 [Cell Signaling Technology, Danvers, MA]), AKT1/2 (A6730, Sigma), or MEK (U0126, Cell Signaling Technology) were added. After incubation at 37°C for 48 hours, invading cells were quantified by counting 5 representative high-powered fields.
Immunofluorescence
Cells were grown on glass coverslips and fixed with 4% paraformaldehyde for 15 minutes at room temperature. Following permeabilization of the cells with 0.5% Triton X-100, the samples were blocked with 0.5% bovine serum albumin. Primary antibodies specific for pPDK1 and Alexa Fluor 488–labeled (Invitrogen, Carlsbad, CA) secondary antibodies were both used at a 1:100 dilution in blocking buffer. Images were captured using a spinning disk confocal microscope.
Site-directed mutagenesis
The sfRon2G point mutation was introduced using the QuickChange Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA), using pMSCV-sfRon plasmid DNA as a template (oligo sequences are in the supplementary material).
GST pull-down assays
GST-Grb2SH2 was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), and pGEX6P-1 p85SH2 was a generous gift from Dr. Deborah Anderson (Saskatchewan Cancer Agency, Saskatoon, SK, Canada). There was 300-µg protein lysate used in each pull down.
Preparation of estrogen pellets
Lyophilized estrogen (Sigma) was dissolved in beeswax (Sigma-Aldrich) in a glass vial at 60°C. Three drops of the melted beeswax solution, which contained approximately 1.2 mg estrogen,33 were pooled onto cooled parafilm to form a pellet and stored at 4°C.
Orthotopic xenografts and experimental metastasis assays
All procedures were approved by the University of Utah Institutional Animal Care and Use Committee. Female NOD/SCID mice were injected at 4 to 6 weeks of age orthotopically with 1 × 106 MCF7, MCF7-Ron, MCF7-sfRon, or sfRon mutant cells suspended in Matrigel. A subcutaneous estrogen pellet was also transplanted behind the shoulder blades. Tumor growth was measured using the Xenogen IVIS System (Caliper Life Sciences, Hopkinton, MA) and calipers. For experimental metastasis assays, 5- to 7-week-old NOD/SCID mice were injected with 1 × 105 MCF7, MCF7-Ron, or MCF7-sfRon cells in HBSS buffer directly into the left cardiac ventricle. Further details are in the supplementary material.
Supplementary Material
Acknowledgments
The authors are grateful to members of the Welm laboratories and the HCI Breast Disease Oriented Team for productive suggestions.
Footnotes
Supplementary material for this article is available on the Genes & Cancer website at http://ganc.sagepub.com/supplemental.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
This work was supported by the Department of Defense Breast Cancer Research Program [grant number 08-1-0109 to A.L.W.]; American Association for Cancer Research/Breast Cancer Research Foundation [grant number 06-60-26-WELM to A.L.W.]; Susan G. Komen for the Cure [grant number KG081251 to A.L.W.]; and the Huntsman Cancer Foundation. The authors are grateful for use of core facilities, including the HCI Tissue Resource and Applications Core [grant number P30 CA042014].
References
- 1. Bacac M, Stamenkovic I. Metastatic cancer cell. Annu Rev Pathol. 2008;3:221-47 [DOI] [PubMed] [Google Scholar]
- 2. Xu AM, Huang PH. Receptor tyrosine kinase coactivation networks in cancer. Cancer Res. 2010;70(10):3857-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gallego MI, Bierie B, Hennighausen L. Targeted expression of HGF/SF in mouse mammary epithelium leads to metastatic adenosquamous carcinomas through the activation of multiple signal transduction pathways. Oncogene. 2003;22(52):8498-508 [DOI] [PubMed] [Google Scholar]
- 4. Peace BE, Toney-Earley K, Collins MH, Waltz SE. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res. 2005;65(4):1285-93 [DOI] [PubMed] [Google Scholar]
- 5. Welm AL, Sneddon JB, Taylor C, et al. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci U S A. 2007;104(18):7570-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maggiora P, Marchio S, Stella MC, et al. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16(22):2927-33 [DOI] [PubMed] [Google Scholar]
- 7. Graveel CR, DeGroot JD, Su Y, et al. Met induces diverse mammary carcinomas in mice and is associated with human basal breast cancer. Proc Natl Acad Sci U S A. 2009;106(31):12909-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15(7):2207-14 [DOI] [PubMed] [Google Scholar]
- 9. Wagh PK, Peace BE, Waltz SE. Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Adv Cancer Res. 2008;100:1-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kretschmann KL, Eyob H, Buys SS, Welm AL. The macrophage stimulating protein/Ron pathway as a potential therapeutic target to impede multiple mechanisms involved in breast cancer progression. Curr Drug Targets. 2010;11(9):1157-68 [DOI] [PubMed] [Google Scholar]
- 11. Lu Y, Yao HP, Wang MH. Multiple variants of the RON receptor tyrosine kinase: biochemical properties, tumorigenic activities, and potential drug targets. Cancer Lett. 2007;257(2):157-64 [DOI] [PubMed] [Google Scholar]
- 12. Bardella C, Costa B, Maggiora P, et al. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell-cell adhesion through E-cadherin transcriptional repression. Cancer Res. 2004;64(15):5154-61 [DOI] [PubMed] [Google Scholar]
- 13. Nishigaki K, Hanson C, Jelacic T, Thompson D, Ruscetti S. Friend spleen focus-forming virus transforms rodent fibroblasts in cooperation with a short form of the receptor tyrosine kinase Stk. Proc Natl Acad Sci U S A. 2005;102(43):15488-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Persons DA, Paulson RF, Loyd MR, et al. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23(2):159-65 [DOI] [PubMed] [Google Scholar]
- 15. Angeloni D, Danilkovitch-Miagkova A, Ivanova T, Braga E, Zabarovsky E, Lerman MI. Hypermethylation of Ron proximal promoter associates with lack of full-length Ron and transcription of oncogenic short-Ron from an internal promoter. Oncogene. 2007;26(31):4499-512 [DOI] [PubMed] [Google Scholar]
- 16. Cardamone MD, Bardella C, Gutierrez A, et al. ERalpha as ligand-independent activator of CDH-1 regulates determination and maintenance of epithelial morphology in breast cancer cells. Proc Natl Acad Sci U S A. 2009;106(18):7420-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penengo L, Rubin C, Yarden Y, Gaudino G. c-Cbl is a critical modulator of the Ron tyrosine kinase receptor. Oncogene. 2003;22(24):3669-79 [DOI] [PubMed] [Google Scholar]
- 18. Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39(3):305-18 [DOI] [PubMed] [Google Scholar]
- 19. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1-2):15-33 [DOI] [PubMed] [Google Scholar]
- 20. Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561-76 [PMC free article] [PubMed] [Google Scholar]
- 21. Vasudevan KM, Barbie DA, Davies MA, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16(1):21-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77(2):261-71 [DOI] [PubMed] [Google Scholar]
- 23. Finkelstein LD, Ney PA, Liu QP, Paulson RF, Correll PH. Sf-Stk kinase activity and the Grb2 binding site are required for Epo- independent growth of primary erythroblasts infected with Friend virus. Oncogene. 2002;21(22):3562-70 [DOI] [PubMed] [Google Scholar]
- 24. Iwama A, Yamaguchi N, Suda T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 1996;15(21):5866-75 [PMC free article] [PubMed] [Google Scholar]
- 25. Gelmann EP, Thompson EW, Sommers CL. Invasive and metastatic properties of MCF-7 cells and rasH-transfected MCF-7 cell lines. Int J Cancer. 1992;50(4):665-9 [DOI] [PubMed] [Google Scholar]
- 26. Zinser GM, Leonis MA, Toney K, et al. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with beta-catenin activation. Cancer Res. 2006;66(24):11967-74 [DOI] [PubMed] [Google Scholar]
- 27. Feres KJ, Ischenko I, Hayman MJ. The RON receptor tyrosine kinase promotes MSP-independent cell spreading and survival in breast epithelial cells. Oncogene. 2009;28(2):279-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang MH, Montero-Julian FA, Dauny I, Leonard EJ. Requirement of phosphatidylinositol-3 kinase for epithelial cell migration activated by human macrophage stimulating protein. Oncogene. 1996;13(10):2167-75 [PubMed] [Google Scholar]
- 29. Kim S, Welm AL, Bishop JM. A dominant mutant allele of the ING4 tumor suppressor found in human cancer cells exacerbates MYC- initiated mouse mammary tumorigenesis. Cancer Res. 2010;70(12):5155-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10 Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54(1):8-29 [DOI] [PubMed] [Google Scholar]
- 32. Micalizzi DS, Christensen KL, Jedlicka P, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J Clin Invest. 2009;119(9):2678-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Markaverich BM, Medina D, Clark JH. Effects of combination estrogen:cyclophosphamide treatment on the growth of the MXT transplantable mammary tumor in the mouse. Cancer Res. 1983;43(7):3208-11 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.